Abstract
Objective
To systematically review and assess the efficacy, different treatment protocols (formulation, dosage, and duration), and safety of nystatin for treating oral candidiasis.
Methods
Four electronic databases were searched for trials published in English till July 1, 2015. Randomized controlled trials comparing nystatin with other antifungal therapies or a placebo were included. Clinical and/or mycological cure was the outcome evaluation. A meta-analysis or descriptive study on the efficacy, treatment protocols, and safety of nystatin was conducted.
Results
The meta-analysis showed that nystatin pastille was significantly superior to placebo in treating denture stomatitis. Nystatin suspension was not superior to fluconazole in treating oral candidiasis in infants, children, or HIV/AIDS patients. The descriptive investigations showed that administration of nystatin suspension and pastilles in combination for 2 weeks might achieve a higher clinical and mycological cure rate, and using the nystatin pastilles alone might have a higher mycological cure rate, when compared with using nystatin suspensions alone. Nystatin pastilles at a dose of 400,000 IU resulted in a significantly higher mycological cure rate than that administrated at a dose of 200,000 IU. Furthermore, treatment with nystatin pastilles for 4 weeks seemed to have better clinical efficacy than treatment for 2 weeks. Descriptive safety assessment showed that poor taste and gastrointestinal adverse reaction are the most common adverse effects of nystatin.
Conclusion
Nystatin pastille was significantly superior to placebo in treating denture stomatitis, while nystatin suspension was not superior to fluconazole in treating oral candidiasis in infants, children, or HIV/AIDS patients. Indirect evidence from a descriptive study demonstrated that administration of nystatin pastille alone or pastille and suspension in combination is more effective than that of suspension alone; prolonged treatment duration for up to 4 weeks can increase the efficacy of nystatin. More well designed and high quality randomized control studies are needed to confirm these findings.
Introduction
Oral candidiasis, which is the most common human fungal infection, is characterized by an overgrowth of Candida species in the superficial epithelium of the oral mucosa.Citation1,Citation2 It has been associated with multiple host risk factors, including impaired salivary gland function, denture wearing, oral mucosa disruption, drug use (long-term administration of broad-spectrum antibiotics, corticosteroids, antidepressants, antineoplastic, drugs, and immunosuppressant), age (common in neonates and the elderly), endocrine alterations (diabetes mellitus, pregnancy, renal failure, and hyperthyroidism), dietary factors (high-carbohydrate diet and iron-deficiency anemia), cancer, and HIV infection.Citation3–Citation5 Elimination of the predisposing factors is an important strategy in treating oral candidiasis.
Various topical and systemic agents are currently available for the treatment of oral candidiasis.Citation3 Systemic antifungal agents, including triazoles, fluconazole, and itraconazole, are appropriate for patients who do not respond to or are intolerant to topical treatment and those at high risk of developing systemic infections.Citation3,Citation6 However, numerous drug interactions and decreased susceptibility of species other than Candida albicans toward azoles limit the application of systemic antifungal agents.Citation7,Citation8 Topical antifungal agents, such as nystatin, amphotericin B, miconazole, and clotrimazole, are recommended typically as the first-line treatment for uncomplicated cases of oral candidiasis.Citation2,Citation9,Citation10
Nystatin is a membrane-active polyene macrolide produced by Streptomyces noursei strains and is available in various forms, such as oral suspension, topical cream, and oral pastille.Citation11–Citation15 Nystatin is not absorbed from gastrointestinal tract when orally administered.Citation5 Therefore, the topical use of nystatin is considered the most common route of administration in dentistry, as systemic exposure is minimal. Further, nystatin also plays an important role in the prophylaxis of oral and systemic candidiasis in full-term and premature newborns, infants, and immunocompromised patients (eg, AIDS patients, cancer patients, and organ transplant recipients), as it is associated with a low incidence of drug interactions and acceptable costs, especially in developing countries.Citation16–Citation19 The common recommended dose for topical use of nystatin is 200,000–600,000 IU qid for children and adults, and 100,000–200,000 IU qid for newborns and infants.Citation18,Citation20 Treatment duration can vary from 1 or 2 to 4 weeks.Citation5,Citation21–Citation23 Up to now, there is no consensus on the formulation, dosage, or treatment duration of nystatin in the treatment of oral candidiasis. The aim of this study was to summarize and assess the efficacy, different treatment protocols (formulation, dosage, and duration), and safety of nystatin in different patient populations with oral candidiasis by a meta-analysis and systematic review.
