Abstract
Background
The prognosis of Japanese patients with COPD who suffer repeated exacerbations is unclear, although Westerners with such episodes have a poor prognosis.
Materials and methods
We conducted a 1-year prospective observational trial involving 90 Japanese patients with COPD: 58 nonexacerbators, 12 infrequent exacerbators, and 20 frequent exacerbators classified on the basis of exacerbation frequency (zero, one, and two or more exacerbations/year), respectively, during the previous year were observed prospectively for 1 year. The characteristics of frequent exacerbators, the frequency of exacerbation, and the period until the first event were then compared among the groups.
Results
A total of 78 patients completed the study. Frequent exacerbators had a significantly higher risk of frequent exacerbation in the following year than the case for nonexacerbators (odds ratio [95% confidence interval] 2.94 [1.21–7.17], P=0.0340), but not in comparison with infrequent exacerbators (1.51 [0.49–4.63], P>0.05). The mean annual frequency of exacerbations in the following year was significantly (P=0.0020) higher in the frequent exacerbators (1.4 exacerbations/year) than in the nonexacerbators (0.4), but not in the infrequent exacerbators (0.9, P>0.05). The mean period until the first exacerbation was significantly shorter in the frequent exacerbators than in the infrequent or nonexacerbators (P=0.0012). Independent risk factors for future frequent exacerbation included the presence of gastroesophageal reflux disease, more severe airflow obstruction, and use of inhaled corticosteroids.
Conclusion
Our present results indicate that Japanese COPD patients suffering frequent exacerbation have a poor prognosis. The characteristics of Japanese and Western COPD patients suffering frequent exacerbation are similar.
Keywords:
Introduction
Exacerbation is an important life-threatening event for patients with COPD, and can lead to hospitalization and death.Citation1–Citation4 Patients who suffer frequent and repeated exacerbations within 1 year have a poor prognosis,Citation5 characterized by worsening of health-related quality of life (HRQoL),Citation6,Citation7 a rapid decline in lung function,Citation8–Citation10 and high mortality.Citation11 Frequent exacerbators also carry a high risk of further exacerbation and hospitalization.Citation11,Citation12 However, it has been suggested that Japanese patients with COPD may have fewer exacerbations, and they also may have a higher proportion of elderly patients, those with emphysema, and those with a lower body mass index in comparison to Westerners.Citation12–Citation15 The prognosis of Japanese patients with COPD who suffer frequent and repeated exacerbations is unclear. We conducted a 1-year prospective observational trial in a daily-life setting involving 90 Japanese patients with COPD to investigate whether previous moderate-to-severe exacerbations are associated with future exacerbations in this patient population.
Materials and methods
Study design
We conducted a 1-year prospective observational trial in accordance with Good Clinical Practice (GCP) guidelines and approved by the ethics committee of Kurume University and Chikugo City Hospital (GCP 11-127, September 2012–August 2014). Consecutive patients for whom medical records were available covering a period of at least 1 year since provision of informed consent were selected for the study; information on previous annual COPD-related exacerbations and hospitalizations was collected on the basis of those medical records. COPD patients were divided into three groups, based on the total number of moderate and severe exacerbations within the last year before enrollment in the study, ie, non- (previous moderate and severe exacerbations, 0/year), infrequent (one exacerbation/year), and frequent (two or more exacerbations/year) exacerbator groups, in accordance with a previous report.Citation16 In addition, patients with previous hospitalizations were classified as having a subphenotype with severe exacerbation (severe exacerbators). The data collected for each patient included baseline data for previous moderate and severe exacerbations and hospitalizations; clinical parameters included age, sex, body mass index, smoking habits, smoking index, comorbidities, duration of COPD, 5-grade modified Medical Research Council (mMRC) dyspnea scale score,Citation17 total COPD Assessment Test (CAT) score,Citation18,Citation19 frequency scale for symptoms of gastroesophageal reflux disease (GERD) (FSSG),Citation20 Center for Epidemiologic Studies Depression (CESD) scale score,Citation21 medications, blood pressure and heart rate, lung function and blood parameters, and chest computed tomography. Duration of COPD was defined as the period (years) since the patient had been diagnosed by a physician as having COPD, emphysema, and/or chronic bronchitis.Citation22,Citation23
After stable status for at least 4 weeks had been confirmed, each patient was required to regularly visit the hospital every 2 months, and to request emergency admission when the symptoms worsened. Regular respiratory medications were not changed during the period of the study, which was conducted in a daily-life setting. However, adherence to inhaled medications was not assessed. All of the patients had received annual influenza-virus vaccinations. None had received regular rehabilitation for COPD.
