Abstract
Introduction
Patients with COPD experience exacerbations that may require hospitalization. Patients do not always feel supported upon discharge and frequently get readmitted. A Self-management Program of Activity, Coping, and Education for COPD (SPACE for COPD), a brief self-management program, may help address this issue.
Objective
To investigate if SPACE for COPD employed upon hospital discharge would reduce readmission rates at 3 months, compared with usual care.
Methods
This is a prospective, single-blinded, two-center trial (ISRCTN84599369) with participants admitted for an exacerbation, randomized to usual care or SPACE for COPD. Measures, including health-related quality of life and exercise capacity, were taken at baseline (hospital discharge) and at 3 months. The primary outcome measure was respiratory readmission at 3 months.
Results
Seventy-eight patients were recruited (n=39 to both groups). No differences were found in readmission rates or mortality at 3 months between the groups. Ten control patients were readmitted within 30 days compared to five patients in the intervention group (P>0.05). Both groups significantly improved their exercise tolerance and Chronic Respiratory Questionnaire (CRQ-SR) results, with between-group differences approaching statistical significance for CRQ-dyspnea and CRQ-emotion, in favor of the intervention. The “Ready for Home” survey revealed that patients receiving the intervention reported feeling better able to arrange their life to cope with COPD, knew when to seek help about feeling unwell, and more often took their medications as prescribed, compared to usual care (P<0.05).
Conclusion
SPACE for COPD did not reduce readmission rates at 3 months above that of usual care. However, encouraging results were seen in secondary outcomes for those receiving the intervention. Importantly, SPACE for COPD appears to be safe and may help prevent readmission with 30 days.
Introduction
Patients with COPD experience exacerbations, some of which require hospitalization,Citation1 which accounts for a significant proportion of the £810–930 million economic cost annuallyCitation2 in the United Kingdom. Furthermore, exacerbations and admissions are associated with reduced physical functioning,Citation3,Citation4 which may contribute to the increased readmission risk.Citation5 The 28-day readmission rates arê33%,Citation2 and hospitals are penalized financially if patients get readmitted within 30 days of discharge.Citation6
A patient surveyCitation6 highlighted that individuals do not always feel able to cope at home postexacerbation. Additionally, patients report that they want more information and advice on practical coping issues,Citation6 highlighting the need for supportive interventions.
Pulmonary rehabilitation (PR) is one intervention that has increasingly been employed to address the reduced exercise capacity associated with exacerbations.Citation7,Citation8 PR consists of exercise and education to promote health-enhancing behaviors.Citation9 Rehabilitation offered early after hospital discharge can reduce readmission rates and improve exercise capacity,Citation7 but other studies have shown that it is difficult to recruit patients,Citation8 indicating that it may not be wholly acceptable during this period. Furthermore, no improvements in readmission rates or physical function were observed for an early intensive rehabilitation intervention over a longer, 12-month period,Citation10 highlighting the need for a different approach.
With finite health care resources, it is important to prevent unnecessary hospital admissions. Given the detrimental physical and emotional effects of hospitalization, it is important to devise an intervention to help reduce the associated impacts, while being safe and acceptable to patients.
Recent attention has been given to self-management interventions, from simple exacerbation management plans to comprehensive behavior-changing programs.Citation11 However, there have been concerns with these types of interventions, particularly after an acute exacerbation, as recent studies have observed increased mortality rates within the intervention arms.Citation10,Citation12 Although reasons for this have not been established, it has been postulated that patients may have misplaced confidence in their self-management skills.
A Self-management Program of Activity, Coping, and EducationCitation13 for COPD (SPACE for COPD) improves clinical and health care utilization outcomes within a stable COPD populationCitation14 but has not been investigated as a stand-alone intervention within an acute setting. SPACE for COPD is a brief intervention containing practical advice, a home-based exercise program, and an exacerbation action plan that aims to support patients to manage their day-to-day activities and promote health-enhancing behaviors.