Materials and methods
This review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.Citation24
Inclusion criteria
Randomized controlled trials that compared nystatin (at any dosage and in any form) with other antifungal therapies or a placebo were included in this review. The diagnosis of oral candidiasis was based on clinical diagnosis with or without confirmation by mycological tests. There were no restrictions on patients’ age, sex, or race. The primary outcome was the clinical cure rate: the patient was considered to be cured if the oral lesion and symptoms had completely resolved. The secondary outcome was the mycological cure rate: the patient was considered to be cured if the smear or culture test showed negative results.
Database and search strategies
Four electronic databases were searched by two independent reviewing authors (XL, CZ): the Cochran Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2015, Issue 6), PubMed (July 1, 2015), EMBASE (July 1, 2015), and Science Citation Index (July 1, 2015). The following terms were searched in combination: (“oral candidiasis” OR “oral candidiases” OR “thrush” OR “oral moniliases” OR “oral moniliasis” OR “oropharyngeal candidiasis” OR “candidal stomatitis” OR “muguet” OR “prosthetic stomatitis” OR “angular cheilitis” OR “rhomboid glossitis”) AND (“nystatin” OR “fungicidin”) AND (“randomized controlled trial” OR “randomized controlled study” OR “RCT”). Manual searches were also conducted as a supplement.
Data extraction and quality assessment
The two review authors (XL, CZ) were independently responsible for scanning titles and abstracts, selecting studies, reading full reports, extracting data, and assessing the quality of studies; these steps were performed in duplicate by each of these authors. All the relevant data of each included study, including author, year of publication, region, risk factors, characteristics of the patients, detailed interventions, outcomes, and adverse effects, were extracted and summarized in a table format. The quality of the included studies was assessed using the Cochrane Handbook for Systematic Review of Interventions and the Rev Man 5.2.0 software. The following assessment criteria were used to assess the quality of the studies: 1) random sequence generation (if the study did not use this method, it was considered to have a selection bias), 2) allocation concealment (selection bias), 3) blinding of participants and personnel (performance bias), 4) blinding of outcome assessment (detection bias), 5) incomplete outcome data (attrition bias), 6) selective reporting (reporting bias), and 7) other biases. The Kappa coefficient was used to calculate inter-rater agreement with regard to study inclusion and quality assessment. A third reviewer (HH or ZMY) was invited to make an assessment if the two review authors could not reach a consensus.
Data synthesis and analysis
The efficacy of nystatin versus placebo and nystatin versus fluconazole was evaluated using the Stata 12.0 (StataCorp LP, College Station, TX, USA) software. Results were expressed as odds ratio (OR) together with the 95% confidence interval (CI), and plotted on a forest plot. The inconsistency index I2 was calculated to assess the variation caused by heterogeneity. When P was >0.10 and I2 was <25%, the fixed-effect model was used, which assumes the same homogeneity of effect size across all studies. When P was <0.10 and I2 was >25%, inter-study heterogeneity was deemed statistically significant, and a random-effects model was employed.Citation25 A descriptive study was conducted on studies evaluating the efficacy of nystatin versus other antifungal treatments due to the limited number of studies or marked heterogeneity in many aspects of the study characteristics.
Results
Results of the search
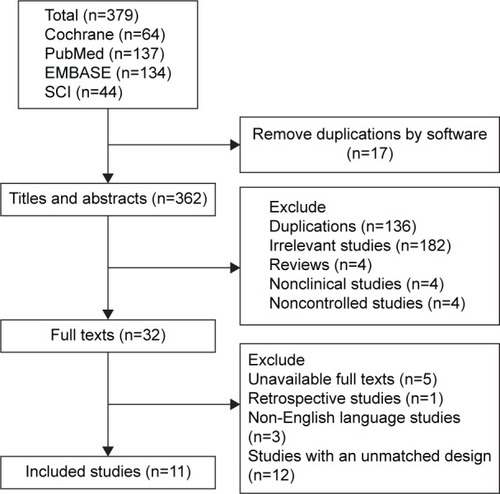
A total of 379 abstracts were extracted from the four databases. Finally, only eleven trials with a total of 1,148 patients were included in the present analysis (). On the basis of the inclusion criteria, 153 duplicate publications, 182 irrelevant studies, four reviews, four nonclinical studies, four noncontrolled studies, one retrospective study, 12 studies with an unmatched study design, five studies with unavailable full texts, and three publications that were not in English were excluded. The Kappa value of inter-reviewer agreement for study inclusion was 0.83. No studies that met the requirements were obtained by the manual search.