Diagnosis and spirometric classification of COPD
The diagnosis of COPD was based on age ≥40 years, smoking index >10 pack-years, forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio of <0.7 after bronchodilator administration, and the spirometric GOLD (Global initiative for chronic Obstructive Lung Disease)-stage classification, ie, stage I (FEV1 ≥80% predicted), II (50% ≤FEV1 <80% predicted), III (30% ≤FEV1 <50% predicted), and IV (FEV1 <30% predicted).Citation1 Chest computed tomography confirmed that all patients had low-attenuation areas.
To exclude any patients with asthma, patients who had past symptoms of repeated spasmodic wheezes and medications for asthma and a classification of FEV1 >200 mL or >12% after bronchodilation were excluded.Citation24 Diagnosis of asthma–COPD overlap syndrome (ACOS) was made on the basis of a history of dyspnea and wheezing attacks at rest, large variations in daily symptoms, a fixed FEV1/FVC ratio of <0.7, marked reversibility of FEV1 after administration of bronchodilators (>15% and >400 mL), and a peripheral eosinophil count of >600/mm3, in accordance with a previous report.Citation25
Assessment of mMRC scale, total CAT, FSSG scale, and CESD
Baseline data for the mMRC scale, total CAT, FSSG scale, and CESD scale scores were obtained only once, based on a self-report completed by each patient after written informed consent had been obtained.Citation17–Citation21
Comorbidities
Information about comorbidities was obtained from the patients by interview, and the diagnoses were confirmed by physicians. However, patients who had moderate-to-severe comorbidities associated with poor prognosis, such as active malignancies, depression (CESD scale >16 points),Citation21 liver cirrhosis, digestive ulcers, persistent arrhythmia, congestive heart disease, coronary artery disease, lung fibrosis and bronchiectasis, chronic renal failure requiring dialysis, and central nervous system disorders, including palsy and dementia, were excluded, in accordance with previous studies.Citation26,Citation27 Hypertension (systolic >140 mmHg or diastolic >90 mmHg blood pressure or use of medications), dyslipidemia (serum low-[≥14] or high- [<4] mg/L density lipoprotein cholesterol, triglycerides >15 mg/L, or use of medications), diabetes (blood hemoglobin A1c ≥6.5% National Glycohemoglobin Standardization Program value or use of medications)Citation28 without triopathy (neuropathy, retinopathy, and nephropathy), and GERD (FSSG scale >7 points)Citation20 were accepted as comorbidities. All data on medications for each patient were obtained from individual medication notebooks.
Frequency and date of mortality, hospitalizations, and exacerbations
Previous and prospective exacerbations documented in the medical records made by physicians were accepted as moderate and severe events. Moderate exacerbations that required a prescription for antibiotics and/or systemic corticosteroids were defined on the basis of symptom-based diagnosis, such as increased cough and sputum production, a change in sputum color, and worsening of dyspnea from a stable state and beyond normal day-to-day variations, ie, showing acute onset and necessitating a change in regular medication, in accordance with previous reports.Citation1,Citation29,Citation30 COPD-related and other causes of death and hospitalization were prospectively followed for 1 year. COPD-related deaths and hospitalizations were considered severe exacerbations. Hospitalization was decided by each examining physician when hypoxemia required additional or intensive oxygen and/or assisted ventilation therapy, performance status was ≥3, and unconsciousness occurred with COPD exacerbation.Citation1,Citation23,Citation31 However, pneumonia was also recognized as exacerbation. Mild and unreported exacerbations were not considered to have been equal in severity to previous and prospective exacerbations. However, mild exacerbations were defined as those that improved naturally without any medication or administration of inhaled short-acting bronchodilators. Unreported exacerbations were considered to be those to which patients had been insensitive, or those that had been self-controlled in spite of worsening of respiratory symptoms. The patient’s self-reported daily journal was not used in the study.
