Abstract
Apoptosis is a highly regulated process crucial for maintaining cellular homeostasis and development. The B-cell lymphoma 2 (Bcl-2) family of proteins play a crucial role in regulating apoptosis. Overexpressed Bcl-2 proteins are associated with the development and progression of several human cancers. Annona muricata is a tropical plant that belongs to the Annonaceae family and is well known for its anticancer properties. In this study, molecular docking and simulations were performed to investigate the inhibitory potential of phytochemicals present in A. muricata against antiapoptotic proteins of the Bcl-2 family including Bcl-2, B-cell lymphoma extra-large (Bcl-Xl), and Mcl-1. Docking results revealed that the acetogenins, such as annomuricin A, annohexocin, muricatocin A, annomuricin-D-one, and muricatetrocin A/B, exhibited strong binding interactions with Bcl-Xl when compared to Bcl-2 and Mcl-1. Binding score and interactions of these acetogenins were notably better than those of currently available synthetic and natural inhibitors. Molecular dynamics simulations of the top-scoring lead molecules established that these molecules could bind strongly and consistently in the active site of Bcl-Xl. These results suggest that acetogenins could be explored as selective natural inhibitors of Bcl-Xl that could assist in promoting the intrinsic pathway of apoptosis.
Introduction
Ever since the discovery of apoptosis in early 1970 by Kerr et al,Citation1 it has been one of the most explored biological processes in the area of molecular research. Apoptosis is a highly regulated and selective process crucial for maintaining cellular homeostasis and development in multicellular organisms.Citation2 It plays an indispensable role in shaping various body parts in early embryonic development and also regulates cellular equilibrium by controlling cell number and proliferation. It also serves as a defense mechanism by discarding defective cells that have undergone major DNA damage and cannot be repaired.Citation3,Citation4 Apoptosis is mainly executed either by the extrinsic pathway/death receptors or by the intrinsic/mitochondrial pathway with the activation of caspases.Citation5,Citation6
Extrinsic pathway is evoked when cytokine ligands bind to cell-surface death receptors of the tumor necrosis factor receptor family. This binding initiates a series of events that eventually result in the catalytic activation of procaspase-8 into caspase-8. Activation of caspase-8 triggers the executional phase of apoptosis. Internal stimuli such as DNA damage, heat, virulence factors, oxidative stress, and growth factor deprivation will take the intrinsic pathway. These stimuli increase the permeability of the outer mitochondrial membrane (OMM) and release cytochrome C into the cytoplasm, which further promote caspase-9 activation and lead to apoptosis.Citation2,Citation7,Citation8 Cells experiencing apoptosis will exhibit several morphological and biochemical changes such as cellular shrinkage, loss of membrane symmetry, membrane blebbing, DNA breakdown, protein cleavage, nuclear condensation, and fragmentation, and eventually these apoptotic bodies will be engulfed by phagocytes. The members of B-cell lymphoma 2 (Bcl-2) family of proteins are key regulators of the intrinsic pathway, and their dynamic binding interplay determines whether the cell survives or dies.Citation9 Failure to maintain the delicate balance between cell survival and cell death will result in oncogenesis and chemoresistance.Citation10,Citation11 Due to their conflicting functions, members of the Bcl-2 family have been grouped into three major classes: prosurvival, proapoptotic, and Bcl-2 homology 3 (BH3)-only proteins. Bcl-2, B-cell lymphoma extra-large (Bcl-Xl), myeloid leukemia 1 (Mcl-1), B-cell lymphoma-w (Bcl-w), B-cell lymphoma 2-related protein (A1), and Bcl-B comprise the group of prosurvival or antiapoptotic proteins that protect the cell from various cytotoxic conditions by inhibiting proapoptotic proteins.Citation12 Major proapoptotic proteins are the Bcl-2 antagonist/killer (Bak) and Bcl-2-associated protein X (Bax) that promote cell death via OMM permeabilization. This causes the release of apoptogenic molecules into the cytosol followed by caspase activation.Citation13,Citation14 BH3-only proteins such as BIM, BID, BAD, HRK, BIK, PUMA, and NOXA possess conserved BH3 domains by which they interact with either proapoptotic or antiapoptotic proteins to promote apoptosis. They act as initial sensors of apoptotic signals that arise from various cellular responses such as DNA damage, endoplasmic reticulum stress, and growth factor deprivation.Citation15 Despite these opposing functions, all Bcl-2 family members share a conserved amino acid sequence motif LXXGD (where X is any amino acid) known as the BH3 motif. Most antiapoptotic Bcl-2 proteins contain four distinct Bcl-2 homology (BH) domains, namely, BH1, BH2, BH3, and BH4, which fold into a hydrophobic groove to which BH3 proteins bind. The hydrophobic membrane spanning domain of these proteins helps them to get embedded in the OMM, endoplasmic reticulum membrane, and the nuclear envelope.Citation16,Citation17 Structural analysis reveals that the proapoptotic and BH3-only proteins interact with the hydrophobic binding pockets (P1–P4), which constitute a large groove on the surface of Bcl-2 family members (Figure S1A).Citation18 Binding in P2 and P4 pockets is crucial for the strong binding of polypeptides on the surface of Bcl-2 proteins.