Characteristics of the included studies
Three trials were performed in patients with denture stomatitis;Citation26–Citation28 three trials were conducted on infants or children;Citation20,Citation29,Citation30 three trials included HIV or AIDS patients;Citation31–Citation33 one trial was on hospitalized cancer patients;Citation34 and one trial was performed in several groups of patients, including those with xerostomia, HIV, immunosuppression in conjunction with organ transplantation, and wearing of dentures.Citation35 Nystatin was used in the suspension and pastille forms; the dosage ranged from 100,000 to 1,100,000 IU three to five times a day; and the treatment duration was 10 to 30 days ().
Table 1 Characteristics of the included studies
Risk of bias and quality of the included studies
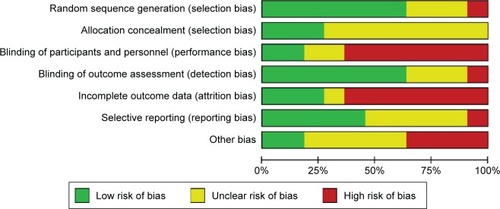
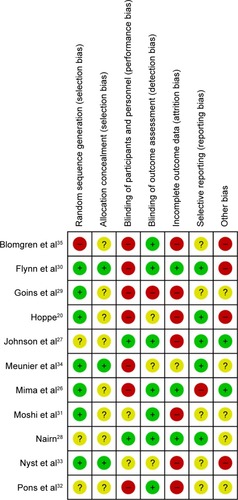
None of the included studies met all the seven assessment criteria (). Most studies were found to have a high risk of a performance and attrition bias and a moderate risk of other biases. The overall risk of each bias is presented in , and the risk of each bias in each of the studies separately is presented in . A 100% agreement was achieved on study quality among the reviewers.
Efficacy assessment
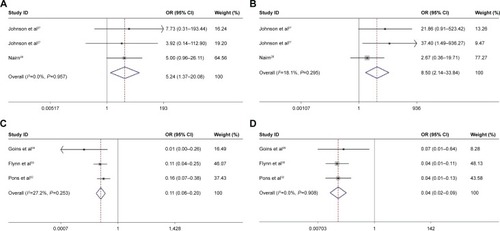
The clinical and mycological cure rates associated with nystatin and the control treatments are summarized in . Two studies comparing the efficacy of nystatin pastilles to the placebo in treating denture stomatitis were analyzed by meta-analysis.Citation27,Citation28 The results showed that the efficacy of nystatin pastilles was significantly superior to that of the placebo treatment (clinical OR =5.24, 95% CI =1.37–20.08, P=0.957; mycological OR =8.50, 95% CI =2.14–33.84, P=0.295; ; ). Three studies evaluating the efficacy of nystatin suspension and fluconazole for treating oral or oropharyngeal candidiasis in infants, children (1 month to 13 years old), and HIV/AIDS patients were also studied by meta-analysis.Citation29,Citation30,Citation32 The results of these three studies demonstrated that the efficacy of the nystatin suspension was significantly inferior to that of fluconazole (clinical OR =0.11, 95% CI =0.06–0.20, P=0.253; mycological OR =0.04, 95% CI =0.02–0.09, P=0.908; ; ).
Table 2 Clinical and mycological efficacy of nystatin and the control treatments
Table 3 Meta-analysis of the efficacy of nystatin compared with that of the placebo and fluconazole
Figure 4 Forest plots evaluating the efficacy of nystatin (fixed-effect model).
Abbreviations: CI, confidence interval; OR, odds ratio.
