Abstract
Background
Microparticles (MPs) are small membrane vesicles of 0.1–1 µm which are released by cells following chemical, physical, and apoptotic stimuli. MPs represent more than a miniature version of the cell. Their composition and function depend not only on cellular origin, but also on stimuli. Chronic obstructive pulmonary disease (COPD) is a lung disease characterized by nearly irreversible lung destruction which results in airway limitation.
Purpose
We investigated the presence and source of MPs in sputum of COPD patients to evaluate if changes in MP number and origin may reflect the pathophysiological conditions of disease and may serve as potential biomarkers for diagnostic and prognostic use.
Methods
Induced sputum samples were collected from 18 male subjects and liquefied with Sputasol. MPs obtained were immunolabeled for leukocyte (CD11a), granulocyte (CD66b), monocyte-macrophage (CD11b), platelets and megakaryocytic cells (CD41), endothelial cells (CD31), and red blood cells (CD235ab) and analyzed by cytofluorimetry.
Results
There was a negative correlation between CD31-MPs and forced expiratory volume in 1 second (R=−53, P<0.05) and CD66b-MP level was correlated with worse performance index of COPD such as the Body mass index airflow Obstruction, Dyspnea, and Exercise capacity (BODE); they were negatively correlated with 6-minute walking test: 0.65 and −0.64, respectively (P<0.05). CD235ab-MPs showed a negative correlation with body mass index (R=−0.86, P<0.05), while there was a positive correlation with dyspnea index (R=0.91, P<0.05).
Conclusion
The main finding of this study was that MPs were detected in the sputum of patients affected by COPD. The phenotype of some of them was related to the main COPD parameters. These results suggest that MPs could be implicated in the pathogenesis of COPD.
Keywords:
Background
Extracellular vesicles have received a great deal of attention during the last decade as a novel approach to detect diseases as messengers or mediators of disease pathophysiology. The main classes of extracellular vesicles generally include exosomes, microvesicles/microparticles (MPs), and apoptotic bodies, which are differentiated by their biogenesis and secretion mechanisms. Exosomes are 50–150 nm in diameter and are characterized by their endosomal origin. Exosomes are released by endocytosis following intracellular assembly in multivesicular bodies that contain intraluminal vesicles.
MPs are shed from the plasma membrane through direct outward budding, and they are larger than exosomes (100–2,000 nm). MPs are enriched in phosphatidylserine and contain a membrane component that is similar to that of the parent cell membrane.Citation1 Apoptotic bodies are 1–4 µm in diameter and are released from the plasma membrane as blebs of cells undergo apoptosis. Apoptotic bodies may contain DNA fragments, noncoding RNAs, and cell organelles.Citation2 Several cell types (such as macrophages, platelets, endothelial cells, granulocytes, monocytes, lymphocytes) release MPs following chemical (cytokines, thrombin, and endotoxin), physical (shear stress and hypoxia), and apoptotic stimuli. MPs play an active role in the initiation and amplification of the coagulation cascade, and a pivotal role has been proposed for them in thrombosis, propagating inflammation, modulating vascular tone, angiogenesis, stem cell engraftment, and tumor metastasis.Citation3 MPs have been isolated from different biological fluids including plasma,Citation4 serum,Citation5 cerebrospinal fluid,Citation6 bronchoalveolar lavage,Citation7 and synovial fluid.Citation8
The phenotype of circulating MPs and, consequently, their origin are different in various pathological conditions, and detection of their cellular origin may serve as a predictor or marker of diseases.Citation9
MPs are more than just a miniature version of the specific cell of origin, although the antigens found on the surface of MPs and their cargo resemble those of their parental cells (eg, lineage markers), as certain MP components are selectively enriched compared to their parental cell.
Recent reports have underlined their role as signaling elements in cell–cell communication. Interest has been attracted to MPs because of their positive correlations with various vascular diseases, but now investigation is also being made of MPs in pulmonary diseases in view of their amplified numbers, procoagulant properties, and participation in inflammatory events.