The Bcl-2 family of proteins are important regulators of the apoptotic pathway, and overexpression of Bcl-2 proteins is a usual scenario in various malignant tumors and chemoresistance.Citation19 Altered Bcl-2 members are involved in the development of numerous cancers by inhibiting the apoptotic pathway.Citation20,Citation21 Upregulation of Bcl-2 proteins has been reported to inhibit Bax-mediated apoptosis and induction of chemoresistance in cancer cells.Citation22,Citation23 This association of Bcl-2 proteins in tumor initiation, disease progression, and drug resistance makes them candidate targets in anticancer therapy.Citation24 Finding and developing efficient inhibitor molecules for antiapoptotic proteins is a longstanding goal in tumor biology.
Since the dawn of medicine, natural products derived from medicinal plants and herbs have been used for treating various disease conditions. Phytocompounds, or compounds from plants, are once again achieving prominence in modern medicine due to their fewer side effects and cost effectiveness. A vast majority of commercially available drugs have been derived from plants. For example, vinblastine and vincristine from Catharanthus roseus (periwinkle) and taxol from the bark of Taxus brevifolia (Pacific yew) are used for treating various forms of cancers including leukemia, lymphomas, as well as breast, lung, and testicular cancers.Citation25,Citation26 Medicinal plants are rich sources of anticancerous compounds.
In this study, a widely studied plant, Annona muricata, was considered.Citation27 It is commonly known as soursop, graviola, or pawpaw and belongs to the family Annonaceae. It is a tree widely found in tropical and subtropical areas of the world. In traditional medicine, these plants are extensively used for treating a wide array of diseases.Citation28,Citation29 It was reported that an aqueous leaf and bark extract of A. muricata produced hypoglycemic effect by improving the pancreatic insulin level in streptozotocin-induced diabetic rats.Citation30,Citation31 The ethanolic leaf extract of A. muricata has been shown to possess anti-inflammatory and anti-arthritic activity in animal models.Citation32 Phytochemical analysis indicates the presence of various compounds such as acetogenins, alkaloids, flavonoids, glycosides, saponins, tannins, and carbohydrates in this plant.Citation33 Acetogenins are white waxy derivatives of long-chain fatty acids (C32 or C34) derived through the polyketide pathway and are exclusively found in the Annonaceae family.Citation34 They share a common structural skeleton characterized by an α,β-unsaturated-γ-lactone moiety and a hydroxylated tetrahydrofuran (THF) ring joined by a long alkyl chain and a long hydrophobic tail.Citation35 These chemicals are well known for their cytotoxic effects on various cancer cell lines and are potent inhibitors of NADH–ubiquinone oxidoreductase (mitochondrial complex I).Citation36 It has also been shown that an ethyl acetate extract of A. muricata leaves induces apoptosis in cancer cell lines by mediating the nuclear factor κB pathway.Citation37 Targeting antiapoptotic proteins by small-molecule inhibitors is challenging in cancer biology research due to the difficulties in targeting many protein–protein interaction sites.Citation38 However, inhibitors such as navitoclax and venetoclax have been developed to inhibit Bcl-2 proteins. Navitoclax is the first orally bioavailable drug currently in Phase II clinical trials.Citation39,Citation40 Natural polyphenols such as quercetin and apogossypol also exhibit significant inhibitory action against Bcl-2 proteins. Quercetin is a dietary polyphenol found in many plants, and apogossypol is a derivate of gossypol found in cotton plant.Citation41–Citation43
Computational methods such as molecular docking and molecular dynamics (MD) have been used extensively to identify new lead compounds in the field of drug discovery.Citation44–Citation46 The present study was designed to investigate whether the phytocompounds present in A. muricata could interact with the antiapoptotic proteins, such as Bcl-2, Bcl-Xl, and Mcl-1, by analyzing their binding interactions and stability through computational approaches including molecular docking and simulation. For a comparative analysis, the potent Bcl-2 inhibitor navitoclax and the natural inhibitors quercetin and apogossypol were used as controls.
Materials and methods
Preparation of protein and ligand structures
In order to ensure the validity of the results, two structures of each of the proteins – Bcl-2, Bcl-Xl, and Mcl-1 – were used. Three-dimensional (3D) structures of these proteins were retrieved from the Protein Data Bank ().Citation47 The protein structures obtained were preprocessed using the Protein Preparation Wizard of Schrödinger Maestro. This step was performed to remove unwanted water molecules, add and optimize hydrogen bonds, simplify multimeric complexes, create disulfide bonds, adjust formal charges and bond orders of atoms that are attached to metal ions and cofactors, fix the orientation of misoriented groups, and, finally, optimize and refine the structure for further analysis.Citation48
Table 1 Antiapoptotic proteins from the Bcl-2 family used in this study
From the literature,Citation28,Citation29 65 natural compounds from A. muricata were identified, and structures of these compounds were retrieved from PubChem and Chemspider databases (Table S1).Citation49 For a comparative study, Bcl-2 inhibitor navitoclax (ABT 263) and the phytochemical inhibitors quercetin and apogossypol were also retrieved and processed.Citation50 Schrödinger’s LigPrep was used to prepare the structures of these ligands, which involved the conversion of ligand two-dimensional (2D) structures to 3D, addition of hydrogen atoms, generation of various ionization states and tautomers, and, lastly, optimization of geometries.Citation51 A total of 378 stereoisomers were generated from these ligands, which were used for the docking study.