A descriptive investigation was conducted on the other seven studies due to the limited number of studies or marked heterogeneity in many aspects of the study characteristics.Citation20,Citation26,Citation28,Citation31,Citation33–Citation35 The efficacy of nystatin pastilles was compared to that of other drugs in two studies.Citation28,Citation34 The results showed that nystatin pastilles had similar clinical efficacy (79.6%–87.5%) to amphotericin B (88.8%)Citation28 and ketoconazole (72.2%).Citation34 The efficacy of nystatin suspension was compared to that of other treatment strategies in four studies.Citation20,Citation26,Citation31,Citation33 These studies showed that the clinical and mycological efficacy of nystatin suspensions (9%–54.1% and 5.6%–13%, respectively) was significantly inferior to that of miconazole (99% and 54.1%, respectively),Citation20 gentian violet (42% and 62%, respectively), and ketoconazole (43% and 57%, respectively)Citation33 in treating oral candidiasis in infants, children, and HIV/AIDS patients, while nystatin suspensions had similar clinical efficacy (53%–63.5%) to photodynamic therapy (45%)Citation26 and sodium benzoate (55.6%).Citation31 These results showed that nystatin pastilles had a relatively high clinical efficacy (79.6%–87.5%) in treating oral candidiasis. However, nystatin suspension seemed to be inferior to miconazole, gentian violet, and ketoconazole in treating oral candidiasis in infants, children, and HIV/AIDS patients (for details, and ).
Formulation, dosage, and duration of nystatin treatment
Due to obvious diversity in study design, the analysis of different treatment protocols (formulation, dosage, and duration) of nystatin was not qualified for meta-analysis; only descriptive investigations were conducted. In the included eleven studies, there are three formulations of nystatin, including suspension form, pastille form, and a combination of the suspension and pastille forms. For patients with denture stomatitis, the clinical cure rate with the suspension form was 53%,Citation26 and the clinical and mycological cure rates with the pastille form only were 14.3%–76.9% and 40%–71.4%, respectively.Citation27,Citation28 Researches on infants, children, and HIV/AIDS patients with oral candidiasis showed that the clinical cure rate was 9%–63.5% and the mycological cure rate was 5.6%–13% with the use of the suspension form.Citation20,Citation29–Citation33 Meunier et alCitation34 found that clinical and mycological cures were achieved in 87.5% and 66% of cancer patients, respectively, with oral candidiasis by using the suspension and pastille forms in combination. The results of these studies indicated that combined administration of nystatin in the suspension and pastille form for 2 weeks might achieve a higher clinical and mycological cure rate than administration of the nystatin suspension alone in the treatment of oral candidiasis. Moreover, use of the pastille alone for 2 weeks might result in a higher mycological cure rate than use of the suspension alone ().
Table 4 Summary of the usage and efficacy of nystatin
Johnson et alCitation27 compared the efficacy of the nystatin pastilles administered in two dosages (200,000 and 400,000 IU) for treating denture stomatitis. No significant difference was found in clinical efficacy (28.6% and 14.3%, OR =5.67, 95% CI =0.56–56.96) between the two dosages, while the mycological efficacy of the 400,000 IU nystatin pastilles was significantly higher than that of the 200,000 IU pastilles (71.4% and 57.1%, OR =28.33, 95% CI =2.95–271.81).
The treatment duration of nystatin was 2 to 4 weeks. Researches on denture stomatitis and oral candidiasis in infants, children, and HIV/AIDS patients showed that the clinical and mycological cure rates were 9%–63.5% and 6%–13%, respectively, with the use of the suspension form for 2 weeks.Citation30–Citation33 Blomgren et alCitation35 found that a 16.7% clinical cure rate was achieved when the suspension form was used for 3 weeks. Studies on denture stomatitis showed that using nystatin pastilles for 2 weeks could achieve a 14.3%–28.6% clinical cure rate and a 57.1%–71.4% mycological cure rateCitation27 and that using the pastille form for 4 weeks could achieve a 76.9% clinical cure rate and a 40% mycological cure rate.Citation28 These findings indicated that nystatin administration of 4 weeks has a better clinical efficacy than 2 weeks in the treatment of denture stomatitis ().