Many studies have been conducted in order to characterize circulating MPs in pulmonary diseases. Mutschler et al showed, for the first time ever, the presence of MPs, derived from platelets, in pulmonary air–liquid interfaces in sedated pigs.Citation8 Recent investigations conducted in bronchoalveolar lavage fluid characterized intra-alveolar procoagulant MPs in patients with acute respiratory distress syndrome and hydrostatic pulmonary edema. In acute respiratory distress syndrome patients, intra-alveolar MPs contain high levels of tissue factor, show a highly procoagulant activity, and likely contribute to intra-alveolar fibrin formation, a critical pathogenic feature of acute lung injury.Citation10,Citation11
Chronic obstructive pulmonary disease (COPD) is a lung disease characterized by irreversible lung destruction which results in airflow limitation. The severity of the disease depends largely on the degree of airflow limitation, which is measured by forced expiratory volume in 1 second (FEV1).Citation12
In 2010, Porro et alCitation13 provided evidence of the presence of MPs in sputum obtained from cystic fibrosis patients and also found that MPs obtained from cystic fibrosis sputum are proinflammatory when injected into the lungs of mice.Citation14
The goal of the present study is to investigate the presence and source of sputum MPs in COPD patients and to correlate the number and source of MPs to the clinical picture. Changes in MP number and composition may reflect the disease pathophysiological conditions and, therefore, could have potential prognostic value for diagnostic use. Understanding MP involvement in COPD may provide insight into disease mechanisms and also aid in the development of novel therapeutic strategies.
Methods
Study patients
The study was approved and performed according to the ethical standards of CE Azienda Ospedaliero-Universitaria Ospedali Riuniti di Foggia on human experimentation. Written informed consent was obtained from each subject.
Induced sputum samples were collected from 18 male subjects with mild to severe COPD (I–IV stages according to Global initiative for chronic Obstructive Lung Disease guidelines) enrolled at the Institute of Respiratory diseases (Ospedali Riuniti of Foggia). All subjects were former smokers, and stopped smoking for at least 1 year. Presence of main comorbidities was also evaluated and it was found that the majority of patients had been affected by cardiovascular diseases (). All subjects completed the study questionnaires on smoking status, COPD Assessment Test score, and general data, and performed standardized spirometry. The equipment was calibrated daily using a 3 L syringe. In accordance with the Global initiative for chronic Obstructive Lung Disease guidelines, the subjects were defined as COPD when FEV1/forced vital capacity was <70% post-bronchodilation. All patients with COPD had stable disease with no exacerbation or respiratory tract infection 2 months before the study. Drugs for COPD (inhaled corticosteroid/long-acting β2-agonist or long-acting muscarinic antagonist) were interrupted at least 7 days before the collection of sputum. The data about the 6-minute walking test (6MWT), and body mass index (BMI), and dyspnea by Borg scaleCitation15 were also collected, and the BODE index was then calculated according to international guidelines were also calculated according to international guidelines.Citation16
Table 1 General characteristics of COPD patients at enrollment in the study
Collection of sputum samples
Sputum induction was achieved by making the patients inhale hypertonic saline solution via UltraNeb DevilBiss (Sunrise Medical, Wollaston, UK) treated with Sputasol (Oxoid, Hampshire, UK) according to European Respiratory Society guidelines.Citation17 The whole expectorate was collected directly into a clear plastic Petri dish where the selection process was performed. We used forceps to pull the sputum out of the surrounding saliva. The selected sputum (plugs) was dissolved in Sputasol. After homogenization, the solution was filtered through a nylon mesh filter (53 µm nylon mesh).
The filtered cell suspension was centrifuged at 600× g for 10 minutes at 4°C–8°C, and the supernatant was aspirated and stored at −80°C for the analysis of MPs, performed later. The total cell count and viability of sputum cells were obtained simultaneously in a Bürker counting chamber. Cytospins were prepared, stained with Diff Quick Stain (Medion Diagnostics, Düdingen, Switzerland), and two researchers with training in reading induced sputum slides independently counted 400 nonsquamous cells on the stained slides.