Statistical analysis
Data are expressed as mean ± standard deviation (SD) and number (percentage) of non-, infrequent, and frequent exacerbators at baseline. Characteristics were compared using analysis of variance, Fisher’s exact test, χ2-test for trend, and Tukey–Kramer honestly significant difference tests. Subsequent moderate and severe exacerbation events during 1 year of prospective observation were recognized as future risk indicators, and baseline parameters were chosen and modified in accordance with a previous report.Citation16 Kaplan–Meier analyses and log-rank tests for subsequent moderate and severe exacerbations were performed in all three groups of patients.
For patients who were observed throughout the study period, the odds ratio (95% confidence interval [CI]) of baseline parameters was determined to predict factors for future risk (subsequent exacerbations once or more and twice or more) using univariate and logistic multivariate regression analyses. Medians of age, body mass index, smoking index, and duration of COPD were 68 years, 21.4 kg/m2, 53.5 pack-years, and 4 years, respectively, and each median was used as the cutoff value for the analysis. Differences at P<0.05 were considered statistically significant. Statistical analysis was performed by the JMP® version 9.0 statistical software package (SAS Institute Inc, Cary, NC, USA).
Results
Study subjects
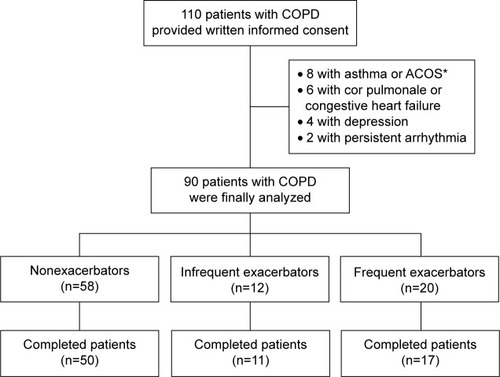
Ninety of 110 patients who provided informed consent were finally analyzed; 32 (35.6%) patients had suffered previous moderate and/or severe exacerbations during the last year. The numbers of patients with non-, infrequent, and frequent exacerbations were 58 (64.4%), 12 (13.3%), and 20 (22.2%), respectively ().
Figure 1 Study design.
Abbreviation: FEV1, forced expiratory volume in 1 second.
Baseline characteristics
At baseline, frequent exacerbators had a significantly lower body mass index, were less likely to be male or current smokers, had higher mMRC-scale grades and higher total CAT score, had a higher proportion of spirometric stages III and IV, GERD, and use of inhaled corticosteroid (ICS)/long-acting muscarinic antagonist or ICS/long-acting β2-agonist combination therapy, and lower lung-function parameters, including FEV1, FVC, and FEV1/FVC ratio before and after bronchodilator use compared with nonexacerbators, but not in comparison with infrequent exacerbators (). Frequent and infrequent exacerbators had significantly higher mMRC-scale grades and total CAT scores compared with nonexacerbators (). There was no change in the frequency of previous annual hospitalizations per patient between those with frequent and infrequent exacerbations ().
Table 1 Characteristics of nonexacerbators and infrequent and frequent exacerbators
Stability of the frequent and severe exacerbation phenotype in patients who completed the study
During the 1-year prospective observation period, one frequent exacerbator, one infrequent exacerbator, and two nonexacerbators died, with causes of death respiratory failure with COPD exacerbation, cerebrovascular attack, and malignancies (small-cell lung cancer and colon cancer), respectively, whereas one frequent exacerbator and six nonexacerbators dropped out for private reasons. As a result, 78 patients (17 frequent, eleven infrequent, and 50 nonexacerbators) completed the study ().
also shows that among the frequent exacerbators, the number of patients who subsequently suffered severe exacerbations (requiring one or more hospitalizations) was two (11.8%), and frequent (two or more exacerbations/year), infrequent (one exacerbation/year), and no moderate or severe exacerbations were seven (42.1%), six (35.3%), and four (23.5%), respectively, whereas seven (63.6%) of eleven infrequent and 37 (74.0%) of 50 nonexacerbators experienced no further exacerbation. The proportions of frequent exacerbators who subsequently experienced frequent and infrequent exacerbations were significantly higher than those of nonexacerbators (odds ratio [95% CI] 2.94 [1.21–7.17], P=0.0340; 2.94 [1.72–5.03], P=0.0004, respectively), but not in comparison with infrequent exacerbators (1.51 [0.49–4.63], P>0.05; 2.01 [0.92–4.80], P=0.053, respectively).
