?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Silymarin is a naturally occurring flavonoid drug; evidence from recent research has highlighted its use as a potential treatment for atopic dermatitis (AD). Both poor water solubility and drug permeability have hindered the percutaneous absorption of silymarin. Formulation of silymarin into pluronic-lecithin organogel (PLO) basis for topical skin delivery is the main aim of this work. Six different PLO formulations were prepared containing various pluronic to lecithin ratios using two cosolvent systems of ethyl alcohol and dimethyl sulfoxide. Formulation 2 (20% pluronic and 3% lecithin) was found to be the optimal base for topical delivery of silymarin as it showed optimum pH, viscosity, drug content, and satisfactory in vitro silymarin permeation. The silymarin PLO formulation significantly relieved inflammatory symptoms of AD such as redness, swelling, and inflammation. These findings warrant the ability for application of these novel silymarin PLO formulations as a novel treatment for AD.
Introduction
Atopic dermatitis (AD) is a chronically relapsing inflammatory disease; AD is characterized by intensely pruritic skin and occurs quite often in children.Citation1 This is associated with increased production of IgE and/or altered pharmacological reactivity.Citation2 The characteristic symptoms of AD include skin dryness, erythema, oozing, and it is occasionally followed by crusting and lichenification of the skin in untreated patients. Pruritus and skin itching are the most annoying symptoms and are responsible for much of the disease burden for patients and their families.Citation3 Therefore, the main goal of the management of AD patients is based on the use of topical anti-inflammatory preparations along with optimum hydration of the skin. However, some patients may require phototherapy or systemic treatment in severe cases.Citation4,Citation5
Treatment of acute flare-ups is still based on topical glucocorticosteroids.Citation6 Relapse after the discontinuation of oral steroids is well known. Long-term therapy of corticosteroids is associated with a series of well-documented side effects such as: increased risk of glaucoma and cataracts,Citation7 in addition to the high incidence of steroid addiction when they are withdrawn.Citation8
Recently, there has been a growing interest in using herbal medicines for the treatment of skin disease.Citation9–Citation12
Silymarin is a natural polyphenolic flavonoid derived from milk thistle seeds. It has long been used as a traditional herbal hepatoprotective agent.Citation13 It is one of the most widely used compounds of flavonoids because of its extensive therapeutic properties.
Zhao et al reported that silymarin has a diverse effect on the skin with chemoprotective and anticancer properties.Citation10 Also, it was found that silymarin plays a role in the prevention of ultraviolet-B-induced skin damage, with a skin whitening effect.Citation11,Citation14 Moreover, silymarin has a positive effect on wound healing and skin burns owing to its anti-inflammatory and antioxidant effects.Citation15–Citation17 In addition, Han et al demonstrated that silymarin can exert a suppressive effect on chemically induced irritant contact dermatitis in an animal model. Furthermore, it has been reported that silymarin might inhibit the expression of cytokines and infiltration of neutrophils, therefore, it has a potential role for prevention and treatment of cutaneous inflammatory diseases.Citation18 It was also found that prophylactic use of silymarin-based cream was associated with both significant decrease in the duration, and maximum severity of dermatitis compared with the standard of care treatment. Therefore, silymarin-based cream is a promising candidate for a safe prophylactic treatment option of radiodermatitis.Citation19 It is well known that the stratum corneum is considered the major obstacle for topical delivery, which controls the drug transport even through the most superficial layers of the epidermis.Citation20 Limited skin permeability of silymarin results from its lipophilicity and limited solubility. Hence, ideal topical drugs must possess both lipoidal and aqueous solubility.Citation21 Spada et al studied the influence of silymarin complexation with cyclodextrin and formulation into oil-in-water emulsion on permeation in porcine skin. The penetration of the silymarin into the stratum corneum was successfully achieved using the phytocomplex; approximately 80% of silymarin was absorbed by the skin within 1 hour, compared with almost 30% permeability reported with uncomplexed silymarin.Citation22
Recently, pluronic-lecithin organogels (PLOs) have gained popularity as transdermal and topical drug delivery systems owing to their biphasic composition and versatility; PLO can enhance solubility of poorly soluble drugs and enhance penetrability of hydrophilic drugs.Citation23
Therefore, this work aimed to formulate and evaluate silymarin PLO as a novel treatment for AD. Specific objectives will look at: studying the effect of different parameters (polymer type and oily phase) on both the drug release and skin permeability. The efficacy of the formulated PLO loaded silymarin for treatment of AD patients was also studied.