MP isolation
The processed sputum was centrifuged at 37× g for 3 minutes. The supernatant was then centrifuged at 253× g for 10 minutes and recentrifuged at 253× g for 20 minutes to remove the cells and large debris, respectively. Two hundred microliters of each MP-containing supernatant was frozen and stored at −80°C until characterization by flow cytometry.
MP characterization
The MP population was characterized in the sputum supernatant according to the expression of membrane-specific antigens. Antihuman CD11a labeling was used to count leukocyte MPs, while counting of granulocyte MPs was performed using antihuman CD66b. Platelets and megakaryocytic MPs were counted using antihuman CD41, platelet–endothelial MPs (EMPs) using antihuman CD31, and MPs from red blood cells were counted using antihuman CD235ab. Human Immunoglobulin M was used as isotype-matched negative control for CD66b, IgG1 was used as isotype-matched negative control for CD11a, CD41, and CD31, while IgG2 was the negative control for CD235ab. For these studies, 10 µL of supernatant MPs was incubated with 10 µL of specific antibody (1 µg/mL; fluorescein iso-thiocyanate conjugated; BioLegend, San Diego, CA, USA). After 15 minutes of incubation at +4°C, the samples were diluted in 500 µL of 0.9% saline solution. Then, 10 µL of flow count beads were added to each sample and analyzed in a flow cytometer (Beckman Coulter Epics XL-MCL, Miami, FL, USA). Sample analysis was stopped after counting 10,000 events.
Statistical analysis
All data are reported as mean ± standard deviation and analyzed by Statistica Software (StatSoft, Inc, Tulsa, OK, USA). Continuous data are presented as mean ± standard deviation in the tables. The relationships between variables were determined by measuring the Pearson’s correlation coefficient or Spearman’s correlation for the variables which were not normally distributed. A P-value of <0.05 was considered statistically significant.
Results
Patients
Patient characteristics are summarized in . Study participants were aged between 51 and 85 years and had a mean FEV1 of 52.39%±22.19%. Induced sputum was characterized by a high level of neutrophils (86.33%±13.98%) and although in two patients we found a higher number of eosinophils, in both cases, the level was lower than 10%, while the prevalence of neutrophils was higher than 85% ().
Table 2 Cellularity of induced sputum in COPD patients
MP phenotype
MPs were found in induced sputum of all 18 subjects enrolled. The MP phenotype was analyzed by evaluating the presence of different antigens representing all cell types. The expression of CD66b-MPs (granulocytes) was higher than that of other MPs, CD235ab (erythrocytes), and CD31-MPs (platelets/endothelial cell adhesion molecules 1) were also frequently found, instead the levels of CD41-MPs and CD11a-MPs were generally low ().
Table 3 Characterization of MPs in COPD sputum
Correlation between main COPD parameters and MP phenotype
summarizes the correlations between the MP phenotype and the main COPD parameters.
Table 4 Main correlations between the MP phenotype and some COPD characteristics
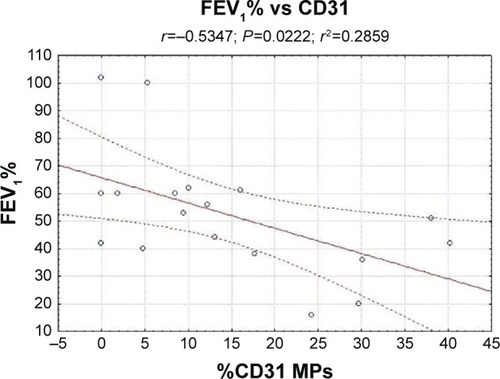
There was a negative correlation between CD31-MPs and FEV1 (R=−0.53, P<0.05; ). CD66b-MPs were correlated with a worse COPD performance index, being positively correlated with the BODE index and negatively correlated with 6MWT: 0.65 and −0.64, respectively (P<0.05). CD235ab-MPs showed a negative correlation with BMI (R=−0.86, P<0.05) and a positive correlation with dyspnea index (R=0.91, P<0.05). CD41-MPs and CD11a-MPs did not show correlations with the other parameters analyzed (data not shown).