Figure 2 Stability of the phenotypes of frequent and infrequent exacerbators, nonexacerbators, and severe exacerbators.
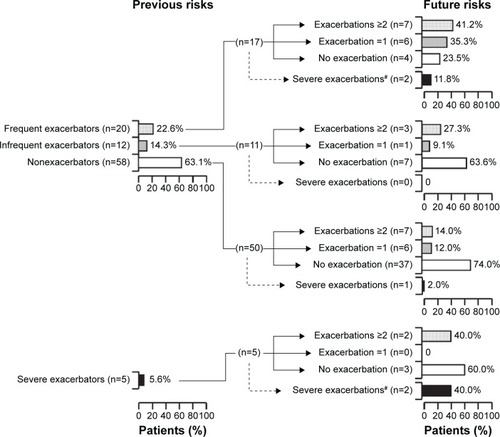
Among five (5.6%) of the severe exacerbators, two (40.0%) had subsequently suffered severe exacerbations. The number of patients who had subsequent frequent, infrequent, and no moderate or severe exacerbations was two (40%), 0, and three (60%), respectively (). In subanalysis, there was no difference in the proportion of patients who had subsequently suffered severe exacerbations between the frequent and severe exacerbator groups (P>0.05). In addition, one severe exacerbator died due to COPD-related respiratory failure during the 1-year prospective observation period.
The numbers of patients who reported pneumonia (n=78) and those who regularly used ICS (n=15) were seven (9%) and three (20%), respectively. Patients who regularly used ICS had higher but not significant contraction of pneumonia than those who did not use ICS (P>0.05).
Comparison of annual exacerbation and hospitalization among non-, infrequent, and frequent exacerbators
shows that the mean annual frequencies (±SD, exacerbations/year) of future total (1.4±1.2, P=0.0020) and moderate (1.3±1.1, P=0.0057) exacerbations in frequent exacerbators were significantly higher than those in nonexacerbators (0.4±0.8 and 0.4±0.7, respectively), but not in infrequent exacerbators (0.9±1.4 and 0.8±1.4, respectively).
Figure 3 Comparison of annual exacerbation and hospitalization among nonexacerbators and infrequent and frequent exacerbators.
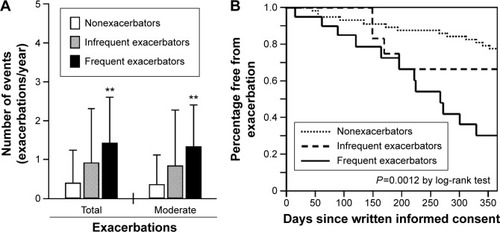
shows the Kaplan–Meier analysis of the period until the first moderate and severe exacerbations. The median (mean ± SD) periods (days) until the first moderate and severe exacerbations for frequent, infrequent, and nonexacerbators were 266 (235±28), 365 (293±30), and 365 (323±13) days, respectively (log-rank test, P=0.0012).
There was no significant difference in the annual frequencies of severe exacerbation (hospitalization) among non- (0.0±0.3), infrequent (0.1±0.39), and frequent (0.1±0.3) exacerbators (P>0.05). The median (mean ± SD) periods until the first severe exacerbation were 365 (363±2), 365 (365±0), and 365 (360±5) days, respectively (P>0.05; data not shown).
Baseline characteristics of patients suffering further exacerbations (one or more/year) and frequent exacerbations (two or more/year)
Univariate analysis revealed that a low FEV1 (<50%) had the highest odds ratio for risk of future exacerbation (one or more exacerbations/year), followed in order by regular use of ICS, two or more previous exacerbations in the last year, presence of GERD, pneumococcal vaccination, one or more previous exacerbations in the last year, low body mass index (≤21.4 kg/m2), a high total CAT score (≥10 points), a high mMRC scale grade (≥2) and older age (≥68 years), whereas in patients who suffered two or more exacerbations, the highest odds ratio was observed due to the presence of GERD, followed in order by regular use of ICS, low FEV1 predicted, pneumococcal vaccination, older age, a high mMRC-scale grade, long duration of COPD (≥4 years), and two or more and one or more previous exacerbations in the last year (). However, 13 (86.7%) of 15 patients who had received ICS had also received pneumococcal vaccination.