Determination of active site and grid generation
Based on the binding of proapoptotic proteins and BH3-only proteins, functional residues involved in the active site regions of antiapoptotic proteins were determined.Citation52 A receptor grid was generated by incorporating all these functional residues. The van der Waals scaling factor for nonpolar atoms and partial charge cutoff values were set to the default values of 1.0 and 0.25, respectively. Rotation of hydroxyl groups in the active site residues was allowed to produce the most favorable interactions. While generating the receptor grid, no constraints were used. The rest of the parameters were set to default. The OPLS 2005 force field was employed for modeling the interactions.Citation53
Molecular docking
Molecular docking of the prepared targets and ligands was carried out using Schrödinger’s Glide. It uses a series of hierarchical filters for searching favorable interactions of ligands in the active site of proteins. The extra precision (XP) dock scoring function was employed in the analysis.Citation54 Flexible ligand sampling was used to generate conformers during docking. To soften the nonpolar parts of ligands, values for the scaling factor and partial charge cutoff were set as 0.80 and 0.15, respectively. In the entire docking procedure, no constraints were used, and 100 docking poses were generated for each ligand. To improve the geometry of the poses, post-docking minimization was also carried out. Top compounds were ranked based on GlideScore, which is the sum of all XP terms.Citation55 The binding pattern and interactions of these compounds were visualized using the Glide XP visualizer.Citation56 The binding energy, based on molecular mechanics generalized Born surface area, was calculated using Schrödinger’s Prime.Citation57
MD simulations
Top-scoring molecules were subjected to MD simulation for a period of 100 nanoseconds using Desmond to investigate the stability and dynamic interactions of these compounds in their binding site.Citation58 For each docked complex, an orthorhombic box was made with a box wall distance of 10 Å, and the system was solvated with single point charge water molecules.Citation59 Neutralization of the entire system was achieved by adding six Na+ ions and using a salt concentration of 0.15 M. The OPLS 2005 force field was used to model all atoms and their interactions. The initial step involved energy minimization of the prepared system for 2,000 steps using the steepest descent method. For equilibrating the entire system, Desmond’s default protocol was used. After this, MD simulations were performed for a period of 100 nanoseconds at 300 K temperature and 1.01325 bar pressure.Citation60 For this, the Nosé–Hoover chain thermostat was used with a relaxation time of 1 ps and the isotropic Martyna–Tobias–Klein barostat was used with a relaxation time of 2 ps.Citation61,Citation62 For short-range Coulombic interactions, a 9 Å cutoff was set, while long-range interactions were evaluated using the smooth particle mesh Ewald method.Citation63 At every 5 ps, frames were captured to form the simulation trajectory. After the entire simulation, root-mean-square deviation and root-mean-square fluctuation of protein–ligand complexes were calculated with reference to the first frame of the simulation trajectory. Hydrogen bonds and other nonbonded interactions formed between the protein and the ligand throughout the simulation were also analyzed. Rescoring of the simulated docked complexes was performed using Glide by extracting a frame every 10 nanoseconds from the 100 nanoseconds simulation trajectory.
Results and discussion
To explore the possible binding patterns and interactions of phytocompounds to Bcl-2, Bcl-Xl, and Mcl-1, molecular docking and simulation approaches were employed. The 65 ligands used for XP docking were evaluated using GlideScore and binding energies. The results were analyzed based on their interactions in the active site of these antiapoptotic proteins. lists the top-ranked molecules in Bcl-Xl and Bcl-2 along with the interacting residues. In Bcl-Xl, five acetogenins, namely, annomuricin A, annohexocin, muricatocin A, muricatetrocin A/B, annomuricin-D-one (), exhibited high GlideScore ranging from −13.00 kcal/mol to −11.21 kcal/mol in one structure (3ZLR) and from −12.59 kcal/mol to −10.60 kcal/mol in the other (4QVX). In Bcl-2 proteins, the acetogenins muricin E, annomuricin A, and annohexocin came on top with Glide-Scores ranging from −9.32 kcal/mol to −8.80 kcal/mol in 2W3L and from −9.89 kcal/mol to −9.58 kcal/mol in 4AQ3.
Table 2 GlideScores and residues in Bcl-Xl and Bcl-2 that formed hydrogen bonds with the shortlisted acetogenins
Figure 1 Chemical structures of (A) lead acetogenins and (B) inhibitors. Chiral centers are marked with an asterisk (*).
