Abstract
Purpose
The outcome of radical surgery for lung cancer was investigated in patients with combined pulmonary fibrosis and emphysema (CPFE).
Methods
A retrospective chart review involved 250 patients with lung cancer who underwent pulmonary resection at Tokyo Women’s Medical University Yachiyo Medical Center between 2008 and 2012. Based on the status of nontumor-bearing lung evaluated by preoperative computed tomography (CT), the patients were divided into normal, emphysema, interstitial pneumonia (IP), and CPFE groups, and their clinical characteristics and surgical outcome were analyzed.
Results
The normal, emphysema, IP, and CPFE groups comprised 124 (49.6%), 108 (43.2%), seven (2.8%), and eleven (4.4%) patients, respectively. The 5-year survival rate of the CPFE group (18.7%) was significantly lower than that of the normal (77.5%) and emphysema groups (67.1%) (P<0.0001 and P=0.0027, respectively) but equivalent to that of the IP group (44.4%) (P=0.2928). In a subset analysis of cancer stage, the 5-year overall survival rate of the CPFE group in stage I (n=8, 21.4%) was also lower than that of the normal group and emphysema group in stage I (n=91, 84.9% and n=70, 81.1%; P<0.0001 and P<0.0001, respectively). During entire observation period, the CPFE group was more likely to die of respiratory failure (27.2%) compared with the normal and emphysema groups (P<0.0001). Multivariate analysis of prognostic factors using Cox proportional hazard model identified CPFE as an independent risk factor (P=0.009).
Conclusion
CPFE patients have a poorer prognosis than those with emphysema alone or with normal lung on CT finding. The intensive evaluation of preoperative CT images is important, and radical surgery for lung cancer should be decided carefully when patients concomitantly harbor CPFE, because of unfavorable prognosis.
Introduction
Combined pulmonary fibrosis and emphysema (CPFE) is a clinical syndrome defined by upper lobe emphysema and lower lobe pulmonary fibrosis on computed tomography (CT).Citation1,Citation2
A previous report found that 8.9% of lung cancer patients had CPFE, and these patients had a poorer prognosis than those with emphysema alone.Citation3 However, the impact of CPFE on the outcomes of lung cancer surgery has not been extensively investigated.Citation4
The purpose of this study was to investigate the incidence of postoperative morbidity and mortality in patients with CPFE and lung cancer who underwent lung cancer surgery and to clarify the impact of the preoperative existence of CPFE on long-term survival after pulmonary resection for lung cancer.
Patients and methods
Patients
This study was approved by the Institutional Review Board of Tokyo Women’s Medical University, and informed consent was waived as the study was retrospective. The study population consisted of 250 consecutive patients with lung cancer who underwent pulmonary resection between April 2008 and January 2012 at Tokyo Women’s Medical University Yachiyo Medical Center. Clinical data were collected from our institutional cancer registry database and from outpatient follow-up visits. The records contained preoperative patient characteristics, disease status, operative procedures, postoperative complications, pathological diagnosis, and follow-up data.
Based on the preoperative chest conventional CT or high-resolution CT, patients were divided into the following four groups: CPFE, emphysema, interstitial pneumonia (IP), and normal. CPFE was defined by the following CT findings: the combination of bilateral positive findings of a low attenuation area, which indicated 2 points or higher by the Goddard classification scoreCitation5 in the upper field, and basal pulmonary fibrosis.Citation1 The emphysema group was defined by diffuse bilateral positive findings of low attenuation area without an interstitial shadow on CT. The IP group was defined by the interstitial shadow without emphysema. The interstitial shadow was defined as fibrosis, subpleural curvilinear traction, bronchiolectasis, ground-glass opacities, and consolidation on CT findings.Citation6 The remaining patients were categorized as the normal group. Of the 250 patients, eleven (4.4%) were categorized as the CPFE group, 108 (43.2%) as the emphysema group, and seven (2.8%) as the IP group. The remaining 124 patients (49.6%) were categorized as the normal group.
