Abstract
Objectives
Emphysema is one of the prognostic factors for rapid lung function decline in patients with COPD, but the impact of incidentally detected emphysema on population without spirometric abnormalities has not been evaluated. This study aimed to determine whether emphysema detected upon computed tomography (CT) screening would accelerate the rate of lung function decline and influence the possibility of future development of airflow limitation in a population without spirometric abnormalities.
Materials and methods
Subjects who participated in a routine screening for health checkup and follow-up pulmonary function tests for at least 3 years between 2004 and 2010 were retrospectively enrolled. The percentage of low-attenuation area below −950 Hounsfield units (%LAA−950) was calculated automatically. A calculated value of %LAA−950 that exceeded 10% was defined as emphysema. Adjusted annual lung function decline was analyzed using random-slope, random-intercept mixed linear regression models.
Results
A total of 628 healthy subjects within the normal range of spriometric values were included. Multivariable analysis showed that the emphysema group exhibited a faster decline in forced vital capacity (−33.9 versus −18.8 mL/year; P=0.02). Emphysema was not associated with the development of airflow limitation during follow-up.
Conclusion
Incidental emphysema quantified using CT scan was significantly associated with a more rapid decline in forced vital capacity in the population with normative spirometric values. However, an association between emphysema and future development of airflow limitation was not observed.
Introduction
Lung function declines with age,Citation1 but certain factors accelerate this rate of decline,Citation2,Citation3 one of which is chronic obstructive pulmonary disease (COPD). COPD is characterized by persistent airflow limitation associated with a mixture of small airway inflammation (obstructive bronchiolitis) and parenchymal destruction (emphysema).Citation2,Citation4 Emphysema is reported to be a prognostic factor for higher mortality rates in COPD patients,Citation5,Citation6 but the influence of emphysema on the rate of lung function decline is controversial.Citation7–Citation11 In Korea, regular health checkup, including computed tomography (CT) screening, is frequently performed because of increased concern over one’s health status and highly accessible medical sources with low economic burden. Consequently, the incidental detection of asymptomatic emphysema has increased. However, according to the current Global Initiative for Chronic Obstructive Lung Disease (GOLD) guideline, classification and treatment of COPD are based on pulmonary function test (PFT) results. Therefore, guidance for populations with emphysema without spirometric abnormalities is not included in the current guideline. There is limited knowledge about the natural course in this population, and whether emphysema affects the rate of decline of lung function or future development of airflow limitation remains undetermined. In addition, many previous studies classify the severity of emphysema on the basis of visual assessment by a small number of radiologists to minimize interobserver variability.Citation7,Citation10 However, poor agreement between radiologists in the visual grading of emphysema has been reported.Citation12 Therefore, quantification of emphysema by objective computerized software may improve the reproducibility.Citation13
The aim of our study was to investigate the association among emphysema quantified by computerized software, the annual decline rate of lung function, and the development of airflow limitation in a population within the normal range of pulmonary function.
Materials and methods
Study participants
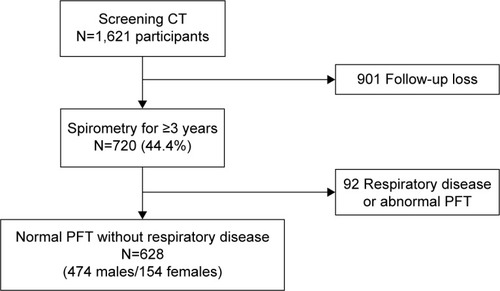
Healthy participants who underwent voluntary baseline CT scans for routine health checkup at Seoul Metropolitan Government – Seoul National University Boramae Medical Center in South Korea and follow-up PFTs for at least 3 years, between January 2004 and December 2010, were retrospectively included. Subjects with known respiratory disease or abnormal PFT results at baseline, including cases with forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) <0.7, FEV1 <80% predicted, or FVC <80% predicted, were excluded. Participants were asked to complete a short questionnaire on respiratory symptoms, smoking history, and medical history. Clinical information including age, sex, height, weight, abdominal circumference, smoking status, smoking amount, other underlying diseases, values of PFT results, and CT image findings were also reviewed. Informed consent was waived due to the retrospective design, and patient records were anonymized and de-identified prior to analysis. The Institutional Review Boards of Seoul Metropilitan Government - Seoul National University Boramae Medical Center, Seoul, Republic of Korea, approved this study (IRB no: 20110407/06-2011-67/106).