In Mcl-1, the phytochemicals failed to generate consistent interactions in the two structures, indicating low specificity (Table S2). Even though Bcl-2 exhibited consistent interactions in both structures, from the GlideScores it is evident that acetogenins interacted much better with Bcl-Xl than Bcl-2. These molecules interacted with key residues in the active site by establishing hydrogen bonding and hydrophobic interactions. Thus, from the GlideScores and binding energies, acetogenins appear to have a higher specificity for Bcl-Xl than Bcl-2 or Mcl-1 ( and S2).
Binding interactions of acetogenins with Bcl-Xl
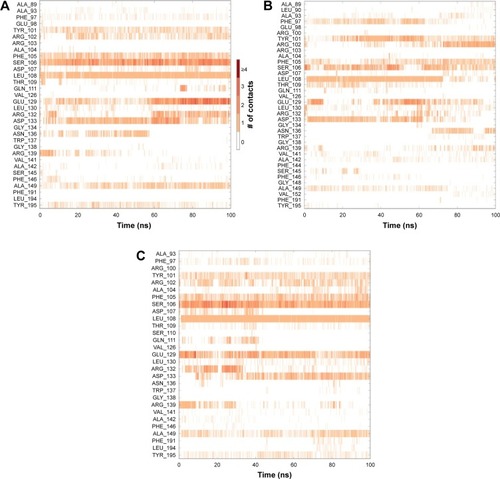
The 3D structure of antiapoptotic/prosurvival protein Bcl-Xl (4QVX and 3ZLR) consists of a common domain formed by assembling two hydrophobic (α5 and α6; Figure S2) and six amphipathic α helices (α1–α4, α7, and α8).Citation64 Four amphipathic helices fold to form a long hydrophobic groove of nearly 20 Å in length (Figure S1A). Structural insights reveal that high-affinity binding of proapoptotic protein, and BH3-only proteins is mediated by interactions in the P2 and P4 pockets of the hydrophobic groove and also by electrostatic interactions between conserved arginine and aspartic acid residues of proapoptotic and prosurvival proteins, respectively.Citation24,Citation52 The P2 and P4 pockets are lined by residues Glu96, Tyr101, Ser106, Asp107, Leu108, Arg139, and Tyr195.Citation24 The P2 hydrophobic pocket is crucial for tight binding of ligand molecules due to its deep and plastic cavity.Citation65 Analysis of the binding pose of annomuricin A in 3ZLR showed that it interacted with the protein by forming six hydrogen bonds and numerous hydrophobic interactions with the critical residues seen in the active site. Annomuricin A is present in the leaves and pericarp of A. muricata, and it has an α,β-unsaturated-γ-lactone moiety and a mono-THF ring with five hydroxyl groups ().Citation28,Citation66 The α,β-unsaturated-γ-lactone moiety of annomuricin A was buried in the P2 pocket, and the hydroxyl group adjacent to this moiety formed a hydrogen bond with the important Ser106 in the P2 pocket that is required for tight binding (Figure S1B).Citation65 The remaining four hydroxyl groups in this molecule interacted with Arg132, Asp133, Asn136, and Arg139 by forming hydrogen bonds. The long hydrophobic tail of the molecule extended into the P4 pocket and formed interactions with several hydrophobic residues, including Tyr101 and Tyr195 ( and S3A).Citation24 Entropic penalty imposed by restraining the ligand in the binding site was overcome by the highly favorable lipophilic and hydrogen-bond interactions in the binding site. A similar binding pattern was also observed in the 4QVX–annomuricin A complex. The hydroxyl groups adjacent to the THF ring region and the alkyl chain formed hydrogen bonds with the key residues such as Ser106, Leu108, and Leu130 in the pockets ( and S4A). In the two Bcl-Xl structures considered, annomuricin A exhibited GlideScores of −13.00 kcal/mol and −12.59 kcal/mol, respectively. Numerous hydrophobic contacts contributed to its binding to Bcl-Xl (Figures S3A and S4A).
Figure 2 Binding interactions of acetogenins in the active site of Bcl-Xl (3ZLR).
Abbreviation: Bcl-Xl, B-cell lymphoma extra-large.
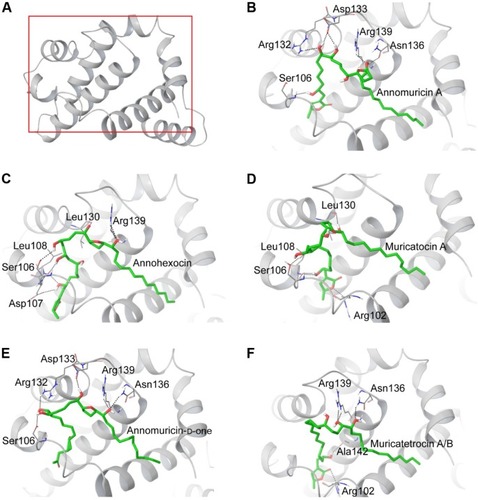
Figure 3 Binding interactions of acetogenins in the active site of Bcl-Xl (4QVX).