Figure 1 Correlation between FEV1% and CD31-microparticles.
Abbreviations: COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; MPs, microparticles.
Finally, no correlation was found between the number of MPs and induced sputum cellularity, or with the number of disease exacerbations.
Discussion
The main result of the present study is the demonstration that in the sputum of patients affected by COPD, it is also possible to detect the presence of MPs. The MPs were obtained with the same protocol used in a previous studyCitation13 and they were identified through measures of cytofluorimetric analysis.
The phenotype of some MPs is related with the main COPD parameters such as FEV1, BODE index, or 6MWT. These results, together with other data, suggest that MPs are likely implicated in the pathogenesis of COPD.
There are various subtypes of MPs that are defined according to specific membrane antigens, such as endothelial/platelet cell adhesion molecule 1 (CD31), leukocytes (CD11a), megakaryocytic (CD41), granulocytes (CD66b), monocyte-macrophages (CD11b), and red blood cells (CD235ab), which have recently been described in a number of diseases including pulmonary hypertension and acute coronary syndrome.Citation18
Different MPs were found in induced sputum of all patients enrolled; the levels of CD41-MPs and CD11a-MPs were low, CD235ab-MPs and CD31-MPs were frequently found, and CD66b-MPs were the most abundant among all other MPs.
No correlation was found between the number of MPs and induced sputum cellularity, as well as the number of exacerbations. This could mean that MPs were not strictly derived from sputum cells or influenced by exacerbations.
Platelet/endothelial cell adhesion molecule 1 (CD31) is a signaling molecule that plays various roles in vascular biology, in particular, in the regulation of platelet function, angiogenesis, T- and B-cell activation, endothelial cell permeability, and transmigration across the endothelium.Citation19–Citation24 PECAM-1 is concentrated at endothelial junctions and is also expressed on the surface of platelets, neutrophils, and subsets of lymphocytes. Unlike vascular endothelial-cadherin, PECAM-1 is located outside the adherence junctions on endothelial cells.Citation25
Takahashi et alCitation26 reported that CD31-EMPs are released from pulmonary microvascular endothelial cells mainly in response to apoptosis induced by stimulation by H2O2 or cigarette smoke extract.
Thus, the released EMPs likely reflect the apoptosis of injured endothelial cells.Citation6 In a recent paper, Thomashow et alCitation27 demonstrated that circulating levels of CD31-MPs were higher in COPD patients compared to control subjects and, moreover, that there was a negative correlation with FEV1 and with the percentage of emphysema. Liu et alCitation28 also found a relationship between CD31+ and the severity of obstruction in animal models.
In our study, we found high levels of CD31-MPs also, in the sputum of COPD patients, the numbers being negatively correlated with the severity of disease. Patients with a worse lung function have highest levels of CD31-MPs ().
Thus, we can hypothesize that CD31-MPs could be directly correlated with lung damage; in fact, these data indicate that the high levels of circulating and local MPs could reflect the decline of small airway function in COPD patients. Moreover, the presence of CD31-MPs in sputum could hypothesize lung epithelium and vascular endothelium damage in COPD patients.
On the other hand, CD66b-MPs and CD235ab-MPs were more strongly correlated with a worsening of the main COPD indexes such as BODE and 6MWT. Moreover, CD235ab-MPs showed a negative correlation with BMI and a positive correlation with dyspnea index. In this case, it is more difficult to explain the relationship with MPs because they are multiparametric indexes and, therefore, different components could be involved in their decline. We can only suppose that during the progression of disease, endothelial activations are increased, and this mechanism could upregulate the expression of a pool of MPs, including CD66b and CD235ab. However, clinical relevance of these correlations should be evaluated in future larger studies.