Pulmonary function testing
All the spirometry tests were conducted using standardized equipment (model 1022; SensorMedics Corp, BD, Franklin Lakes, NJ, USA) by two qualified technicians following the American Thoracic Society/European Respiratory Society guidelines.Citation14 Spirometry was repeated at least three times to ensure reproducibility and validity. Calculation of PFT values, in relation to reference values, was performed using computer programs and reviewed by trained physicians. Reference values were calculated using Morris’s predictive equation.Citation15,Citation16 As only participants without spirometric abnormalities were enrolled in the study, postbronchodilator testing was not performed, and all measures were based on prebronchodilator values. COPD was defined as occurrence of airflow limitation (FEV1/FVC <0.7) during the follow-up period according to GOLD statements.
CT protocol and image analysis
The baseline chest CT scans were used for analysis, which were obtained using a low-radiation dose technique without intravenous contrast material. Chest CT scans were acquired in the supine position with breath held at full inspiration by using a LightSpeed Pro 16 (General Electric Medical Systems, Milwaukee, WI, USA). Technical parameters were as follows: 40–60 mAs, 120 kVp tube voltage, and 360 mm field of view. Effective milliampere-second was selected based on the patients’ body mass index (BMI) (40 mAs for BMI ≤30 kg/m2 and 60 mAs for BMI >30 kg/m2). Tube current modulation or iterative reconstruction was not used. The scan was performed from the lung apex to diaphragm, and respiratory gating was not used. Transverse data sets were reconstructed with 2.5 mm thickness at 2.5 mm increments, using a standard reconstructing algorithm. The percentage of the low-attenuation area (%LAA), which indicates emphysematous destruction, was automatically calculated by Extended Brilliance Workspace (Version 3.0; Philips, Best, The Netherlands). As −950 Hounsfield units (HU) has been suggested to be the optimal threshold for quantification of emphysematous destruction,Citation17,Citation18 the extent of low-attenuation area below −950 HU (%LAA−950) was measured for emphysema scoring. Emphysema was defined as calculated emphysema scores (%LAA−950) exceeding 10%, which was the same criteria used in the COPDGene and ECLIPSE cohort studies.Citation19 Several studies also showed an increased mortality in cases with %LAA−950 >10%.Citation5,Citation6 Since minimal differences were reported in the quantification of emphysema between standard radiation dose and low radiation dose CT techniques, we used values of emphysema scoring calculated from our low radiation dose CT protocol without modification, which is fitted for lung cancer screening.Citation20–Citation23
Statistical analysis
A Student’s t-test was used to compare continuous variables, and chi-square tests were used for between-group comparisons. The effects of emphysema on the FEV1 or FVC (mL/year) annual decline rate were analyzed using a random-slope, random-intercept mixed linear regression model with variables including emphysema, time of visit in years, and emphysema–by-time interaction and covariates including age, sex, height, BMI, smoking status, and baseline FEV1 or FVC.Citation24–Citation29 The occurrence of airflow limitations was calculated using the Kaplan–Meier method, and Cox proportional hazards regression was used to assess risk factors for multivariate analysis. All statistical analyses were performed using Stata (Stata 13.1; StataCorp LP, College Station, TX, USA) and SPSS software (Version 12.0K; SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics
A total of 628 subjects who met the inclusion criteria were enrolled in the study; 474 (75.5%) were males and 271 (43.2%) were current smokers (). The baseline median age was 48 years (interquartile range [IQR], 40–56). The mean PFT values were as follows: FVC, 4.02±0.84 L (% predicted, 98.0±11.0); FEV1, 3.26±0.69 L (% predicted, 105.7±12.4); and FEV1/FVC, 81.2%±5.5%. The median %LAA−950 was 1.8% (IQR, 0.6–7.0). Eighty-five subjects (13.5%) were allocated to the emphysema group (%LAA−950 >10%). The participants’ demographic and clinical characteristics including pulmonary functions are summarized in . Emphysema score (%LAA−950) was not significantly associated with baseline spirometric pulmonary function or smoking amount.