Table 2 Baseline characteristics of patients with future exacerbation (once or more) and frequent exacerbation (twice or more) by univariate analysis
The independent risk factors for both one or more and two or more future exacerbations were low FEV1 predicted, presence of GERD, and regular use of ICS, whereas a low body mass index was an independent risk factor for one or more but not for two or more future exacerbations ().
Table 3 Baseline characteristics of patients with future exacerbation (once or more) and frequent exacerbation (twice or more) by multivariate analysis
Discussion
The frequent-exacerbator phenotype is important to consider in the management of COPD patients, who require future exacerbation-related hospitalization associated with high mortality.Citation16 A previous Western study suggested that frequent exacerbators tend to have had exacerbations during the previous year, a lower FEV1, more severe HRQoL, a history of GERD, and a higher peripheral white blood-cell count in comparison with infrequent exacerbators and nonexacerbators.Citation12 Another Western study demonstrated that the characteristics of frequent exacerbators include a more severe mMRC-scale grade, lower FEV1 predicted, comorbid cardiovascular disease, depression or osteoporosis, and female sex as independent risk factors.Citation32,Citation33 We conducted the present study to observe moderate and severe exacerbations 1 year before and after baseline to investigate the characteristics of Japanese COPD patients who were frequent exacerbators. Based on exacerbations during the previous year, we found that frequent exacerbators were more likely to be female, have a lower body mass index, have a significantly lower FEV1 predicted, have a higher mMRC-scale grade (lower exercise tolerance), and have a lower total CAT score (lower HRQoL). We also found that the characteristics of frequent exacerbators were similar between Japanese and Westerners, except for body mass index, as reported previously.Citation12,Citation16,Citation32 Interestingly, univariate analysis did not show that previous frequent exacerbators would become future frequent exacerbators (). Indeed, 60% of previous frequent exacerbators did not suffer subsequent exacerbations, whereas conversely 14% of patients who had not previously suffered exacerbations subsequently did so (). Investigation of factors predicting the change in frequency of exacerbations is critically important, as they are still unclear.Citation34
A total of 78 (86.7%) of our 90 patients completed the 1-year prospective study period. Among five severe exacerbators, one (20%) died, two (40%) again developed severe exacerbation, and two (40%) became frequent exacerbators in the following year. Therefore, severe exacerbators appear to have a poor prognosis. However, our study-sample size was small and the observational period short. Further analysis will therefore be needed to verify this issue. Frequent exacerbators had a significantly higher frequency of future exacerbations and a shorter period until the next exacerbation than nonexacerbators, and also had a significantly poorer prognosis than the latter, thus confirming the findings of a previous study.Citation12
Previous studies have demonstrated that over half of COPD patients have unreported exacerbations. Such unreported exacerbations are thought to be an important component of HRQoL decline.Citation7,Citation35,Citation36 In our present study, to make the conditions for previous and future exacerbations uniform, daily journals for symptoms were not accepted. Therefore, mild and unreported exacerbations were unclear. Previous studies have demonstrated that the frequency of annual moderate or severe exacerbations per patient in Japanese individuals may be lower than that in the USA and Europe.Citation13,Citation14,Citation35–Citation37 In our study, the mean (± SD [range]) of previous annual total and severe exacerbations were 0.84±1.48 (0–6) and 0.06±0.23 (0–1) exacerbations/year, respectively for all patients (data not shown), whereas those of future total and severe exacerbations were 0.71±1.08 (0–4) and 0.06±0.28 (0–2) exacerbations/year, respectively. Our data for the frequency of exacerbations seem to indicate a slightly lower incidence in Japanese than in Westerners.Citation12–Citation15,Citation31,Citation32,Citation38 Previous Japanese reports on the frequency of exacerbations have been scarce.Citation13,Citation14 Japanese COPD patients may be slightly older and thinner on average than Westerners.Citation12–Citation15,Citation31,Citation32,Citation38 Although the discrepancy between Japanese and Westerners is still unclear, the difference in the frequency of exacerbations may be associated with different populations of phenotypes with emphysema, locality, and understanding of both the physician and the patient about diseases and exacerbations, such as convenient use of ICS, and in definition of exacerbation.