Preoperative evaluation for all patients included a detailed medical history, physical examination, blood and urine examinations, and a 12-lead electrocardiogram. Use of incentive nebulizers using distilled water with or without bronchodilator was routinely encouraged for enhancing lung expansion and airway clearance for ∼1 week after surgery. Neoadjuvant or adjuvant therapy, consisting of platinum agent-based chemotherapy and radiation, was prescribed mainly for the patients who had locally advanced disease or multiple nodal involvement or who underwent incomplete resection. The postoperative morbidities were considered as those occurring within 30 days from the operation or during a longer period if the patient was still in the hospital. These included bacterial pneumonia (confirmed by infiltrative shadows on chest radio-graph, positive sputum culture, body temperature ≥37.5°C, and white blood cell count >10,000/μL), acute lung injury (aggravation of dyspnea on exertion, deterioration of respiratory function as indicated by arterial blood gas analysis, and diffuse interstitial abnormalities compatible with acute IP or exacerbation of IP in the CPFE group on chest radiograph and CT), mechanical ventilation for >3 days, bronchial stump dehiscence, empyema and bronchial fistula (positive bacterial infection of a pleural effusion), tracheostomy, and postoperative home oxygen therapy for patients with partial pressure of oxygen (PaO2) <55 mmHg at rest or <60 mmHg on exercise at the time of hospital discharge.Citation7
After discharge from the hospital, patients visited our outpatient clinic regularly every 1–6 months, unless the tumor recurred or the patient had any health problems. The overall survival was analyzed for each group of patients.
Statistical analysis
Data were analyzed using JMP11 software (SAS Institute Inc., Cary, NC, USA). To compare differences between the CPFE group and the other three groups, the Tukey–Kramer test was used to analyze continuous variables, and the Pearson’s chi-square test was used for categorical variables. Survival curves were estimated using the Kaplan–Meier method, and differences in survival times between the CPFE group and the other three groups were calculated by the log-rank test. The Cox proportional hazards regression analysis was used to estimate the hazard ratio and the 95% confidence interval of each factor. Univariate Cox regression analysis model was used to identify significant factors. Multivariate Cox regression analysis model, with significant factor which had P<0.05 in univariate analysis, was used to identify prognostic factors.
Results
Preoperative patient characteristics
The preoperative patient characteristics are summarized in . Males were more predominant in the CPFE and emphysema groups than in the normal group, and the smoking index was higher in the CPFE and emphysema groups than in the normal group (P<0.05).
Table 1 Preoperative characteristics of lung cancer patients
Although the percentage of forced vital capacity (%FVC) and forced expiratory volume in 1 second (FEV1.0)/FVC in the CPFE group was similar to that in the normal groups, %FEV1.0 and percentage of diffusion capacity of the lung for carbon monoxide (%DLCO) in the CPFE group were significantly lower than that in the normal groups.
Perioperative characteristics
Perioperative characteristics are summarized in . Squamous cell carcinoma was predominant in the CPFE group, whereas adenocarcinoma was predominant in the other groups (P<0.001). The type of operative procedure, pathological tumor status, pathological nodal status, and pathological staging were not different among the four groups.
Table 2 Perioperative characteristics of lung cancer patients
Postoperative morbidity and mortality
Postoperative pulmonary complications within 30 days and the 30-day and 90-day mortality are summarized in . Pulmonary complications occurred in 15 of 250 patients (6%) overall and in two of eleven patients (18.2%) in the CPFE group. There is significant difference among the four groups in postoperative bacterial pneumonia. CPFE patients and IP patients had high risk of postoperative pneumonia (9.0% and 14.2%, respectively). The 30-day mortality was 0%, and the 90-day mortality was 9.0% in the CPFE group. The 90-day mortality in the CPFE group was higher than other groups, but there is no significant difference among the four groups.
Table 3 Postoperative pulmonary complications and mortality
Cause of death
The causes of death in all groups are summarized in . The causes of death were divided into primary cancer, respiratory failure, and others. Death due to respiratory failure was associated with bacterial pneumonia or acute exacerbation (AE) of IP. Three CPFE patients died of respiratory failure, which was significantly different from the other three groups (P=0.0001). The frequency of respiratory failure death in CPFE was also higher than that in the emphysema group (P<0.0001) (). Two of the patients in the CPFE group experienced bacterial pneumonia or AE two or more times prior to their death. The time to AE ranged from 3 days to 1.5 years after surgery. There were no significant differences in the proportions of patients who died of their primary cancer or other causes among the four groups of patients (P=0.080 and P=0.315, respectively).