Materials and methods
Materials
Silymarin was a generous gift from SEDICO Pharmaceutical Company (Cairo, Egypt). Lecithin was purchased from MP Biomedicals (LCC, Santa Ana, CA, USA). Isopropyl myristate (IPM), pluronic F-127, and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). Potassium dihydrogen orthophosphate, disodium hydrogen orthophosphate, and sodium chloride were purchased from ADWIC (El-Nasr Pharmaceutical Co., Cairo, Egypt).
Methods
Analytical method validation of silymarin
A spectrophotometric method was developed and validated for determination of silymarin. Validation parameters included linearity, accuracy, and precision. The spectrophotometric method was validated by measuring absorbance at different concentration ranges and plotting calibration curves. The average accuracy and standard deviation (SD) were calculated. Precision was validated by measuring absorbance at six concentrations. Precision was expressed by the SD and relative SD of measured response.
Preparation of silymarin PLO gels
Different PLO formulations were prepared and shows the composition of the different PLO formulations. The oil phase, containing soya lecithin was prepared by dissolving its components in an appropriate quantity of IPM overnight at room temperature. For preparing the aqueous phase, the calculated amounts of pluronic F-127 and ascorbic acid were dissolved in cold water and stored in a refrigerator (at 4°C). Silymarin was dissolved in either ethyl alcohol or DMSO, and mixed with the prepared aqueous phase. The aqueous phase (80%) was slowly added drop-wise to the oil phase (20%) with continuous stirring (1,000 rpm) for 1 minute at room temperature. The pH of the final formulated organogels was recorded using a digital pH meter (HANNA Instruments, Woonsocket, RI, USA).
Table 1 Composition of different PLO gels loaded with silymarin
Characterization of the prepared PLO formulation
Fourier transform infrared spectroscopy (FTIR)
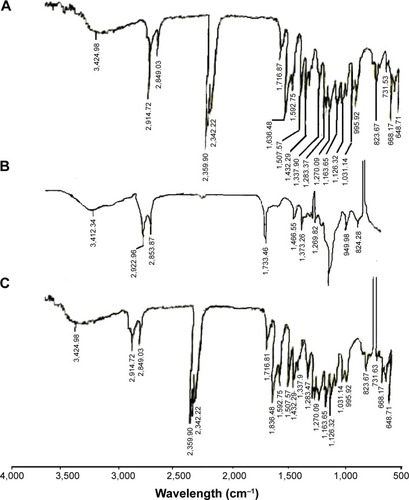
Samples (silymarin, plain PLO gel and drug loaded PLO gel [formulation 2 {F2}]), 1 mg were dried, mixed with potassium bromide, compressed at a pressure of 6 ton/cm2 into discs, and scanned using a Fourier transform infrared spectrophotometer (PerkinElmer Inc., Waltham, MA, USA) over the range of 4,000–500 cm−1. A blank KBr pellet was used as a reference.
Drug content
For determining the drug content of the prepared PLO gels, a sample of 1 g of each formulation was transferred into a 50 mL volumetric flask. The volume was made up with ethyl alcohol and shaken at 100 rpm for 10 minutes at room temperature to dissolve the drug. The samples were filtered through 0.22 μm filter discs. One mL of the filtrate was withdrawn into a 10 mL volumetric flask and diluted to 10 mL with ethyl alcohol. The content of the drug was estimated spectrophotometrically at λmax 286 nm using a spectrophotometer (Spectronic Genesys®, with Winspec Software; Spectronic 700, NY, USA).
Viscosity and rheology study
The viscosity and rheological studies of the samples were carried out at 25°C±0.1°C using a Brookfield Rheometer (model DV-I+; Brookfield Engineering Laboratory, Inc, Middleboro, MA, USA). The shear stress was increased automatically by the instrument over a period of 30 seconds. The viscosities of the formulations were recorded before and after gradual increase in the angular velocity from 10 rpm to 100 rpm. The average of three readings was used to calculate the viscosity. The relationship between shear stress and shear rate of each formulation was determined using power law described in the following equation:
Stability study
Stability of the prepared formulations was also studied by monitoring the optical quality for any phase separation and viscosity over 3 months at 25°C±5°C and around 50% relative humidity. The rheological parameters were also evaluated as previously mentioned.
In vitro release studies
In vitro release of silymarin PLO formulations was studied using phosphate buffer of pH 7.4 containing 20% ethyl alcohol as a release medium. The release study was performed under sink conditions. A cellophane membrane of molecular weight cut-off of 6,000–8,000 Da and presoaked in the release medium was used to cover one end of a cylindrical glass tube with a surface area of 2.8 cm2. One gram of the examined formulation was placed over the inner side of the cellophane membrane and the whole assembly was immersed in 100 mL of the release media at 37°C±0.5°C and subjected to shaking at 100 strokes per minute. Samples of 5 mL each were drawn at the specified time intervals. Each sample was replaced by an equal volume of fresh phosphate buffer. The concentration of silymarin was analyzed and validated spectrophotometrically at λmax 322 nm.