Patients with COPD have a wide variety of numerous comorbidities, which are strongly associated with mortality and exacerbation.Citation1,Citation12,Citation26 We assessed baseline comorbidities based on interviews with patients and the physicians’ diagnosis, and partly through examinations or questionnaires. Several comorbidities, such as depressionCitation21,Citation39 assessed by the CESD questionnaire, ACOSCitation27,Citation40 based on previous criteria, and congestive status with heart failure and cor pulmonale based on history or medical signs,Citation41 were carefully excluded. GERD, although not moderate to severe, was a common major comorbidity, and the proportion of frequent exacerbators with GERD was significantly higher than that of nonexacerbators. GERD was an independent, and the highest, risk factor for future frequent exacerbations, although all patients with GERD were receiving proton-pump inhibitors. Our results confirmed previous reports.Citation12,Citation42
A low FEV1 predicted was an independent risk factor for future exacerbations. The guidelinesCitation1,Citation43 recommend long-term treatment with ICS and pneumococcal vaccination for patients with severe and very severe COPD, and frequent exacerbations may not be adequately controlled by long-acting bronchodilators. In our study, most patients who regularly used ICS also received pneumococcal vaccination. Regular use of ICS, but not pneumococcal vaccination, was an independent risk factor for future frequent exacerbations. Sixteen (17.8%) of our patients used ICS, although none had received ICS without long-acting bronchodilators at baseline. Users of ICS included four nonexacerbators with GOLD stage II, seven patients with frequent exacerbations, and the remaining five had severe or very severe disease. The risk of ICS for respiratory infection including pneumonia is a concern.Citation44–Citation46 Among four nonexacerbators with GOLD stage II and users of ICS during the prospective observation period, two patients had frequent exacerbations and one had infrequent exacerbations. This risk must be weighed against the benefits when prescribing ICS to patients with COPD.
Our study had some limitations. First, the study population was small and the observation period rather short for analyzing severe exacerbations and mortality. Balcells et al demonstrated that female sex was the highest risk factor for exacerbation,Citation33 although in the present study there was no difference in the frequency of exacerbations between males and females. This discrepancy may have been attributable to sample size. Second, our protocol was not double-blinded. Both physicians and patients were aware of the severity and frequency of previous exacerbations at baseline. Third, dealing with comorbidity was difficult. We carefully removed severe comorbidities, such as cardiovascular disease and depression, in accordance with previous reports.Citation26,Citation27 However, patients with asthma and ACOS may have been included, because we did not investigate airway responsiveness and inflammation or serum total immunoglobulin (Ig) E levels, although we carefully excluded patients with asthma based on symptoms and spirometry. However, no patients had received medications for osteoporosis and low peripheral lymphocyte counts, although latent osteoporosis and HIV infections were not tested for. Fourth, the contents of medicines could not be ethically unified in a clinical setting in Japan, and in our study adherence to pharmacological medicines and compliance with inhalation techniques were not assessed or included. The effects of respiratory medications, including inhaled medicines, on previous and future exacerbations were investigated. Further trials will be necessary to clarify the limitations of our approach.
Conclusion
Our results indicate that Japanese COPD patients with frequent exacerbations have a poor prognosis. The characteristics of frequent exacerbators among Japanese and Western patients are similar. The presence of GERD, regular use of ICS, and low FEV1 may be associated with frequent exacerbations.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
The authors are grateful to Masaharu Kinoshita, MD, PhD, Yanagawa Nagata Hospital, and Tatsuya Mukaino, MD, Social Insurance Tagawa Hospital, for collection of data on the study subjects. The authors are also grateful to Prof Tatsuyuki Kakuma, Biostatistics Center, Kurume University School of Medicine, for support with statistical analysis. The authors are also grateful to Dr Howard A Young, National Cancer Institute – Frederick, for reading our manuscript and providing English-language editing.