Table 4 Comparison of cause of death between CPFE and emphysema groups
Survival analyses
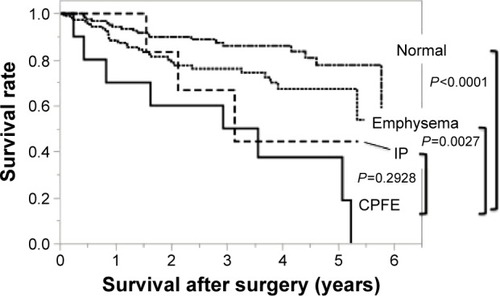
shows the overall survival after surgery. The mean follow-up period was 922 days in the CPFE group, 979 days in the emphysema group, 1,086 days in the IP group, and 1,117 days in the normal group. The cumulative survival at 3 years and 5 years was 50% and 18.7% in the CPFE group, 76.0% and 67.1% in the emphysema group, 44.4% and 44.4% in the IP group, and 85.9% and 77.5% in the normal group, respectively. Comparing with the CPFE group, there was significant difference in the normal group (P<0.0001, log-rank test) and the emphysema group (P=0.0027, log-rank test). There was no significant difference between the CPFE group and the IP group (P=0.2928, log-rank test) ().
Figure 1 Overall survival in the four groups: Kaplan–Meier curves for OS after surgery.
Abbreviations: OS, overall survival; CPFE, combined pulmonary fibrosis and emphysema; IP, interstitial pneumonia.
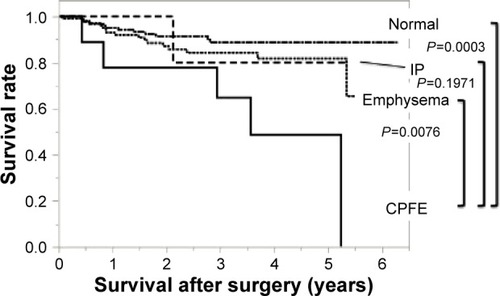
The disease-specific survival at 5 years was 48.6% in the CPFE group, 81.7% in the emphysema group, 80% in the IP group, and 88.6% in the normal group, respectively (). Comparing with the CPFE group, there was also significant difference in the normal group (P=0.0003, log-rank test) and the emphysema group (P=0.0076, log-rank test) but no difference in the IP group (P=0.1971).
Figure 2 Disease-specific survival in the four groups: Kaplan–Meier curves for disease-specific survival.
Abbreviations: CPFE, combined pulmonary fibrosis and emphysema; IP, interstitial pneumonia.
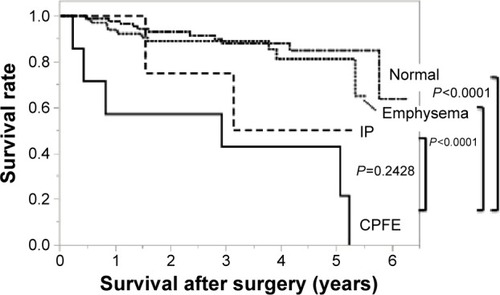
In patients with pathological stage I disease, the cumulative survival at 5 years was 21.4% in the CPFE group, 81.1% in the emphysema group, 50% in the IP group, and 84.9% in the normal group, respectively (). Comparing with the CPFE group, there was significant difference in the normal group (P<0.0001, log-rank test) and the emphysema group (P<0.0001, log-rank test) but no difference in the IP group (P=0.2428, log-rank test).
Figure 3 Overall survival in the four groups with pathological stage I disease: Kaplan–Meier curves for overall survival with pathological stage I disease.
Abbreviations: CPFE, combined pulmonary fibrosis and emphysema; IP, interstitial pneumonia.
We also attempted to clarify risk factors for long-term overall mortality ( and ). Univariate analysis identified age, sex, smoking index, %FVC, %DLCO, %DLCO/alveolar volume (VA), type of operative procedure, and pathological staging as significant factors (). Using these factors, we identified CPFE as an independent risk factor according to the Cox proportional hazard model (P=0.009). Age, %FVC, pathological stage III, and pathological stage IV were also independent risk factors (P=0.007, 0.006, 0.012, and 0.002, respectively).