Recent studies, in fact, demonstrated that the main causes of death in COPD patients are not respiratory events, but cardiovascular events such as ischemic heart disease and stroke.Citation29 Vascular abnormalities in the endothelium have been reported in both pulmonaryCitation30 and systemic vasculaturesCitation31 in COPD patients. Impaired endothelial function, as assessed by flow-mediated dilation of the brachial artery, is associated with a low FEV1 in COPD patients.Citation6,Citation32 Endothelial injury in the pulmonary capillary vasculature leads to lung destruction, and since cardiovascular diseases are the main cause of death among individuals with COPD, EMPs are now receiving attention as potential biomarkers for COPD.
The number of circulating EMPs is increased in patients with vascular disorders, such as acute coronary disease,Citation33,Citation34 renal failure,Citation35 and metabolic diseases,Citation36 and reflects the endothelial damage occurring in these patients. Moreover, the number of EMPs is a sensitive marker of pulmonary capillary endothelial damage induced by smoking in healthy active smokers.Citation37
The number of apoptotic epithelial and endothelial cells is increased in emphysematous lung as compared to normal lung.Citation38 The senescence of alveolar epithelial and endothelial cells is accelerated in patients with emphysema.Citation39 Greater numbers of apoptotic lung cells are observed in lung tissues from COPD patients than in those from smokers without COPD.Citation40–Citation42 Furthermore, morphological and biochemical markers of autophagy are increased in the lungs of patients with COPD compared with normal lung tissue. These results indicate the importance of injured cells in the pathophysiology of lung destruction and COPD.Citation6
MPs are not passive agents induced from activated or injured cells, but rather active modulators that promote both proinflammatory and anti-inflammatory signals.Citation43 MPs contain proteins and microRNAs and can deliver those components to distant endothelial cells.Citation44 Therefore, increased EMPs may influence vascular function and systemic inflammation under COPD exacerbation.Citation17
Increased DNA fragmentation in the pulmonary capillaries and arteriolar endothelium of individuals with COPD was shown by Segura-Valdez et al.Citation45 In addition, Kasahara et alCitation38,Citation46,Citation47 reported increased septal cell death (endothelial and epithelial cells) in human emphysematous lungs compared with lungs of nonsmokers or smokers without emphysema.Citation37
Conclusion
The main result of our study is not only the presence of MPs in COPD patients’ sputum, but also the relation between the number of EMPs and FEV1. This indicates that endothelial injury is closely connected to the pathophysiology of COPD.
Since COPD is a heterogeneous disease characterized by various combinations with small airway disease and emphysema, the relationships between the severity of the emphysema and the EMP count are of great interest. Moreover, as quicker responses can be seen in circulating EMP levels compared with an annual FEV1 decline, monitoring EMP levels is valuable as a means of estimating COPD progression.
Simple and noninvasive biomarkers in COPD are needed to monitor disease progression, identify exacerbations, and evaluate the efficacy of novel therapies. Sputum is a rich, noninvasive source of biomarkers of inflammation and infection, and has been used extensively to assess inflammation in lung airways pathologies. The presence of CD31-MPs in COPD sputum could be a new noninvasive method to monitor the disease course.
Main limitations of this study are that only a limited number of subjects with lung diseases were enrolled, so it was not possible to evaluate different expression of MPs according to severity of the disease, as well as the obvious absence of a control group with healthy subjects in whom it would be difficult to obtain sputum even if induced. However, our preliminary data suggest that high levels of MPs reflect the presence of endothelial inflammation. CD31-MPs, CD66b-MPs, and CD235ab-MPs could be good new candidates for the study of pulmonary endothelial injury and COPD progression.
Future studies could aim to evaluate if different stages of diseases can influence the phenotype of MPs and define the possible role of them in monitoring the effectiveness of medication.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
We thank Prof MC Martinez for careful and critical reading of the manuscript. This work was supported by a grant of Foggia University (Fondo per i Progetti di Ricerca di Ateneo, PRA).
Disclosure
The authors report no conflicts of interest in this work.