Our hypothesis was that a structured self-management strategy (SPACE for COPD) employed upon hospital discharge would reduce readmissions for patients with COPD, compared to usual care. We also investigated the effect of SPACE for COPD on exercise tolerance, psychological impact, health-related quality of life, and disease knowledge.
Methods
Design
A prospective, two-center, single-blinded randomized controlled trial was conducted during January 2013–September 2014. Participants provided written informed consent, and ethical approval was granted by National Research Ethics Service Committee West Midlands – Solihull, reference 12/WM/0106, trial registration ISRCTN84599369.
Population
Participants were recruited from University Hospitals Coventry and Warwickshire and University Hospitals of Leicester NHS Trusts. Participants were included if they had an established diagnosis of COPD and grade 2–5 dyspnea according to the Medical Research Council. Individuals were excluded if their reason for admission was not an acute exacerbation of COPD or if they were 1) unable to safely participate in unsupervised exercise (ie, due to psychiatric, locomotive, cardiac, or neurological impairments), 2) involved in other research, 3) unable to read English, 4) had previously received SPACE for COPD or completed PR within the previous 6 months, or 5) had four or more admissions in the previous 12 months.
Randomization
Participants were randomized to receive usual care or SPACE for COPD via a web-based, concealed allocation program (www.sealedenvelope.com) using simple random permuted block 1:1 randomization by VJ-W. Randomization was performed after the participants completed the baseline assessment, with treatment allocation prior to hospital discharge.
Usual care
All participants received usual care during the study period. This consisted of a follow-up appointment with the community COPD team or telephone follow-up after an inpatient review by a respiratory nurse specialist and an outpatient consultant review. Due to waiting times, participants did not receive PR during the study period.
SPACE for COPD
SPACE for COPD has previously been described;Citation13 briefly, it comprises written educational information and a home-based exercise program (consisting of a daily walking-based aerobic program and thrice weekly resistance training using free weights of the upper and lower limbs). Participants were introduced to the manual and exercises by a trained physiotherapist (VJ-W) in a one-to-one session lasting 30–45 minutes, using motivational interviewing techniques to facilitate behavior change, goal setting, and problem solving. Participants were advised how to progress and that the manual could be valuable for the future to reinforce any life-long lifestyle changes. Participants received structured phone calls within 72 hours and at 2 weeks, 4 weeks, 6 weeks, 8 weeks, and 10 weeks from hospital discharge with the aim of reinforcing skills, helping identify and manage exacerbations, promoting an active lifestyle, and providing encouragement, while tailoring to patient needs. If participants get readmitted during the 3-month follow-up period, they continued the intervention as planned.
Outcome measures
The primary prespecified outcome measure was respiratory-related hospital readmission at 3 months. Secondary outcomes were the Chronic Respiratory Questionnaire – self reported (CRQ-SR),Citation15 Hospital Anxiety and Depression Score,Citation16 Bristol COPD Knowledge Questionnaire,Citation17 Incremental Shuttle Walk Test (ISWT),Citation18 Endurance Shuttle Walking Test (ESWT),Citation19 Pulmonary Rehabilitation Adapted Index of Self-Efficacy,Citation20 and the “Ready for Home” survey.Citation6 All outcomes were measured at baseline (during admission but as close to discharge as possible) and 3 months after randomization, by a clinician blinded to treatment allocation. Mortality and readmission data were collected from hospital and primary care databases.
Sample size
Based on the primary outcome measure of readmission at 3 months, 36 participants were required in each arm to detect a fall in readmissions comparable to Seymour et al.Citation21 Calculations were based on 5% significance (alpha 0.05), 80% power, and two-tailed test using the SAS system.
Statistical analysis
Data were tested for normality and appropriate parametric or nonparametric tests used. For binary variables (including primary outcome measure), Fisher’s exact test was used to compare differences between the two groups. Odds ratios were calculated for 30-day readmissions. Independent t-tests or Kruskal–Wallis tests were used to compare between-group differences. Paired t-tests or Wilcoxon signed rank tests were used to compare within-group changes. Statistical significance was accepted if P<0.05. Analysis was conducted on an intention-to-treat basis. Number (and percentages) of those achieving the known minimal clinically important differences of 0.5 for CRQ-SR,Citation22 −1.5 for Hospital Anxiety and Depression Score,Citation23 47.5 m for ISWT,Citation24 and 186 seconds for ESWTCitation25 was calculated.