Table 5 Univariate analysis of prognostic factors using Cox proportional hazard model
Table 6 Multivariate analysis of prognostic factors using Cox proportional hazard model
Discussion
The present study found that CPFE was an independent prognostic factor for patients with lung cancer undergoing surgery. The CPFE group had a poorer prognosis than any other groups in terms of overall survival. These results were consistent with a previous report.Citation8 There was a significant difference among the four groups for respiratory failure as a cause of death but no significant difference in the rates of cancer death. However, the disease-specific survival of CPFE patients was also poorer than that of normal and emphysema groups. Kumagai et al pointed out that patients with CPFE had a high prevalence of cancer recurrence.Citation8 They reported poor progression-free survival after operation and poor overall survival after recurrence in lung cancer patients with CPFE who underwent surgery. They suggested that CPFE patients have risk factors for lung cancer progression, including smoking, emphysema, and fibrosis. CPFE patients often cannot undergo an optimal chemotherapy after cancer recurrence because of a poor Eastern Cooperative Oncology Group Performance Status and AE after the administration of chemotherapy. Since our study found that four of eight CPFE patients with stage I disease experienced cancer recurrence, CPFE might have a high risk of cancer recurrence, as previously reported.Citation8 Furthermore, five CPFE patients had chemotherapy or radiation because of cancer recurrence and two of five patients died due to respiratory failure after these therapies. Therefore, careful treatment planning should be undertaken for CPFE patients with any stage of lung cancer, because surgery and chemotherapy may lead to respiratory deficiency.
Assessment of respiratory function is useful for the prediction of prognosis of CPFE patients.Citation9 The results of lung function testing in patients with CPFE differed markedly from that of patients with idiopathic pulmonary fibrosis (IPF) and that of patients with emphysema.Citation1 The mean values of lung volumes of CPFE patients are near normal, but the DLCO values are markedly decreased. Hyperinflation and high compliance of the emphysematous areas of the lungs probably compensate for the volume loss due to fibrosis of the lower lobes, while pulmonary emphysema and fibrosis may have synergistic effects on DLCO and exercise hypoxemia.
CPFE might be underrecognized in patients with subnormal or normal spirometry if DLCO and/or exercise blood gases are not measured.Citation9 Compared with the normal group, CPFE patients had low %FEV1.0 and %DLCO and similar %FVC and FEV1.0/FVC. In addition, patients with CPFE tended to die of respiratory failure more often than the normal and emphysema patients ( and ).
DLCO measures the ability of gas to diffuse across the alveolar–capillary membrane and assesses the surface area and pulmonary capillary blood volume available for gas exchange.Citation10 The preoperative DLCO expressed as percentage of predicted has a higher correlation with postoperative deaths than the FEV1.0 expressed as percentage of predicted.Citation11 Our study showed %DLCO was a significant factor for overall survival in the univariate analysis. Some studies found that a reduced predicted postoperative DLCO was strongly associated with the risk of pulmonary morbidity, mortality, and poor long-term survival following lung resection.Citation12,Citation13
Similar to previous reports, our study showed no significant difference in long-term survival between the CPFE and IP groups.Citation3,Citation8,Citation14,Citation15 This suggests that IP has a higher impact on the outcome of lung cancer patients than emphysema. However, Schmidt et al clarified the importance of evaluation for emphysema in CPFE patients.Citation9 They reported the efficiency of the composite physiological index that was calculated from the following formula: 91 − (0.65× %predicted DLCO) − (0.53× %predicted FVC) + (0.34× %predicted FEV1.0) in IPF. This index was a powerful predictor of mortality in IPF patients. They showed that change in FEV1.0 was the best predictor of mortality in CPFE. Therefore, evaluation of the air obstruction status in CPFE patients may still be important.
Our study showed that CPFE patients with lung cancer also had a high risk of death due to respiratory failure caused by bacterial infection or AE. In this study, one patient with CPFE died due to AE of IP, and two died due to bacterial pneumonia. These types of death might be associated with impaired immunity, which is involved in the pathogenesis of CPFE. Aging lung and cellular senescence have been reported to be involved in the pathogenesis of IPF and emphysema.Citation16 Exposure to a variety of toxic substances such as those in smoking can contribute to the acceleration of pulmonary senescence. Premature aging can impair lung function by inducing systemic and/or local changes in the immune system, impairing the complex pulmonary mechanisms of defense against infection and stimulating a local and/or systemic inflammatory condition. This phenomenon and surgical stress may lead to deterioration of the pulmonary immune responses.