Abbreviation: Bcl-Xl, B-cell lymphoma extra-large.
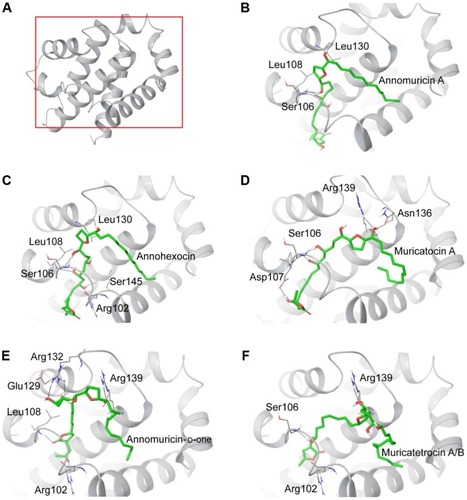
Annohexocin, present in the leaves of A. muricata, is a C-35 mono-THF acetogenin with six hydroxyl groups.Citation28,Citation67 In the 3ZLR–annohexocin complex, the hydroxyl groups in the compound formed six hydrogen bonds with Ser106, Asp107, Leu108, Leu130, and Arg139. The α,β-unsaturated-γ-lactone moiety of annohexocin occupies the P2 pocket, and the hydrophobic tail region of the molecule extends into the P4 pocket by forming hydrophobic contacts. The interactions with these important residues in the active site contribute to their high binding score ( and S3B). Similar interactions were also observed in the 4QVX–annohexocin complex. The hydroxyl groups in the alkyl chain formed strong hydrogen bonds with Arg102, Ser106, Leu108, and Ser145, while the hydroxyl group adjacent to THF ring interacted with Leu130. The hydrophobic tail extended into the P4 region ( and S4B).
Muricatocin A, found in the leaves of A. muricata, is a mono-THF acetogenin with five hydroxyl groups.Citation68 It interacted with 3ZLR by forming five hydrogen bonds with Arg102, Ser106, Leu108, and Leu130 ( and S3C). In the 4QVX–muricatocin A complex, it formed five hydrogen bonds with Ser106, Asp107, Asn136, and Arg139 ( and S4C).
In both structures, the three molecules, namely, annomuricin A, annohexocin, and muricatocin A, displayed consistent interactions in the active site, with GlideScores ranging from −13.00 kcal/mol to −12.58 kcal/mol. All three structures are C35 mono-THF ring acetogenins with identical spacing between the α,β-unsaturated-γ-lactone moiety and the THF ring. Even though there is a minor variation in the number and position of the hydroxyl groups, all three bound strongly in the P2 pocket by forming hydrogen bonds with critical residues such as Ser106, Asp107, and Leu108. This suggests that the number and position of hydroxyl groups do not have a major impact on the binding efficiency of these compounds (Figures S3 and S4). Furthermore, this is supported by molecular mechanics generalized Born surface area calculations, which show that lipophilic interactions contribute the most to the total binding energy (Table S3).
Compared to the aforementioned three compounds, annomuricin-D-one and muricatetrocin A/B displayed lower GlideScore in both Bcl-Xl structures. These two compounds consist of a mono-THF ring with four hydroxyl groups ().Citation69,Citation70 The interactions between Bcl-Xl and these compounds are shown in and F, 3E and F, S3D and E, and S4D and E. The length of the alkyl chain that connects the α,β-unsaturated-γ-lactone moiety and THF ring is shorter when compared to those in annomuricin A, annohexocin, and muricatocin A. This suggests that the length of alkyl chain plays a role in the binding efficiency of these compounds; longer the alkyl chain, the stronger the interactions.