Safety assessment
Eight out of the eleven studies reported the adverse effects of nystatin.Citation20,Citation28–Citation33,Citation35 Two of the eight studies reported that nystatin had no adverse effects.Citation29,Citation33 One study did not show the details of the adverse effects.Citation31 Poor taste (the incidence was 61.5% in one study)Citation28 and gastrointestinal adverse reactions, including vomiting, nausea, diarrhea, anorexia, and abdominal pain (the incidence was 0.01%–0.06% in four studies), were the most common adverse effects reported ().Citation20,Citation30,Citation32,Citation35
Table 5 Adverse effects of nystatin and the control treatments
Discussion
Oral candidiasis is an opportunistic infection of oral cavity. It is common among the elderly and infants, particularly in those elderly patients who wear dentures. It can also be a mark of some systemic disease, such as diabetes mellitus, cancer, and immunodeficiency diseases. The prevalence and incidence of all forms of oral candidiasis have increased in recent decades. Approximately 54% of people who wear removable dentures suffer from oral candidiasis.Citation36 Thrush occurs in ~1% to 37% of systematically healthy infants.Citation29 In all, 15%–60% of cancer patients will develop oral candidiasis because of immunosuppression.Citation2 More than 90% of patients with AIDS may suffer from oral candidiasis at some time during their illness.Citation37 Although the appearance and development of azoles and echinocandins antifungal agents, which had better tastes and less gastrointestinal adverse reactions, provided more clinical options, topical therapy, such as nystatin, is still one of the main recommended treatments for oral candidiasis due to its high efficacy, low cost, and less side effects, especially in developing countries.Citation11,Citation18 One study showed that nystatin was the most commonly prescribed antifungal agent for the treatment of oral candidiasis in Jordan (78.2%).Citation38
In 2009, the Infectious Diseases Society of America updated its clinical practice guidelines for the management of candidiasis. In this guideline, nystatin suspension at a concentration of 100,000 U/mL and a dosage of 4–6 mL qid, or one to two nystatin pastilles (200,000 U each) administered qid for 7–14 days, is recommended for mild oropharyngeal candidiasis.Citation39 In addition, the World Health Organization recommended that topical therapy with nystatin suspension or pastilles can be an alternative to oral fluconazole for treating oropharyngeal candidiasis in HIV-positive children and adults.Citation40 Even so, few evidences were found on the efficacy of nystatin for oral candidiasis in clinical practice. Furthermore, the applications of nystatin were varied among different patient populations and countries. So, ensuring the detailed indications of nystatin for different types of oral candidiasis is of great importance.
In the present review, the meta-analysis of the limited studies showed that the efficacy of nystatin pastilles was significantly superior to placebo in treating denture stomatitis, while nystatin suspension was not superior to fluconazole in treating oral candidiasis in infants, children, or HIV/AIDS patients. Further, the descriptive investigations showed that administration of nystatin suspension and pastilles in combination for 2 weeks might achieve a higher clinical and mycological cure rates (87.5% and 66%) than if nystatin suspension alone is used. Nystatin pastilles at a dose of 400,000 IU resulted in a significantly higher mycological cure rate than the dose of 200,000 IU. With regard to treatment duration, administration of nystatin pastilles for 4 weeks showed better clinical efficacy (76.9%) than its administration for 2 weeks. Poor taste and gastrointestinal adverse reaction were the most common adverse effects of nystatin.
Polyene antibiotics exert their antifungal effects by interacting with ergosterol in the fungal cell membrane, creating pores, and subsequently increasing the inflow and outflow of many materials.Citation41 However, the treatment is effective only if these antibiotics are administered over a sufficient period of time. Nystatin suspension was not a good choice for infants, children, and HIV/AIDS patients with oral candidiasis, probably because of its short-term action on the oral mucosa.Citation5 Moreover, exposure to nystatin at a concentration 0.25 to 1 times the minimum inhibitory concentration value for 30 minutes resulted in a postantifungal effect with an average duration range of 3.1 to 6.3 hours in several Candida isolates.Citation16 Nystatin shows a remarkable postantifungal effect, which is defined as the delay in fungal regrowth that persists after a brief exposure to an antifungal agent.Citation16 Therefore, nystatin in the topical pastille form seems to be more effective in treating oral candidiasis than oral nystatin suspension.
Candida species colonize in the oral mucosa via adhesion to buccal epithelial cells, germ tube formation, and relative cell surface hydrophobicity.Citation42–Citation45 Therefore, topical drugs that get absorbed into the oral epithelium are necessary for killing yeast hyphae growing within the tissue.Citation23 In previous studies, the treatment duration of nystatin varied from 1 to 6 week(s). Richardson and JonesCitation8 proposed that nystatin solutions need to be used for at least 1 week after resolution of symptoms, usually for 4 weeks for the primary treatment of oral candidiasis. Moreover, in recurrent cases, the duration of treatment should be at least 4 to 6 weeks.Citation27 In agreement with these findings, in this review, our descriptive investigation showed that 4 weeks of nystatin administration seemed to have better clinical efficacy than 2 weeks of nystatin usage.