Protocol changes
Ethical approval was granted to reduce the time given to participants to consider study participation from 24 hours to whenever they felt they fully understood. This was to allow the inclusion of patients with a short hospital stay (ie, those with mild COPD exacerbations) or those discharged over a weekend.
Results
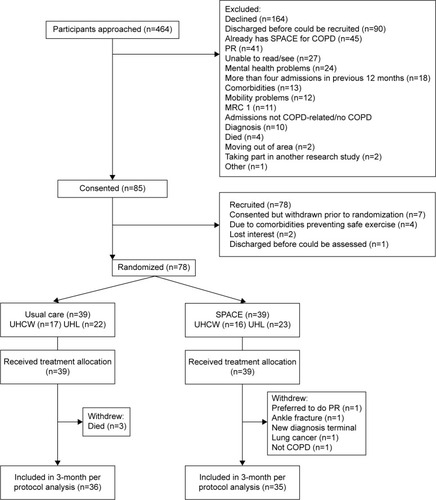
The consort diagram () describes the trial recruitment. Eighty-five patients consented, of whom 78 were randomized (39 to each arm) and included in the intention-to-treat analysis with 36 in usual care and 35 in SPACE for COPD available with follow-up data.
Figure 1 CONSORT diagram.
shows the baseline characteristics. No significant differences existed in baseline measures between groups (P>0.05) or outcome measures between sites (P>0.05). Twenty-two patients used home oxygen (ten used both long-term oxygen therapy and ambulatory, eight used solely long-term oxygen therapy, two ambulatory, and two palliative oxygen) at a mean (standard deviation) flow rate of 1.52 (0.57) L.
Table 1 Baseline characteristics of the study population
Primary outcome measure
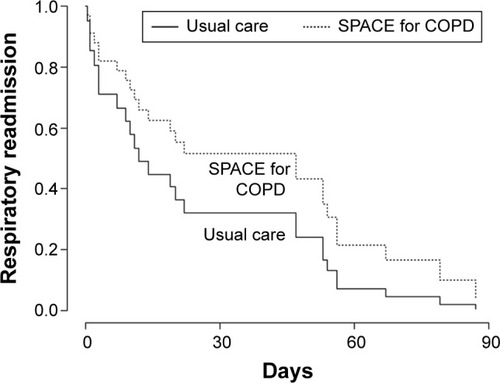
Twenty-five patients (32.05%) were readmitted for respiratory reasons during the 3-month follow-up period: 13 receiving usual care and 12 receiving the intervention (33.33% vs 30.77%, P=0.808). shows the Kaplan–Meier curves.
Health care utilization
Readmission data were nonnormally distributed. Thirty-one patients (14 controls and 17 receiving the intervention, P=0.488) were readmitted for any reason during the 3-month period, with 44 admissions (21 in usual care and 23 in intervention, P=0.726). Respiratory reasons accounted for 79.55% of these readmissions (19 in usual care and 16 in SPACE for COPD, P=0.674).
shows the hospital length of stay for those who got readmitted. Median (interquartile range) days to first respiratory readmission was 14 (4–39) for usual care compared to 47 (4.5–55.5) for the intervention (P=0.341).
Table 2 Hospital length of stay (for readmission)
Ten usual care patients were readmitted within 30 days for respiratory reasons compared to five patients receiving the intervention (25.64% vs 12.82%, P=0.151), odds ratio (95% confidence interval) 0.426 (0.131–1.391), P>0.05.
All participants were offered PR after the study period. Fourteen patients expressed an interest (seven in each group).
Mortality
Within the 3-month study period, three usual care patients died (all due to respiratory reasons, median 65 days to death), whereas no patients receiving SPACE for COPD died, P=0.077. Data censored on September 30, 2014 (mean 339 days to censoring), revealed that seven usual care patients and three patients receiving SPACE for COPD had died, P=0.176.