Table 1 Baseline characteristics at the initial visit
Annual decline rate of lung function and occurrence of COPD: longitudinal analysis
The median follow-up period was 4 years (IQR, 3–5), and the median number of spirometry tests was three (IQR, 2–4). The adjusted annual rate of FEV1 decline was apparently higher in the emphysema group, but the difference was not statistically significant (−26.3±5.7 versus −17.5±2.6 mL/year; mean difference, 8.8 mL/year; P=0.14). The adjusted annual rate of FVC decline was significantly higher in the emphysema group (−18.8±2.4 versus −33.9±6.0 mL/year; mean difference, 15.2 mL/year; P=0.02). There were no statistically significant differences in the adjusted annual rate of FEV1/FVC decline between the two groups (P=0.35; ). Sex (FVC, P=0.37; FEV1, P=0.08), obesity (FVC, P=0.64; FEV1, P=0.73), and abdominal obesity (FVC, P=0.33; FEV1, P=0.85) were not significantly associated with the rate of lung function decline. History of smoking exhibited a tendency toward accelerated annual rate of FEV1 decline (−15.0 versus −21.0 mL/ year), although not statistically significant (P=0.15), and had no influence on the annual rate of FVC decline (−20.9 versus −20.4 mL/year; P=0.91). As smoking is an important risk factor for COPD,Citation30 we compared the subgroup characteristics according to smoking status. In the current-smoker subgroup, the presence of emphysema did not demonstrate statistically significant difference in the adjusted annual decline rates of FVC (P=0.50) and FEV1 (P=0.79). However, the adjusted annual rate of FVC decline was significantly accelerated by the presence of emphysema in the noncurrent-smoker subgroup (−18.0±3.0 versus −35.8±6.7 mL/year; mean difference, 17.8 mL/year; P=0.02), while the adjusted annual rate of FEV1 decline also showed a tendency toward acceleration with emphysema but did not reach statistical significance (−18.2±3.3 versus −30.3±6.4 mL/year; mean difference, 12.1 mL/year; P=0.07).
Table 2 Adjusted decline rate of lung function according to the presence of emphysema
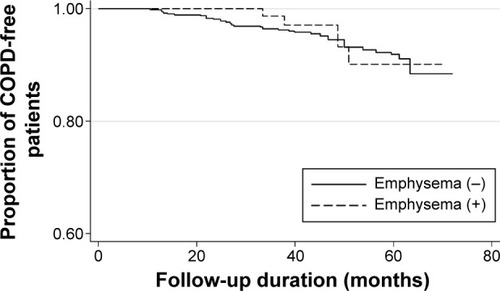
Occurrence of airflow limitation (FEV1/FVC <0.7) during follow-up was observed in 5.5% of participants without emphysema and 4.7% of participants with emphysema. Kaplan–Meier analysis revealed that emphysema was not associated with the development of airflow obstruction (log-rank test, P=0.76; ). Multivariable Cox proportional hazards regression model adjusted for age, sex, height, BMI, and current smoking status showed that emphysema was also not a significant predictor for development of airflow limitation (P=0.47). There was no statistically significant interaction between emphysema and current smoking status (P=0.98) for the development of airflow limitation.
Discussion
In South Korea, possibly owing to low medical costs and increase in attention to health status, CT screening for medical checkup has recently become popular.Citation31 Physicians often encounter incidental emphysema cases detected on CT without spirometric abnormalities. For the first time, our study shows that emphysema found incidentally was associated with a more rapid decline of lung function, particularly FVC. Although a statistically significant difference was not found, the emphysema group showed a faster decline in FEV1 (adjusted mean FEV1 decline rate: −26.3 mL/year), which was slightly lower than that of COPD patients in the ECLIPSE cohort (−33 mL/year).Citation32 In fact, previous studies with COPD patientsCitation8,Citation11 report that emphysema is a risk factor for the rapid decline of FEV1. However, in our study, incidental emphysema did not increase the risk of detection of airflow limitation during follow-up. This was because the rate of decline in FVC (mean difference in slope, −15.2 mL/year; P for interaction, 0.029) was more significant than the rate of decline in FEV1 (mean difference in slope, −8.8 mL/year; P for interaction, 0.153). Similarly, Mohamed Hoesein et al reported that a one-point decrease in total lung emphysema severity was associated with a 64 mL/year decline in FEV1 and a 165 mL/year decline in FVC.Citation33
A plausible mechanism for the more significant decline in FVC is not clear, but it could be explained by an increase in residual volume by hyperinflation. As emphysema is known to be a characteristic pathological finding in COPD patients,Citation2 it contributes to airflow limitation by the mechanism of the equal pressure point theory: loss of elastic recoil contracting the airway and increase in expiration time.Citation34 Airflow limitation induces hyperinflation in emphysema patients, and the increased residual volume can decrease the functional residual capacity and further cause FVC to decrease. Therefore, we cannot assume that emphysema is not associated with further lung function aggravation just because we did not find an association between the presence of emphysema and the future development of airflow limitation.