There are two limitations to this analysis. First, very few clinical trials with heterogeneity were available. Second, several studies were considered to be at a high risk of performance and attrition bias, and at a moderate risk of other biases. Moreover, 72.7% of the studies did not provide enough information about allocation concealment. The inconsistent quality of the included studies would impact the credibility of the results. Therefore, clinicians need to view the results of this research with caution. All these deficiencies indicate that well designed and high quality randomized controlled trial a study are needed in the future.
Conclusion
Nystatin pastille was significantly superior to placebo in treating denture stomatitis, while nystatin suspension was not superior to fluconazole in treating oral candidiasis in infants, children, or HIV/AIDS patients. Indirect evidence from a descriptive study demonstrated that administration of nystatin pastille alone or pastille and suspension in combination is more effective than that of suspension alone; prolonged treatment duration for up to 4 weeks can increase the efficacy of nystatin. More well designed and high quality randomized control studies are needed to confirm these findings.
Acknowledgments
This work was supported by the Program for New Clinical Techniques and Therapies of Peking University School and Hospital of Stomatology (PKUSSNCT-14A01) and Natural Science Foundation of China (81000441, 81570985).
Supplementary material
Table S1 Risk of bias and quality assessment of the included studies
Disclosure
The authors report no conflicts of interest in this work.
References
- MelkoumovAGoupilMLouhichiFRaymondMde RepentignyLLeclairGNystatin nanosizing enhances in vitro and in vivo antifungal activity against Candida albicansJ Antimicrob Chemother20136892099210523620465
- AkpanAMorganROral candidiasisPostgrad Med J20027892245545912185216
- EpsteinJBPolskyBOropharyngeal candidiasis: a review of its clinical spectrum and current therapiesClin Ther199820140579522103
- Coronado-CastelloteLJimenez-SorianoYClinical and microbiological diagnosis of oral candidiasisJ Clin Exp Dent201355e279e28624455095
- SamaranayakeLPKeung LeungWJinLOral mucosal fungal infectionsPeriodontol 2000200949395919152525
- HoepelmanIMDupontBOral candidiasis: the clinical challenge of resistance and managementInt J Antimicrob Agents19966315515918611703
- RichardsonMDWarnockDWFungal Infection: Diagnosis and ManagementOxfordBlackwell Publishing2003
- RichardsonMDJonesBLTherapeutic Guidelines in Systemic Fungal Infections3rd edLondonRemedica Publishing2007
- Antifungal chemotherapy in patients with acquired immunodeficiency syndromeBritish Society for Antimicrobial Chemotherapy Working PartyLancet199234088206486511355218
- ComoJADismukesWEOral azole drugs as systemic antifungal therapyN Engl J Med199433042632728272088
- KaurIPKakkarSTopical delivery of antifungal agentsExpert Opin Drug Deliv20107111303132720961206
- EpsteinJBAntifungal therapy in oropharyngeal mycotic infectionsOral Surg Oral Med Oral Pathol199069132412404226
- GuidaRACandidiasis of the oropharynx and esophagusEar Nose Throat J198867118328348368388403073941
- GreenspanDTreatment of oropharyngeal candidiasis in HIV-positive patientsJ Am Acad Dermatol1994313 Pt 2S51S557915732
- WongSSWSamaranayakeLPSeneviratneCJIn pursuit of the ideal antifungal agent for Candida infections: high-throughput screening of small moleculesDrug Discov Today201419111721173024952336
- Fernandez CamposFCalpena CampmanyACRodriguez DelgadoGLopez SerranoOClares NaverosBDevelopment and characterization of a novel nystatin-loaded nanoemulsion for the buccal treatment of candidosis: ultrastructural effects and release studiesJ Pharm Sci2012101103739375222777575
- HowellAIsaacsDHallidayRAustralasian Study Group For Neonatal IOral nystatin prophylaxis and neonatal fungal infectionsArch Dis Child Fetal Neonatal Ed2009946F429F43319321509
- SklenarZScigelVHorackovaKSlanarOCompounded preparations with nystatin for oral and oromucosal administrationActa Pol Pharm201370475976223923400