Serious adverse events
Only hospitalizations and mortality were reported as serious adverse events (SAEs). No other SAEs were found.
Exercise and questionnaire data
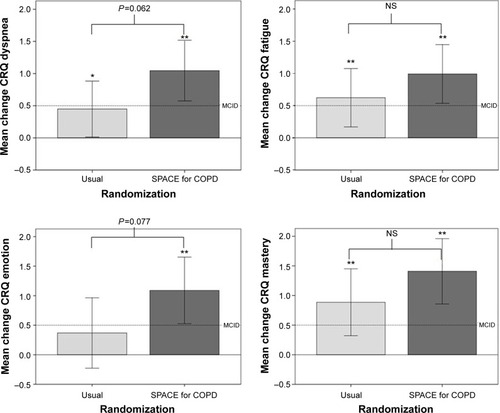
ISWT and ESWT were nonnormally distributed. and show within- and between-group differences for quality of life, disease knowledge, exercise tolerance, and self-efficacy. Within-group changes (P<0.05) were seen for both groups for all CRQ-SR domains except emotion for usual care (P=0.216). Between-group differences approached statistical significance for CRQ-dyspnea (P=0.062) and -emotion (P=0.077) domains, in favor of the intervention. Both groups significantly increased their exercise tolerance (P<0.05). Disease-specific knowledge increased from baseline for those who received SPACE for COPD (P<0.05) but not for usual care. shows that more patients who received the intervention achieved the minimal clinically important difference for CRQ-dyspnea (P=0.039).
Table 3 Baseline and change in secondary outcome measures at 3 months
Table 4 Number (%) of participants who achieved the MCID
Figure 3 Change in Chronic Respiratory Questionnaire – self reported data from baseline to 3 months.
Abbreviations: NS, not significant; SPACE, Self management Programme of Activity Coping and Education; CRQ, Chronic Respiratory Questionnaire.
At 3 months, shows how people felt upon their (initial) discharge from hospital from the Ready for Home survey. More patients following SPACE for COPD felt confident that medications could help and were reassured that good support was available at home compared to usual care (P<0.05). shows that more patients in the SPACE for COPD arm felt that they were better able to arrange their life to cope with COPD, knew when to seek help about feeling unwell, and more often took their medications on time as prescribed, compared to usual care (all P<0.05).
Table 5 How people feel upon discharge from hospital following treatment for their COPD (% per group)
Table 6 Effect of (baseline) hospitalization on how people felt they changed and consequently managed their COPD (%)
Discussion
The supported self-management program, SPACE for COPD, delivered at the time of an acute exacerbation, did not reduce respiratory-related hospital readmissions at 3 months. However, benefits in quality of care and potential improvements in health-related quality of life, delaying time to first readmission, and reducing hospital length of stay were observed for those receiving the intervention. We did not find an increased mortality rate, and thus, SPACE for COPD appears a safe intervention in this population.
Within-group changes were observed for most outcomes for both groups. This gives further support that patients, after an acute exacerbation requiring hospitalization, experience a period of natural recovery.Citation10 There were encouraging trends for improved outcomes in those receiving SPACE for COPD compared to those receiving usual care, especially for CRQ-SR (with dyspnea and emotion scores improving by more than double for those receiving the intervention compared to usual care) and time to first readmission, although many did not reach statistical significance. This is likely due to the relatively small number of participants, so these secondary outcomes are likely to be underpowered.
Although not statistically significant, there were more admissions within the 30-day postdischarge period for respiratory reasons (attracting financial penalties of ~£2,000Citation26 each) in the group receiving usual care compared to the self-management group. Therefore, SPACE for COPD may be a feasible, brief intervention implementable immediately upon hospital discharge to help reduce this financial consequence and provide some benefits in quality of life and emotional support. Patients could then attend more intensive interventions when stable and natural recovery has plateaued, such as outpatient PR, which has established health and economic benefits.Citation27 However, we found that only 14 participants expressed an interest in attending PR. Furthermore, 164 patients declined to take part in this study. Reasons for this varied mainly from none being given to having done similar previous research or PR before, preferring to wait for PR, feeling “too old” or not well enough.