Ex vivo permeability study
Selected formulations (F2 and *F2) were subjected to ex vivo permeability studies using excised rabbit skin. The test was carried out as mentioned in the section, In vitro diffusion study, except for using excised skin from the rabbit ear. The excised skin was cleaned by proper washing with cold running water and then treated to remove any fatty material by soaking the whole skin membranes in water at 60°C for 70 seconds, followed by blunt dissection and they were then folded in aluminum foil. The epidermal membranes were prepared and then mounted with the epidermal side facing the donor solution.
Clinical evaluation of the prepared PLO formulations
The clinical study was conducted according to approval from the Research Ethics Committee, Faculty of Medicine, Minia University, Minia, Egypt.
The present study was conducted on 15 patients (eight males [53.3%] and seven females [47.7%]) with varying degrees of AD severity, attending the outpatient clinic of Dermatology, STDs, and Andrology Department (Minia University Hospital). The age of the patients ranged from 1 year to 30 years with a mean and SD of 9.3±8.7. The severity of AD was assessed using the SCORing Atopic Dermatitis (SCORAD) index.
The study was approved by the committee for postgraduate studies and research of Minya University.
Inclusion criteria
Fifteen patients with AD were recruited by the outpatient clinic of the Department of Dermatology, STDs, and Andrology, Minia University Hospital. The inclusion criteria were:
All patients showing manifestation of AD in a bilateral distribution.
All ages, both sexes, and different degrees of severity of AD as were assessed by the SCORAD index were included.
Patients received no topical or systemic treatment for at least 3 months before starting the study.
Written consent was obtained from all patients who participated in the present study including for the use of the photographs.
Treatment of the patient groups
The PLO containing silymarin (2.5 mg drug/dose) was applied on the right side and PLO base free from silymarin was applied on the left side. All patients were subjected to full history taking, dermatological examination, skin biopsy, and photographing as in the following sections.
Patient follow-up
Patients’ symptoms and signs such as skin itching were assessed using an improvement score as follows:
No change (0%–25%);
Mild improvement (26%–50%);
Moderate improvement (51%–75%);
Good result (76%–99%);
Complete cure (100%).
Photography
Photographs of the treated lesions were obtained for all patients using a high resolution digital camera. Photographs were obtained before and 3 months after starting treatment.
Skin biopsy
Skin biopsies were obtained from patients using sterile 2 mm punches after informed consent.
Skin biopsies were taken from lesional skin and from apparently normal non-lesional skin.
Each biopsy was immediately fixed in 10% formalin, embedded in paraffin, and sectioned by the ordinary microtome into 5 μm thick sections. These were stained with hematoxylin and eosin and then utilized for histopathological examination. A light microscope with a built-in camera (Nikon Optiphot; Nikon Corporation, Tokyo, Japan) was used to examine and photograph these sections.
Statistical analysis
Data were collected and tested for statistically significant difference using SPSS (version 11.0; SPSS Inc., Chicago, IL, USA). The numerical data were expressed as mean ± SD. Student’s t-test was used to compare numerical values. The (Student’s t-test) values were expressed in terms of P-values. The level of significance was determined as follows: P>0.05= not significant; P<0.05= significant; P<0.001= highly significant.
Results and discussion
Analytical method validation of silymarin
The λmax of silymarin in ethyl alcohol and PBS was found to be 286 nm and 322 nm, respectively. The drug followed linearity in the concentration range 2–20 μg/mL with a correlation coefficient value of 0.998 and 0.999 in ethyl alcohol and PBS, respectively. The accuracy of the method was checked by determining percentage recovery. The percentage recovery was found to be in the range of 99.54%–100.98% in ethyl alcohol and 98.94%–101.4% in PBS, respectively. The precision of the method was studied according to intraday: interday variations, and repeatability. The percentage relative SD value <2 indicates that the method is precise. Limit of detection and limit of quantification were evaluated from signal-to-noise ratio and they were 0.3 μg/mL and 1 μg/mL, respectively.
Optimization of PLO formulations
The composition of different PLO formulations of silymarin is shown in . It was found that the key factor for preparation of stable organogel was the percentage of pluronic. By increasing the ratio of pluronic from 10% to 30%, the stability of the organogel was enhanced. Therefore, F1 was ruled out from our study due to instability and phase separation. Additionally, preliminary work revealed that the best ratio of the oil phase (lecithin and IPM) to the aqueous phase (pluronic solution) was 20%:80%. The best ratio of aqueous phase mixture was 70% water to 30% ethyl alcohol for complete solubilization of silymarin. The pH was found to be in the range of 6.1–6.8 reflecting suitability for topical application without any signs of irritation.