- GotzschePCJohansenHKNystatin prophylaxis and treatment in severely immunodepressed patientsCochrane Database Syst Rev20149CD00203325188770
- HoppeJETreatment of oropharyngeal candidiasis and candidal diaper dermatitis in neonates and infants: review and reappraisalPediatr Infect Dis J19971698858949306485
- NiimiMFirthNACannonRDAntifungal drug resistance of oral fungiOdontology2010981152520155503
- WilliamsDWKuriyamaTSilvaSMalicSLewisMACandida biofilms and oral candidosis: treatment and preventionPeriodontol 2000201155125026521134239
- RautemaaRRamageGOral candidosis – clinical challenges of a biofilm diseaseCrit Rev Microbiol201137432833621777047
- ShamseerLMoherDClarkeMPreferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanationBMJ2015349g764725555855
- XieSXuHShanXLiuBWangKCaiZClinicopathological and prognostic significance of survivin expression in patients with oral squamous cell carcinoma: evidence from a meta-analysisPLoS One2015102e011651725710884
- MimaEGVerganiCEMachadoALComparison of photodynamic therapy versus conventional antifungal therapy for the treatment of denture stomatitis: a randomized clinical trialClin Microbiol Infect20121810E380E38822731617
- JohnsonGHTaylorTDHeidDWClinical evaluation of a nystatin pastille for treatment of denture-related oral candidiasisJ Prosthetic Dent1989616699703
- NairnRINystatin and amphotericin B in the treatment of denture-related candidiasisOral Surg Oral Med Oral Pathol197540168751097985
- GoinsRAAscherDWaeckerNArnoldJMoorefieldEComparison of fluconazole and nystatin oral suspensions for treatment of oral candidiasis in infantsPediatr Infect Dis J200221121165116712506950
- FlynnPMCunninghamCKKerkeringTOropharyngeal candidiasis in immunocompromised children: a randomized, multicenter study of orally administered fluconazole suspension versus nystatin. The Multicenter Fluconazole Study GroupJ Pediatr199512723223287636666
- MoshiAHJorgensenAFPallangyoKTreatment of oral candidiasis: a study to determine the clinical response of sodium benzoate compared with nystatin suspensionAIDS19981216223722389833873
- PonsVGreenspanDLozada-NurFOropharyngeal candidiasis in patients with AIDS: randomized comparison of fluconazole versus nystatin oral suspensionsClin Infect Dis1997246120412079195083
- NystMJPerriensJHKimputuLLumbilaMNelsonAMPiotPGentian violet, ketoconazole and nystatin in oropharyngeal and esophageal candidiasis in Zairian AIDS patientsAnn Soc Belg Med Trop199272145521567268
- MeunierFGérainJSnoeckROral treatment of oropharyngeal candidiasis with nystatin versus ketoconazole in cancer patientsDrug Invest1990227175
- BlomgrenJBerggrenUJontellMFluconazole versus nystatin in the treatment of oral candidosisActa Odontol Scand19985642022059765010
- CummingCGWightCBlackwellCLWrayDDenture stomatitis in the elderlyOral Microbiol Immunol19905282852087353
- FeigalDWKatzMHGreenspanDThe prevalence of oral lesions in HIV-infected homosexual and bisexual men: three San Francisco epidemiological cohortsAIDS1991555195251863403
- Al-ShayyabMHAbu-HammadOAAl-OmiriMKDar-OdehNSAntifungal prescribing pattern and attitude towards the treatment of oral candidiasis among dentists in JordanInt Dent J201565421622626148537
- PappasPGKauffmanCAAndesDClinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of AmericaClin Infect Dis200948550353519191635
- WHO Guidelines Approved by the Guidelines Review CommitteeGuidelines on the Treatment of Skin and Oral HIV-Associated Conditions in Children and AdultsGenevaWorld Health Organization Copyright (c) World Health Organization 20142014
- KinoshitaHYoshiokaMIharaFNihiraTCryptic antifungal compounds active by synergism with polyene antibioticsJ Biosci Bioeng Epub2015829
- EllepolaANJosephBKChandyRKhanZUThe postantifungal effect of nystatin and its impact on adhesion attributes of oral Candida dubliniensis isolatesMycoses2014571566323773155
- JayatilakeJASamaranayakeLPExperimental superficial candidiasis on tissue modelsMycoses201053428529520406388
- SitheequeMASamaranayakeLPChronic hyperplastic candidosis/candidiasis (candidal leukoplakia)Crit Rev Oral Biol Med200314425326712907694
- SamaranayakeLPFidelPLNaglikJRFungal infections associated with HIV infectionOral Dis20028Suppl 215116012164650