Previous studiesCitation10,Citation12 have found an increased mortality rate in self-management interventions, which, although not fully understood, has caused safety concerns in delivering these types of interventions. We did not show an increased mortality rate (nor other SAEs) for those who received SPACE for COPD, compared to usual care; therefore, this particular intervention appears to be safe, at least in the short term. Previous authorsCitation10 reasoned that their observed increased mortality rates could be due to either chance, failure to intervene or alterations in health behavior, which delay patients seeking medical advice. In our study, patients who received the self-management program reported that they better knew when to seek medical advice when feeling unwell, suggesting that they would not delay seeking advice. Furthermore, patients receiving SPACE for COPD also reported more often taking their medications on time as prescribed. These are positive behavior change perceptions; however, this did not translate into preventing readmissions.
Limitations to this study include recruitment constraints. Due to available resources, there was not complete coverage to recruit during peak admission periods on both sites. In addition, some inpatient stays were so brief that being able to perform all research procedures within a busy, acute clinical setting was difficult.
This study, along with others, has shown that it can be difficult to prevent hospital readmission in a sick population. To take on board all information during a relatively short introduction to our self-management program, while patients are unwell and may have impaired cognition,Citation28 may have contributed to the limited effectiveness of this intervention. Furthermore, all participants received specialist, usual care follow-up, and so their care could already be optimum. However, SPACE for COPD may help increase patient’s self-management ability and confidence in the short term as displayed by the delay in time to readmission. It may be unreasonable to expect a reduction in readmission rates and arguably should not be seen as a negative outcome for the trial; it may be more realistic to anticipate a change in other aspects of successful disease management, for example, health-related quality of life. Analyses of qualitative interviews and health economic data may provide further insight into this and participants’ compliance and adherence to the intervention.
Conclusion
In conclusion, we found that SPACE for COPD, delivered upon hospital discharge and supported postdischarge, did not reduce readmission rate at 3 months compared to usual care alone. However, we did find that this supported self-management intervention provided some potential benefits in health-related quality of life and delaying time to first readmission, which appears safe, as additional mortality was not incurred, in contrast to findings from other recent studies.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work. SJS and KR were supported by the Collaboration for Leadership in Applied Health Research and Care, East and West Midlands, respectively and the NIHR Leicester Respiratory Biomedical Research Unit (BRU). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Acknowledgments
The authors thank the British Lung Foundation for funding, Dr Matthew Richardson for statistical advice, and Leicestershire, Northamptonshire, and Rutland and West Midlands (South) Comprehensive Local Research Networks for screening.
Presented as an oral presentation in mini symposium B16 at the American Thoracic Society 18th May 2015 – A Supported Self-Management Programme for Chronic Obstructive Pulmonary Disease (COPD) Upon Hospital Discharge: A Randomized Controlled Trial.
British Lung Foundation grant RB11-2.
Disclosure
The authors report no conflicts of interest in this work.