Since deregulation of apoptosis is a common scenario in many forms of cancers, several drugs have been developed to inhibit the Bcl-2 family proteins. It was reported that the natural molecules quercetin and apogossypol also interact directly with Bcl-Xl and trigger apoptotic activity. For a comparative analysis, the Bcl-Xl inhibitor navitoclax and these natural molecules were used as controls ().Citation39,Citation40 Docking results (Figure S5) showed that acetogenins exhibited better GlideScore than these inhibitors (). Pharmacokinetic properties computed for the acetogenins using Schrödinger QikProp were also notably better than those of navitoclax (Table S4). These acetogenins have also been shown to exhibit significant cytotoxicity against several cancer cell lines including MCF-7 (breast), A549 (lung), HT-29 (colon), and MIA PaCa-2 (pancreatic).Citation28,Citation66–Citation70
Table 3 GlideScores and Bcl-Xl residues that formed hydrogen bonds with the known inhibitors
MD simulations
In order to analyze structural dynamics and stability of annomuricin A, annohexocin and muricatocin A bound to Bcl-Xl, MD simulations were performed for a period of 100 nanoseconds each. During the initial period of the simulations, Bcl-Xl exhibited some degree of fluctuation, indicating that the protein was undergoing structural adaptations. After this initial period, the protein structure stabilized, providing an equilibrated system. Most of the fluctuations were observed in the loops and turns, whereas the other secondary structures remained intact throughout the simulations. During the course of the simulation, annomuricin A stayed firmly in the binding pocket of Bcl-Xl by forming hydrogen bonds, hydrophobic interactions, and water bridges with the critical residues Tyr101, Ser106, Leu108, and Arg139. Besides maintaining these crucial interactions, it also formed new interactions in the active site (). Annohexocin also adapted well to the binding pocket of Bcl-Xl by maintaining the important interactions with the residues in the P2 pocket. Throughout the simulation, annohexocin retained the hydrogen-bond interactions with Ser106, Asp107, and Leu108 residues (). These sustained interactions confirmed the strong binding of the molecule in the active site of Bcl-Xl. Muricatocin A also remained in the P2 pocket by intermittently forming and breaking hydrogen bonds with Phe105, Ser106, Asp107, and Leu108 residues (). In all three docked complexes, water molecules assisted in forming numerous water bridges, thereby making the interactions stronger. To study how the water molecules could contribute to the binding energy, frames were extracted every 10 nanoseconds from the MD simulations. These were then rescored using the same protocol as used for molecular docking. In the case of annomuricin A, average rescored GlideScore of all frames analyzed was −18.10±1.57 kcal/mol and the final frame produced a GlideScore of −19.15 kcal/mol. The average GlideScores of annohexocin and muricatocin A were −19.67±1.50 kcal/mol and −18.98±2.07 kcal/mol, respectively. All three lead molecules exhibited significant improvement from the initially docked GlideScore (). During the course of the simulation, water molecules played a crucial role by forming a number of direct interactions and bridges between the ligand and amino acids in the binding site of the protein (Figure S6). This contributed to the significant improvement in the binding scores and assisted the acetogenins to bind more favorably in the active site of Bcl-Xl. This finding highlights the significance of considering water molecules while evaluating protein–ligand interactions, especially in drug discovery.
Conclusion
Overexpression of antiapoptotic proteins is typical in many forms of cancer. In this study, molecular docking and simulations were employed to identify potential lead compounds from A. muricata that could be inhibitors of these antiapoptotic proteins. The results revealed that the acetogenins such as annomuricin A, annohexocin, muricatocin A, annomuricin-D-one, and muricatetrocin A/B exhibited high docking score in Bcl-Xl but not in Bcl-2 and Mcl-1. Structural and interaction analysis showed that these molecules bound stably in the active site of Bcl-Xl largely through lipohillic interactions. In comparative docking studies using natural and synthetic inhibitors of Bcl-Xl, acetogenins exhibited better binding scores and intermolecular interactions. Docking scores were also significantly improved when the protein–ligand complexes were rescored using frames from MD simulations, suggesting that water molecules play an important role in the binding site interaction. Thus, this study provides insights into how acetogenins from A. muricata could be the base for designing potential Bcl-Xl-selective inhibitors for the treatment of cancer.
Acknowledgments
This work was supported by an interdisciplinary research grant from the Zayed Bin Sultan Center for Health Sciences, United Arab Emirates University.
Disclosure
The authors report no conflicts of interest in this work.
References