References
- RaposoGStoorvogelWExtracellular vesicles: exosomes, microvesicles, and friendsJ Cell Biol2013200437338323420871
- BeyerCPisetskyDSThe role of microparticles in the pathogenesis of rheumatic diseasesNat Rev Rheumatol201061212919949432
- BenameurTAndriantsitohainaRMartínezMCTherapeutic potential of plasma membrane derived microparticlesPharmacol Rep2009611495719307692
- SinningJMLoschJWalentaKBöhmMNickenigGWernerNCirculating CD31+/AnnexinV+ microparticles correlate with cardiovascular outcomesEur Heart J201132162034204121186238
- BakaZSenoltLVencovskyJIncreased serum concentration of immune cell derived microparticles in polymyositis/dermatomyositisImmunol Lett2010128212413020043950
- MorelNMorelOPetitLGeneration of procoagulant microparticles in cerebrospinal fluid and peripheral blood after traumatic brain injuryJ Trauma200864369870418332810
- MutschlerDKLarssonAOBasuSNordgrenAErikssonMBEffects of mechanical ventilation on platelet microparticles in bronchoalveolar lavage fluidThromb Res2002108421522012617984
- BoilardENigrovicPALarabeeKPlatelets amplify inflammation in arthritis via collagen-dependent microparticle productionScience2010327596558058320110505
- MartínezMCTesseAZobairiFShed membrane microparticles from circulating and vascular cells in regulating vascular functionAm J Physiol Heart Circ Physiol20052883H1004H100915706036
- BastaracheJAFremontRDKropskiJABossertFRWareLBProcoagulant alveolar microparticles in the lungs of patients with acute respiratory distress syndromeAm J Physiol Lung Cell Mol Physiol20092976L1035L104119700643
- TakahashiTKuboHThe role of microparticles in chronic obstructive pulmonary diseaseInt J Chron Obstruct Pulmon Dis2014930331424707174
- TakahashiTKobayashiSFujinoNIncreased circulating endothelial microparticles in COPD patients: a potential biomarker for COPD exacerbation susceptibilityThorax201267121067107422843558
- PorroCLeporeSTrottaTIsolation and characterization of microparticles in sputum from cystic fibrosis patientsRespir Res2010119420618958
- PorroCDi GioiaSTrottaTPro-inflammatory effect of cystic fibrosis sputum microparticles in the murine lungJ Cyst Fibros201312672172823567201
- BorgGAPsychophysical bases of perceived exertionMed Sci Sports Excerc1982145377381
- CelliBRCoteCGMarinJMThe Bode Mass Index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary diseaseN Engl J Med2004350101005101214999112
- DjukanovićRSterkPJFahyJVStandardised methodology of sputum induction and processingEur Respir J Suppl2002371s2s12361359
- AmabileNHeissCChangVIncreased CD62e(+) endothelial microparticle levels predict poor outcome in pulmonary hypertension patientsJ Heart Lung Transplant200928101081108619782291
- LeyKLaudannaCCybulskyMINoursharqhSGetting to the site of inflammation: the leukocyte adhesion cascade updatedNat Rev Immunol20077967868917717539
- PatilSNewmanDKNewmanPJPlatelet endothelial cell adhesion molecule-1 serves as an inhibitory receptor that modulates platelet responses to collagenBlood20019761727173211238114
- CicmilMThomasJMLeducMBonCGibbinsJMPlatelet endothelial cell adhesion molecule-1 signaling inhibits the activation of human plateletsBlood200299113714411756163
- FalatiSPatilSGrossPLPlatelet PECAM-1 inhibits thrombus formation in vivoBlood2006107253554116166583
- MullerWALeukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory responseTrends Immunol200324632733412810109
- WoodfinAVoisinMBNoursharghSPECAM-1: a multi-functional molecule in inflammation and vascular biologyArterioscler Thromb Vasc Biol200727122514252317872453
- NewmanPJBerndtMCGorskiJPECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamilyScience19902474947121912221690453