Our study had several strengths. This study targeted the population with emphysema but normal spirometric values. Previously, subjects with morphological emphysema but no definite spirometric abnormalities were excluded in COPD studies based on current diagnostic criteria. Yuan et al performed a similar study on 143 subjects without airflow limitations. However, they did not reveal the relationship between emphysema on CT and the annual rate of FEV1 decline, although only half of the participants were scanned twice, and the difference in the annual FVC decline rates was also not analyzed.Citation9 More recently, a subgroup analysis of cohorts for the Dutch Belgian Randomized Lung Cancer Screening Trial (NELSON) was reported; the study subjects included 1,391 participants without airflow limitation on baseline spirometry. PFTs were performed twice, and the subjects who later developed airway obstruction had lower HU point below which 15% of the voxels are distributed (Perc 15).Citation11 However, this study did not evaluate the FVC decline rate, and patients with restrictive lung diseases were included in this subgroup. Compared with previous reports, our study included a larger number of subjects in this population with a longer follow-up duration. The annual decline rate of FEV1 in the emphysema group in our study was similar to that of COPD controls (−30 mL/year) in the UPLIFT trial.Citation25 Thus, the lung function of this population with incidentally detected emphysema actually declined rapidly, similar to COPD patients. However, owing to a simultaneous decrease in FVC, airflow obstruction was not detected during follow-up in the majority of this population. As these patients with emphysema may not be diagnosed with COPD by spirometry, they may miss opportunities for intervention. Out study emphasizes the importance of asymptomatic emphysema irrespective of PFT results. In addition, we quantified emphysema using an automatic computerized system to control discrepancies between observers. Previous studies usually measured the extent of emphysema by visual assessment, despite a report that visual assessment of emphysema revealed low inter- and intra-operator agreement.Citation12 The results with computerized software could be applied to many other centers with high reproducibility.
For the correct interpretation of the present results, the limitation of this study should be noted. First, we did not compare the baseline characteristics among subjects who did not attend follow-up spirometry, and this could have led to bias from loss to follow-up, particularly in the emphysema group. Second, a notable number of nonsmokers were included in the emphysema group in our population; this could be explained by other causes of lung injury, such as exposure to biomass fumes and respiratory infection.Citation35 However, we could not get the complete information about these other causes. For example, we do not have information about history of residence, which was associated with the degree of exposure to air pollution. Third, we did not evaluate postbronchodilator FEV1 and FVC, which are more commonly used as lung function variables in COPD patients.Citation3,Citation36 However, as our study enrolled only subjects with normative values of pulmonary function at initial spirometry, this could be somewhat overlooked. Fourth, although we enrolled the study population retrospectively, there are still some concerns about radiation hazard. Fifth, we could not find a plausible mechanism for the rapid decline in FVC. To understand the natural course of the emphysema population with a normal range of PFT results, further detailed larger trials are needed to evaluate their role in COPD progression, exacerbation, and mortality.
Conclusion
Although quantified emphysema without spirometric abnormalities was not associated with future development of airflow limitation, it was significantly associated with a more rapid decline in FVC.
Disclosure
The authors report no conflicts of interest in this work.