- KerrJFRWyllieAHCurrieARApoptosis: a basic biological phenomenon with wide-ranging implications in tissue kineticsBr J Cancer19722642392574561027
- WongRSApoptosis in cancer: from pathogenesis to treatmentJ Exp Clin Cancer Res20113018721943236
- SaikumarPVenkatachalamMAApoptosis and cell deathCaglePTAllenTCBasic Concepts of Molecular PathologyBerlinSpringer2009147151
- TaylorRCCullenSPMartinSJApoptosis: controlled demolition at the cellular levelNat Rev Mol Cell Biol20089323124118073771
- ElmoreSApoptosis: a review of programmed cell deathToxicol Pathol200735449551617562483
- SuZYangZXuYChenYYuQApoptosis, autophagy, necroptosis, and cancer metastasisMol Cancer20151414825743109
- GhobrialIMWitzigETAdjeiAATargeting apoptosis pathways in cancer therapyCA Cancer J Clin200555317819415890640
- NagasakaAKawaneKYoshidaHNagataSApaf-1-independent programmed cell death in mouse developmentCell Death Differ201017693194119960021
- FrenzelAGrespiFChmelewskijWVillungerABcl2 family proteins in carcinogenesis and the treatment of cancerApoptosis200914458459619156528
- PlatiJBucurOKhosravi-FarRDysregulation of apoptotic signalling in cancer: molecular mechanisms and therapeutic opportunitiesJ Cell Biochem200810441124114918459149
- LoweSWLinAWApoptosis in cancerCarcinogenesis200021348549510688869
- AdamsJMCorySThe Bcl-2 apoptotic switch in cancer development and therapyOncogene20072691324133717322918
- TzifiFEconomopoulouCGourgiotisDArdavanisAPapageorgiouSScorilasAThe role of BCL2 family of apoptosis regulator proteins in acute and chronic leukemiasAdv Hematol20122012115
- KorsmeyerSJBCL-2 gene family and the regulation of programmed cell deathCancer Res1999597 Suppl1693s1701s10197582
- YouleRJStrasserAThe BCL-2 protein family: opposing activities that mediate cell deathNat Rev Mol Cell Biol200891475918097445
- MichelsJKeppOSenovillaLFunctions of BCL-X L at the interface between cell death and metabolismInt J Cell Biol2013201370529423533418
- Shamas-DinAKaleJLeberBAndrewsDWMechanisms of action of Bcl-2 family proteinsCold Spring Harb Perspect Biol201354a00871423545417
- ZhouHChenJMeagherJLDesign of Bcl-2 and Bcl-xL inhibitors with subnanomolar binding affinities based upon a new scaffoldJ Med Chem2012554664468222448988
- Del BufaloDBiroccioALeonettiCZupiGBcl-2 overexpression enhances the metastatic potential of a human breast cancer lineFASEB J199711129479539337147
- YipKWReedJCBcl-2 family proteins and cancerOncogene200827506398640618955968
- AklHVervloessemTKiviluotoSA dual role for the anti-apoptotic Bcl-2 protein in cancer: mitochondria versus endoplasmic reticulumBiochim Biophys Acta20141843102240225224768714
- TeijidoODejeanLUpregulation of Bcl2 inhibits apoptosis-driven BAX insertion but favors BAX relocalization in mitochondriaFEBS Lett2010584153305331020621101
- DongJZhaoYPZhouLZhangTPChenGBcl-2 upregulation induced by miR-21 via a direct interaction is associated with apoptosis and chemoresistance in MIA PaCa-2 pancreatic cancer cellsArch Med Res201142181421376256
- TaoZHasvoldLWangLDiscovery of a potent and selective BCL XL inhibitor with in vivo activityACS Med Chem Lett201451088109325313317
- AhmedMKhanRAKhanIKhanMRRole of medicinal plants in oxidative stress and cancerSci Rep2013264
- RashedKNMedicinal plants as a safe target for treatment of cancerNat Prod Chem Res2014226836
- YangCGundalaSRMukkavilliRVangalaSReidMDAnejaRSynergistic interactions among flavonoids and acetogenins in Graviola (Annona muricata) leaves confer protection against prostate cancerCarcinogenesis201536665666525863125
- MoghadamtousiSFadaeinasabMNikzadSMohanGAliHKadirHAnnona muricata (Annonaceae): a review of its traditional uses, isolated acetogenins and biological activitiesInt J Mol Sci2015167156251565826184167
- GajalakshmiSVijayalakshmiSDevi RajeswariVPhytochemical adn pharmacological properties of Annona muricata: a reviewInt J Pharm Pharm Sci2012421316
- AdewoleSCaxton-MartinsEMorphological changes and hypoglycemic effects of Annona muricata Linn. (Annonaceae) leaf aqueous extract on pancreatic β-cells of streptozotocin-treated diabetic ratsAfr J Biomed Res200993173187
- AhalyaBShankarKRKiranmayiGVNExploration of anti- hyperglycemic and hypolipidemic activities of ethanolic extract of Annona muricata bark in alloxan induced diabetic ratsInt J Pharm Sci Rev Res20142522127
- FoongCPHamidRAEvaluation of anti-inflammatory activities of ethanolic extract of Annona muricata leavesRev Bras Farmacogn201222613011307
- VijayameenaCSubhashiniGLoganayagiMRameshBPhytochemical screening and assessment of antibacterial activity for the bioactive compounds in Annona muricataInt J Curr Microbiol Appl Sci20132118
- KojimaNTanakaTMedicinal chemistry of annonaceous acetogenins: design, synthesis, and biological evaluation of novel analoguesMolecules20091493621366119783948
- MiyoshiHOhshimaMShimadaHAkagiTIwamuraHMcLaughlinJLEssential structural factors of annonaceous acetogenins as potent inhibitors of mitochondrial complex IBiochim Biophys Acta1998136534434529711297
- Zafra-PoloMCGonzálezMCEstornellESahpazSCortesDAcetogenins from Annonaceae, inhibitors of mitochondrial complex IPhytochemistry19964222532718688168