Disclosure
Tomotaka Kawayama received grants from AstraZeneca, Japan; MSD KK (Merck), Japan; and Novartis Pharmaceuticals, Japan; and lecture fees from Novartis Pharmaceuticals, Japan; GSK, Japan; Boehringer Ingelheim, Japan; and AstraZeneca, Japan. Tomoaki Hoshino received a grant from GSK, Japan; Novartis Pharmaceuticals, Japan and Chugai Pharmaceutical Co Ltd., Japan. The other authors report no conflicts of interest in this work.
References
- GOLD (Global initiative for chronic Obstructive Lung Disease)Global Strategy for Diagnosis, Management, and Prevention of COPDBethesda (MD)GOLD2015
- SinganayagamASchembriSChalmersJDPredictors of mortality in hospitalized adults with acute exacerbation of chronic obstructive pulmonary diseaseAnn Am Thorac Soc2013102818923607835
- Bustamante-FermoselADe Miguel-YanesJMDuffort-FalcóMMuñozJMortality-related factors after hospitalization for acute exacerbation of chronic obstructive pulmonary disease: the burden of clinical featuresAm J Emerg Med200725551552217543654
- GroenewegenKHScholsAMWoutersEFMortality and mortality-related factors after hospitalization for acute exacerbation of COPDChest2003124245946712907529
- SeemungalTAHurstJRWedzichaJAExacerbation rate, health status and mortality in COPD – a review of potential interventionsInt J Chron Obstruct Pulmon Dis2009420322319554195
- SpencerSCalverleyPMBurgePSJonesPWImpact of preventing exacerbations on deterioration of health status in COPDEur Respir J200423569870215176682
- SeemungalTADonaldsonGCPaulEABestallJCJeffriesDJWedzichaJAEffect of exacerbation on quality of life in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med19981575 Pt 1141814229603117
- CelliBRThomasNEAndersonJAEffect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH studyAm J Respir Crit Care Med2008178433233818511702
- MakrisDMoschandreasJDamianakiAExacerbations and lung function decline in COPD: new insights in current and ex-smokersRespir Med200710161305131217112715
- DonaldsonGCSeemungalTABhowmikAWedzichaJARelationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary diseaseThorax2002571084785212324669
- Soler-CataluñaJJMartínez-GarcíaMARomán SánchezPSalcedoENavarroMOchandoRSevere acute exacerbations and mortality in patients with chronic obstructive pulmonary diseaseThorax2005601192593116055622
- HurstJRVestboJAnzuetoASusceptibility to exacerbation in chronic obstructive pulmonary diseaseN Engl J Med2010363121128113820843247
- SuzukiMMakitaHItoYMNagaiKKonnoSNishimuraMClinical features and determinants of COPD exacerbation in the Hokkaido COPD cohort studyEur Respir J20144351289129724232696
- FukuchiYFernandezLKuoHPEfficacy of tiotropium in COPD patients from Asia: a subgroup analysis from the UPLIFT trialRespirology201116582583521539680
- TatsumiKKasaharaYKurosuKTanabeNTakiguchiYKuriyamaTClinical phenotypes of COPD: results of a Japanese epidemiological surveyRespirology20049333133615363004
- BeehKMGlaabTStowasserSCharacterisation of exacerbation risk and exacerbator phenotypes in the POET-COPD trialRespir Res20131411624168767
- BestallJCPaulEAGarrodRGarnhamRJonesPWWedzichaJAUsefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary diseaseThorax199954758158610377201
- TsudaTSuematsuRKamoharaKDevelopment of the Japanese version of the COPD Assessment TestRespir Investig20125023439
- JonesPWHardingGBerryPWiklundIChenWHKline LeidyNDevelopment and first validation of the COPD Assessment TestEur Respir J200934364865419720809
- KusanoMShimoyamaYSugimotoSDevelopment and evaluation of FSSG: frequency scale for the symptoms of GERDJ Gastroenterol200439988889115565409
- ItoKKawayamaTShojiYDepression, but not sleep disorder, is an independent factor affecting exacerbations and hospitalization in patients with chronic obstructive pulmonary diseaseRespirology201217694094922564039