References
- National Institute for Health and Clinical ExcellenceChronic Obstructive Pulmonary Disease Management of Chronic Obstructive Pulmonary Disease in Adults in Primary and Secondary Care (Partial Update)LondonNational Institute for Health and Clinical Excellence2010
- Department of HealthConsultation on a Strategy for Services for Chronic Obstructive Pulmonary Disease (COPD) in EnglandLondonDepartment of Health2010
- PittaFTroostersTProbstVSSpruitMADecramerMGosselinkRPhysical activity and hospitalization for exacerbation of COPDChest2006129353654416537849
- SpruitMAGosselinkRTroostersTMuscle force during an acute exacerbation in hospitalised patients with COPD and its relationship with CXCL8 and IGF-IThorax200358975275612947130
- Garcia-AymerichJFarreroEFelezMAEstudi del Factors de Risc d’Agudització de la MPOC investigatorsRisk factors of readmission to hospital for a COPD exacerbation: a prospective studyThorax200358210010512554887
- British Lung Foundation and British Thoracic SocietyReady for Home? Improving Hospital Discharge Care for People Living with COPDLondonBritish Thoracic Society2010
- EatonTYoungPFergussonWDoes early pulmonary rehabilitation reduce acute health-care utilization in COPD patients admitted with an exacerbation? A randomized controlled studyRespirology200914223023819272084
- ManWDPolkeyMIDonaldsonNGrayBJMoxhamJCommunity pulmonary rehabilitation after hospitalisation for acute exacerbations of chronic obstructive pulmonary disease: randomised controlled studyBMJ20043297476120915504763
- SpruitMASinghSJGarveyCATS/ERS Task Force on Pulmonary RehabilitationAn official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitationAm J Respir Crit Care Med20131888e13e6424127811
- GreeningNJWilliamsJEHussainSFAn early rehabilitation intervention to enhance recovery during hospital admission for an exacerbation of chronic respiratory disease: randomised controlled trialBMJ2014349g431525004917
- WaggKUnravelling self-management for COPD: what next?Chron Respir Dis2012915722308549
- FanVGazianoJLewRA comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations: a randomized controlled trialAnn Intern Med20121561067368322586006
- AppsLDMitchellKEHarrisonSLThe development and pilot testing of the self-management program of activity, coping and education for chronic obstructive pulmonary disease (SPACE for COPD)Int J Chron Obstruct Pulmon Dis2013831732723874093
- MitchellKEJohnson-WarringtonVAppsLDA self-management programme for COPD: a randomised controlled trialEur Respir J20144461538154725186259
- WilliamsJESinghSJSewellLGuyattGHMorganMDDevelopment of a self-reported Chronic Respiratory Questionnaire (CRQ-SR)Thorax2001561295495911713359
- ZigmondASSnaithRPThe hospital anxiety and depression scaleActa Psychiatr Scand19836763613706880820
- WhiteRWalkerPRobertsSKaliskySWhitePBristol COPD knowledge questionnaire (BCKQ): testing what we teach patients about COPDChron Respir Dis2006312313116916006
- SinghSJMorganMDScottSWaltersDHardmanAEDevelopment of a shuttle walking test of disability in patients with chronic airways obstructionThorax19924712101910241494764
- RevillSMMorganMDSinghSJWilliamsJHardmanAEThe endurance shuttle walk: a new field test for the assessment of endurance capacity in chronic obstructive pulmonary diseaseThorax199954321322210325896
- VincentESewellLWaggKDeaconSWilliamsJSinghSMeasuring a change in self-efficacy following pulmonary rehabilitation: an evaluation of the PRAISE toolChest201114061534153921737490
- SeymourJMMooreLJolleyCJOutpatient pulmonary rehabilitation following acute exacerbations of COPDThorax201065542342820435864
- JaeschkeRSingerJGuyattGHMeasurement of health status. Ascertaining the minimal clinically important differenceControl Clin Trials1989104074152691207
- PuhanMAFreyMBüchiSSchünemannHJThe minimal important difference of the hospital anxiety and depression scale in patients with chronic obstructive pulmonary diseaseHealth Qual Life Outcomes200864618597689
- SinghSJJonesPWEvansRMorganMDMinimum clinically important improvement for the incremental shuttle walking testThorax20086377577718390634
- PepinVLavioletteLBrouillardCSignificance of changes in endurance shuttle walking performanceThorax20116611512021148135
- National Institiute for Health and Clinical ExcellenceChronic Obstructive Pulnmonary Disease Costing Report NICE Clinical Guidelines 101LondonNational Institiute for Health and Clinical Excellence2011
- GriffithsTLBurrMLCampbellIAResults at 1 year of outpatient multidisciplinary pulmonary rehabilitation: a randomised controlled trialLancet2000355920136236810665556
- DoddJWCharltonRAvan den BroekMDJonesPWCognitive dysfunction in patients hospitalized with acute exacerbation of COPDChest2013144111912723349026