- MoghadamtousiSZKadirHAPaydarMRouhollahiEKarimianHAnnona muricata leaves induced apoptosis in A549 cells through mitochondrial-mediated pathway and involvement of NF-κBBMC Complement Altern Med201414129925127718
- MullardAProtein–protein interaction inhibitors get into the grooveNat Rev Drug Discov201211317317622378255
- WentDMThe discovery of navitoclax, a Bcl-2 family inhibitorWendtMDTopics in Medicinal Chemistry Protein-Protein Interactions8BerlinSpringer2012231258
- WongMTanNZhaJNavitoclax (ABT-263) reduces Bcl-xL-mediated chemoresistance in ovarian cancer modelsMol Cancer Ther20121141026103522302098
- MantzarisMDHatzimichaelEShinJChiSDirect binding of Bcl 2 family proteins by quercetin triggers its pro-apoptotic activityACS Chem Biol20149121115
- OliverCLMirandaMBShangarySLandSWangSJohnsonDE(−)-Gossypol acts directly on the mitochondria to overcome Bcl-2- and Bcl-X(L)-mediated apoptosis resistanceMol Cancer Ther200541233115657350
- WeiJKitadaSRegaMFApogossypol derivatives as antagonists of anti-apoptotic Bcl-2 family proteinsMol Cancer Ther20098490491319372563
- AntonyPVijayanRIdentification of novel aldose reductase inhibitors from spices: a molecular docking and simulation studyPLoS One2015109e013818626384019
- SinghSPKonwarBKMolecular docking studies of quercetin and its analogues against human inducible nitric oxide synthaseSpringerplus2012116923556141
- AzamFAmerAMAbulifaARElzwawiMMGinger components as new leads for the design and development of novel multi-targeted anti-Alzheimer’s drug: a computational investigationDrug Des Devel Ther2014820452059
- SussmanJLLinDJiangJProtein Data Bank (PDB): database of three-dimensional structural information of biological macromoleculesActa Crystallogr D Biol Crystallogr199854Pt 6 Pt 11078108410089483
- SchrödingerSchrödinger Suite 2014–Protein Preparation Wizard; Epik version 3.0Schrödinger, LLCNew York, NY2014
- Impact version 6.5Schrödinger, LLCNew York, NY2014
- Prime version 3.8Schrödinger, LLCNew York, NY2014
- WangYXiaoJSuzekTOZhangJWangJBryantSHPubChem: a public information system for analyzing bioactivities of small moleculesNucleic Acids Res200937623633
- WilsonWHConnorOAOCzuczmanMSSafety, pharmacokinetics, pharcodynamics, and activity of navitoclax, a targeted high affinity inhibitor of BCL-2, in lymphoid malignanciesLancet Oncol201111121149115921094089
- SchrödingerLigPrep, version 3.2Schrödinger, LLCNew York, NY2014
- LesseneGCzabotarPESleebsBEStructure-guided design of a selective BCL-XL inhibitorNat Chem Biol20139639039723603658
- BanksJLBeardHSCaoYIntegrated modeling program, applied chemical theory (impact)J Comput Chem200526161752178016211539
- FriesnerRAMurphyRBRepaskyMPExtra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexesJ Med Chem200649216177619617034125
- HalgrenTAMurphyRBFriesnerRAGlide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screeningJ Med Chem20044771750175915027866
- FriesnerRABanksJLMurphyRBGlide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracyJ Med Chem20044771739174915027865
- SchrödingerPrime version 3.8Schrödinger, LLCNew York, NY2014
- Desmond Molecular Dynamics System, version 4.1D. E. Shaw ResearchNew York, NY2014
- Maestro-Desmond Interoperability Tools, version 4.1SchrödingerNew York, NY2014
- BerendsenHJCPostmaJPMvan GunsterenWFHermansJInteraction models for water in relation to protein hydrationPullmanBIntermolecular ForcesDordrechtReidel1981331342
- ShivakumarDWilliamsJWuYDammWShelleyJShermanWPrediction of absolute solvation free energies using molecular dynamics free energy perturbation and the OPLS force fieldJ Chem Theory Comput2010651509151926615687
- MartynaGJKleinMLTuckermanMNosé–Hoover chains: the canonical ensemble via continuous dynamicsJ Chem Phys19929726352643
- MartynaGJTobiasDJKleinMLConstant pressure molecular dynamics algorithmsJ Chem Phys199410141774189
- EssmannUPereraLBerkowitzMLDardenTLeeHPedersenLGA smooth particle mesh Ewald methodJ Chem Phys19951031985778593
- SouersAJLeversonJDBoghaertERABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing plateletsNat Med201319220220823291630
- CzabotarPELesseneGStrasserAAdamsJMControl of apoptosis by the BCL-2 protein family: implications for physiology and therapyNat Rev Mol Cell Biol2013151496324355989
- WuFEGuZMZengLTwo new cytotoxic monotetrahydrofuran Annonaceous acetogenins, annomuricins A and B, from the leaves of Annona muricataJ Nat Prod19955868308367673926
- ZengLWuFEMcLaughlinJLAnnohexocin, a novel mono-THF acetogenin with six hydroxyls, from Annona muricata (Annonaceae)Bioorg Med Chem Lett199551618651868
- WuFEZengLGuZMNew bioactive monotetrahydrofuran Annonaceous acetogenins, annomuricin C and muricatocin C, from the leaves of Annona muricataJ Nat Prod19955869099157673936
- ZengLWuFEOberliesNHMcLaughlinJLSastrodihadjoSFive new monotetrahydrofuran ring acetogenins from the leaves of Annona muricataJ Nat Prod19965911103510428946744
- RieserMJFangXPAndersonJEMiesbauerLRSmithDLMcLaughlinJLThree new cytotoxic monotetrahydrofuran-ring acetogenins from Annona muricataHelv Chim Acta199376724332444