References
- JanssensJPPacheJCNicodLPPhysiological changes in respiratory function associated with ageingEur Respir J19991319720510836348
- RoisinRRGlobal Strategy for the Diagnosis, Management and Prevention of COPD Updated 20112015 Available from: http://www.goldcopd.orgAccessed November 26, 2015
- ItoKBarnesPJCOPD as a disease of accelerated lung agingChest200913517318019136405
- HoggJCPathophysiology of airflow limitation in chronic obstructive pulmonary diseaseLancet200436470972115325838
- ZuluetaJJWisniveskyJPHenschkeCIEmphysema scores predict death from COPD and lung cancerChest20121411216122322016483
- JohannessenASkorgeTDBottaiMMortality by level of emphysema and airway wall thicknessAm J Respir Crit Care Med201318760260823328525
- StolkJPutterHBakkerEMProgression parameters for emphysema: a clinical investigationRespir Med20071011924193017644366
- NishimuraMMakitaHNagaiKHokkaido COPD Cohort Study InvestigatorsAnnual change in pulmonary function and clinical phenotype in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2012185445222016444
- YuanRHoggJCParéPDPrediction of the rate of decline in FEV(1) in smokers using quantitative computed tomographyThorax20096494494919734130
- Remy-JardinMEdmeJLBoulenguezCLongitudinal follow-up study of smoker’s lung with thin-section CT in correlation with pulmonary function testsRadiology200222226127011756735
- HoeseinMFAde HoopBZanenPCT quantified emphysema in male heavy smokers: association with lung function declineThorax20116678278721474499
- MascalchiMDiciottiSSverzellatiNLow agreement of visual rating for detailed quantification of pulmonary emphysema in whole-lung CTActa Radiol201253536022114019
- GouldGAMacNeeWMcLeanACT measurements of lung density in life can quantitate distal airspace enlargement–an essential defining feature of human emphysemaAm Rev Respir Dis19881373803923341629
- American Thoracic SocietySingle-breath carbon monoxide diffusing capacity (transfer factor). Recommendations for a standard technique – 1995 updateAm J Respir Crit Care Med1995152218521988520796
- MorrisJFKoskiAJohnsonLCSpirometric standards for healthy nonsmoking adultsAm Rev Respir Dis197110357675540840
- LeeCHLeeJYJangEJNew predictive equations for spirometric reference values and comparison with Morris equation in a Korean populationRespirology20081336537118399858
- GevenoisPADe VuystPde MaertelaerVComparison of computed density and microscopic morphometry in pulmonary emphysemaAm J Respir Crit Care Med19961541871928680679
- WangZGuSLeaderJKOptimal threshold in CT quantification of emphysemaEur Radiol20132397598423111815
- HershCPMakeBJLymchDANone-emphysematous chronic obstructive pulmonary disease is associated with diabetes mellitusBMC Pulm Med20141416425341556
- GieradaDSPilgramTKWhitingBRComparison of standard- and low-radiation-dose CT for quantification of emphysemaAJR Am J Roentgenol20071881424717179344
- WangRSuiXSchoepfUJUltralow-radiation-dose chest CT: accuracy for lung densitometry and emphysema detectionAJR Am J Roentgenol2015204474374925794063
- HagueCJKrowchukNAlhassanDQualitative and quantitative assessment of smoking-related lung disease: effect of iterative reconstruction on low-dose computed tomographic examinationsJ Thorac Imaging201429635035625314025
- MetsOMSchmidtMBuckensCFDiagnosis of chronic obstructive pulmonary disease in lung cancer screening computed tomography scans: independent contribution of emphysema, air trapping and bronchial wall thickeningRespir Res2013145923711184
- CelliBRThomasNEAndersonJAEffect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH studyAm J Respir Crit Care Med200817833233818511702
- DecramerMCelliBKestenSUPLIFT InvestigatorsEffect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trialLancet20093741171117819716598
- TashkinDPCelliBSennSUPLIFT Study InvestigatorsA 4-year trial of tiotropium in chronic obstructive pulmonary diseaseN Engl J Med20083591543155418836213
- PauwelsRALöfdahlCGLaitinenLALong-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European respiratory society study on chronic obstructive pulmonary diseaseN Engl J Med19993401948195310379018
- Lung Health Study Research GroupEffect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary diseaseN Engl J Med20003431902190911136260
- BurgePSCalverleyPMJonesPWRandomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trialBMJ20003201297130310807619
- FletcherCPetoRThe natural history of chronic airflow obstructionBr Med J1977116451648871704
- AhnHSKimHJWelchHGKorea’s thyroid-cancer “epidemic” – screening and overdiagnosisN Engl J Med2014371191765176725372084
- VestboJEdwardsLDScanlonPDECLIPSE InvestigatorsChanges in forced expiratory volume in 1 second over time in COPDN Engl J Med20113651184119221991892
- Mohamed HoeseinFAvan RikxoortEvan GinnekenBComputed tomography-quantified emphysema distribution is associated with lung function declineEur Respir J20124084485022323577
- MeadJTurnerJMMacklemPTSignificance of the relationship between lung recoil and maximum expiratory flowJ Appl Physiol196722951086017658
- SalviSSBarnesPJChronic obstructive pulmonary disease in nonsmokersLancet200937473374319716966
- ChenCZOuCYWangWLUsing post-bronchodilator FEV1 is better than pre-bronchodilator FEV1 in evaluation of COPD severityCOPD2012927628022360379