- TakahashiTKobayashiSFujinoNDifferences in the released endothelial microparticle subtypes between human pulmonary microvascular endothelial cells and aortic endothelial cells in vitroExp Lung Res2013394–515516123550836
- ThomashowMAShimboDParikhMAEndothelial microparticles in mild chronic obstructive pulmonary disease and emphysema. The multi-ethnic study of atherosclerosis chronic obstructive pulmonary disease studyAm J Respir Crit Care Med20131881606823600492
- LiuHDingLZhangYCirculating endothelial microparticles involved in lung function decline in a rat exposed in cigarette smoke maybe from apoptotic pulmonary capillary endothelial cellsJ Thorac Dis20146664965524976986
- YoungRPHopkinsREatonTEForced expiratory volume in one second: not just a lung function test but a marker of premature death from all causesEur Respir J200730461662217906084
- PeinadoVIPizarroSBarberàJAPulmonary vascular involvement in COPDChest2008134480881418842913
- HunninghakeDBCardiovascular disease in chronic obstructive pulmonary diseaseProc Am Thorac Soc200521444916113468
- BarrRGMesia-VelaSAustinJHImpaired flow-mediated dilation is associated with low pulmonary function and emphysema in ex-smokers: the emphysema and cancer action project (EMCAP) studyAm J Respir Crit Care Med2007176121200120717761614
- NozakiTSugiyamaSKogaHSignificance of a multiple biomarkers strategy including endothelial dysfunction to improve risk stratification for cardiovascular events in patients at high risk for coronary heart diseaseJ Am Coll Cardiol200954760160819660689
- LacknerPDietmannABeerRCellular microparticles as a marker for cerebral vasospasm in spontaneous subarachnoid hemorrhageStroke201041102353235720814009
- AmabileNGuérinAPLeroyerACirculating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failureJ Am Soc Nephrol200516113381338816192427
- PirroMSchillaciGPaltricciaRIncreased ratio of CD31+/CD42− microparticles to endothelial progenitors as a novel marker of atherosclerosis in hypercholesterolemiaArterioscler Thromb Vasc Biol200626112530253516946129
- GordonCGudiKKrauseACirculating endothelial microparticles as a measure of early lung destruction in cigarette smokersAm J Respir Crit Care Med2011184222423221471087
- KasaharaYTuderRMCoolCDLynchDAFloresSCVoelkelNFEndothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysemaAm J Respir Crit Care Med20011633 Pt 173774411254533
- TsujiTAoshibaKNagaiAAlveolar cell senescence in patients with pulmonary emphysemaAm J Respir Crit Care Med2006174888689316888288
- HensonPMVandivierRWDouglasISCell death, remodeling, and repair in chronic obstructive pulmonary disease?Proc Am Thorac Soc20063871371717065379
- HodgeSHodgeGHolmesMReynoldsPNIncreased airway epithelial and T-cell apoptosis in COPD remains despite smoking cessationEur Respir J200525344745415738287
- VandivierRWHensonPMDouglasISBurying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung diseaseChest200612961673168216778289
- MorelOTotiFMorelNFreyssinetJMMicroparticles in endothelial cell and vascular homeostasis: are they really noxious?Haematologica200994331331719252173
- MartinezMCTual-ChalotSLeonettiDAndriantsitohaninaRMicroparticles: targets and tools in cardiovascular diseaseTrends Pharmacol Sci2011321165966521794929
- Segura-ValdezLPardoAGaxiolaMUhalBDBecerrilCSelmanMUpregulation of gelatinases A and B, collagenases 1 and 2, and increased parenchymal cell death in COPDChest2000117368469410712992
- KasaharaYTuderRMCoolCDVoelkelNFExpression of 15-lipoxygenase and evidence for apoptosis in the lungs from patients with COPDChest20001175 Suppl 1260S10843941
- KasaharaYTuderRMTaraseviciene-StewartLInhibition of VEGF receptors causes lung cell apoptosis and emphysemaJ Clin Invest2000106111311131911104784