- SuetomoMKawayamaTKinoshitaTCOPD assessment tests scores are associated with exacerbations in Japanese patients with chronic obstructive pulmonary diseaseRespir Investig2014525288295
- TashkinDPCelliBSennSA 4-year trial of tiotropium in chronic obstructive pulmonary diseaseN Engl J Med2008359151543155418836213
- Global Initiative for AsthmaGlobal Strategy for Asthma Management and PreventionBethesda (MD)GINA2015
- Soler–CataluñaJJCosíoBIzquierdoJLConsensus document on the overlap phenotype COPD-asthma in COPDArch Bronconeumol201248933133722341911
- DivoMCoteCde TorresJPComorbidities and risk of mortality in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2012186215516122561964
- CharlsonMEPompeiPAlesKLMacKenzieCRA new method of classifying prognostic comorbidity in longitudinal studies: development and validationJ Chronic Dis19874053733833558716
- Japan Diabetes SocietyEvidence-based practice guideline for the treatment of diabetes in Japan 20132014 Available from: http://www.jds.or.jp/modules/en/index.php?content_id=44Accessed December 23, 2015
- Rodriguez-RoisinRToward a consensus definition for COPD exacerbationsChest20001175 Suppl 2398S401S10843984
- CalverleyPMAndersonJACelliBSalmeterol and fluticasone propionate and survival in chronic obstructive pulmonary diseaseN Engl J Med2007356877578917314337
- KesslerRFallerMFourgautGMennecierBWeitzenblumEPredictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med199915911581649872834
- McGarveyLLeeAJRobertsJGruffydd-JonesKMcKnightEHaughneyJCharacterisation of the frequent exacerbator phenotype in COPD patients in a large UK primary care populationRespir Med2015109222823725613107
- BalcellsEAntóJMGeaJCharacteristics of patients admitted for the first time for COPD exacerbationRespir Med200910391293130219427776
- DonaldsonGCMüllerovaHLocantoreNFactors associated with change in exacerbation frequency in COPDRespir Res2013147923899210
- XuWColletJPShapiroSNegative impacts of unreported COPD exacerbations on health-related quality of life at 1 yearEur Respir J20103551022103019897555
- LangsetmoLPlattRWErnstPBourbeauJUnderreporting exacerbation of chronic obstructive pulmonary disease in a longitudinal cohortAm J Respir Crit Care Med2008177439640118048806
- WiseRAAnzuetoACottonDTiotropium Respimat inhaler and the risk of death in COPDN Engl J Med2013369161491150123992515
- TanabeNMuroSHiraiTImpact of exacerbations on emphysema progression in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2011183121653165921471102
- AtlantisEFaheyPCochraneBSmithSBidirectional associations between clinically relevant depression or anxiety and COPD: a systematic review and meta-analysisChest2013144376677723429910
- MenezesAMMontes de OcaMPérez-PadillaRIncreased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthmaChest2014145229730424114498
- FisherKAStefanMSDarlingCLessardDGoldbergRJImpact of COPD on the mortality and treatment of patients hospitalized with acute decompensated heart failure: the Worcester Heart Failure StudyChest2015147363764525188234
- TeradaKMuroSSatoSImpact of gastrooesophageal reflux disease symptoms on COPD exacerbationThorax2008631195195518535116
- Japanese Respiratory SocietyGuidelines for the diagnosis and treatment of COPD (chronic obstructive pulmonary disease)3rd edTokyoMedical Review Co2009 Japanese
- CrimCDransfieldMTBourbeauJPneumonia risk with inhaled fluticasone furoate and vilanterol compared with vilanterol alone in patients with COPDAnn Am Thorac Soc2015121273425490706
- DiSantostefanoRLSampsonTLeHVHindsDDavisKJBakerlyNDRisk of pneumonia with inhaled corticosteroid versus long-acting bronchodilator regimens in chronic obstructive pulmonary disease: a new-user cohort studyPLoS One201495e9714924878543
- KewKMSeniukovichAInhaled steroids and risk of pneumonia for chronic obstructive pulmonary diseaseCochrane Database Syst Rev20143CD01011524615270