This study has several limitations. First, this was a retrospective and uncontrolled study performed at a single institution. The results may have been subject to selection and treatment bias. The indications for therapy were not uniform for all patients, thereby limiting the evaluation of the effects of treatment. Second, this study included a relatively small number of patients with CPFE. Therefore, there may be several confounding variables. Third, high-resolution chest CT was not always utilized, and the pathological evaluation of fibrotic lesions was not always performed.
Conclusion
In conclusion, lung cancer patients with CPFE who undergo surgery have a worse prognosis than surgical lung cancer patients with emphysema alone or surgical lung cancer patients with normal lung because they tend to die of respiratory failure. The intensive evaluation of preoperative CT images and assessment of DLCO are important for planning cancer treatment for CPFE patients, and great care should be taken when deciding on specific treatments.
Acknowledgments
The abstract of this paper was presented at the 23rd European Conference on General Thoracic Surgery Conference as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in Interactive CardioVasc Thoracic Surgery: Hyperlink (http://icvts.oxfordjournals.org/content/21/suppl_1/S27.4.full). The actual paper, however, has never been published.
Disclosure
YS received a Grant-in-Aid for Scientific Research (26462139) from the Ministry of Education, Culture, Sports, Science and Technology of Japan in support of this work. The authors report no other conflicts of interest in this work.
References
- CottinVNunesHBrilletPYCombined pulmonary fibrosis and emphysema: a distinct underrecognised entityEur Respir J200526458659316204587
- BourosDCombined pulmonary fibrosis and emphysema syndromePneumon2009222128130
- UsuiKTanaiCTanakaYNodaHIshiharaTThe prevalence of pulmonary fibrosis combined with emphysema in patients with lung cancerRespirology201116232633121114711
- SekineYSakairiYYoshinoMThe impact of combined pulmonary fibrosis and chronic obstructive pulmonary disease on long-term survival after lung cancer surgeryThorac Cardiovasc Surg201462433233724879070
- GoddardPRNicholsonEMLaszloGWattIComputed tomography in pulmonary emphysemaClin Radiol19823343793877083738
- SuzukiHSekineYYoshidaSRisk of acute exacerbation of interstitial pneumonia after pulmonary resection for lung cancer in patients with idiopathic pulmonary fibrosis based on preoperative high-resolution computed tomographySurg Today201141791492121748606
- SekineYChiyoMIwataTImpact of interstitial lung disease on surgical morbidity and mortality for lung cancer: analyses of short and long-term outcomesJ Thorac Cardiovasc Surg200312641141114614566260
- KumagaiSMarumoSYamanashiKPrognostic significance of combined pulmonary fibrosis and emphysema in patients with resected non-small-cell lung cancer: a retrospective cohort studyEur J Cardiothorac Surg2014466113119
- SchmidtSLNambiarAMTayobNPulmonary function measures predict mortality differently in IPF versus combined pulmonary fibrosis and emphysemaEur Respir J201138117618321148225
- LiptayMJBasuSHoaglinMCDiffusion lung capacity for carbon monoxide (DLCO) is an independent prognostic factor for long-term survival after curative lung resection for cancerJ Surg Oncol2009100870370719798693
- FergusonMKLittleLRizzoLDiffusing capacity predicts morbidity and mortality after pulmonary resectionJ Thorac Cardiovasc Surg19889668949003193801
- FergusonMKReederLBMickROptimizing selection of patients for major lung resectionJ Thorac Cardiovasc Surg199510922752817853881
- SantiniMFiorelloAVicidominiGDi CrescenzoVGLaperutaPRole of diffusing capacity in predicting complications after lung resection for cancerThorac Cardiovasc Surg200755639139417721850
- MinegishiYKokuhoNMiuraYClinical features, anti-cancer treatments and outcomes of lung cancer patients with combined pulmonary fibrosis and emphysemaLung Cancer201485225826324894326
- PapirisSATriantafillidouCManaliEDCombined pulmonary fibrosis and emphysemaExpert Rev Respir Med201371193123362797
- ChilosiMCarloniARossiAPolettiVPremature lung aging and cellular senescence in the pathogenesis of idiopathic pulmonary fibrosis and COPD/emphysemaTransl Res2013162315617323831269