Characterization of silymarin PLO
Macroscopic examination of the prepared organogels indicated that the formulated organogels had a yellowish white to yellow color and had a good texture, free from grittiness. These results reflect acceptability by the patients.
FTIR study
FTIR of silymarin, plain PLO gel, and drug loaded PLO gel is shown in . FTIR of silymarin loaded PLO gel showed that the characteristic peaks of silymarin appear without any change in the spectrum of the drug. These results indicated that there was no interaction which could be detected between the drug and the components of the base.
Drug content
Drug content of the prepared formulations was found to range from 98.2% to 101.2% as shown in . These results indicate complete distribution of the drug throughout the PLO gel base.
Table 2 Physicochemical properties of different PLO gels loaded with silymarin
Viscosity and rheology study
shows the viscosity values of the prepared PLO formulations. The results showed that increasing the concentration of lecithin led to a significant increase in the gel viscosity (). Parsaee et al stated that long, flexible, and cylindrical giant micelles were formed upon increasing the concentration of lecithin in the formulation.Citation24 Upon the addition of polar solvents, cylindrical reverse micelles started growing until they intertwined to form a 3-dimensional network in the organogels due to its amphiphilic nature.Citation25
Figure 2 Rheology behaviors of different PLO gels loaded with silymarin.
Abbreviations: PLO, pluronic-lecithin organogel; DMSO, dimethyl sulfoxide.
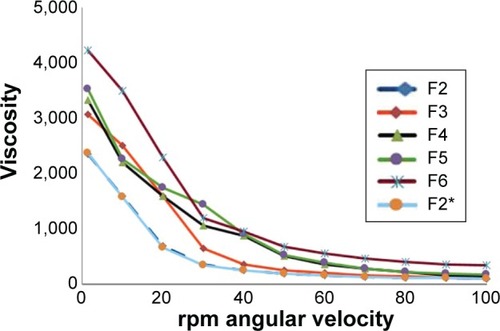
However, a significant increase in the viscosity of the PLO from 2,350 to 4,420 (cps) was observed with increasing concentration of pluronic from 20% to 30% (w/v), respectively. These results could be ascribed to formation of a complex network. Moreover, no statistical significant difference was recorded between F2 and F2* and this indicated that type of solvent has no effect on the viscosity of the formulation.
As shown in , all the developed formulations exhibited non-Newtonian behavior with pseudoplastic flow and shear thinning behavior. Application of the power law model to the rheological properties of the formulation enabled the calculation of the consistency (k) and flow index (n). The values of flow index (n) were found to be less than 1 (0.67) for the gel, confirming a shear thinning behavior of the gel. At the same time, PLO gels retained their initial viscosity at the end of the test (). This clearly indicates the better thixotropic properties which are preferable for topical use.
Stability study
Visual inspection of all prepared formulations showed no phase separation after storage for 3 months. In addition, there were no significant changes in the viscosity or rheological parameters after storage.
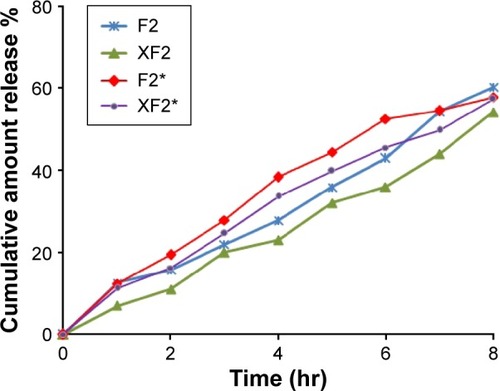
In vitro release studies and release kinetics
In vitro release profiles of PLO formulations containing 0.2% (w/w) silymarin are shown in . The results showed that the developed formulations exhibited sustained release of the drug. Data shown in also confirmed that the release rate of silymarin significantly decreased by increasing the lecithin ratio from 3% to 9% (% w/w). As mentioned before, the viscosity of the organogel was increased from 2,350 to 3,530 cps with increasing lecithin ratio from 3% to 9%, respectively, which affected the release rate of the drug. As expected, at a higher lecithin concentration, there is more extensive entanglement of long cylindrical micelles, forming a network-like structure with very high viscosity, hence, a smaller amount of free drug is available for release.Citation27
Figure 3 In vitro release of silymarin from different formulations of PLO gels.
Abbreviations: PLO, pluronic-lecithin organogel; hr, hours; DMSO, dimethyl sulfoxide.
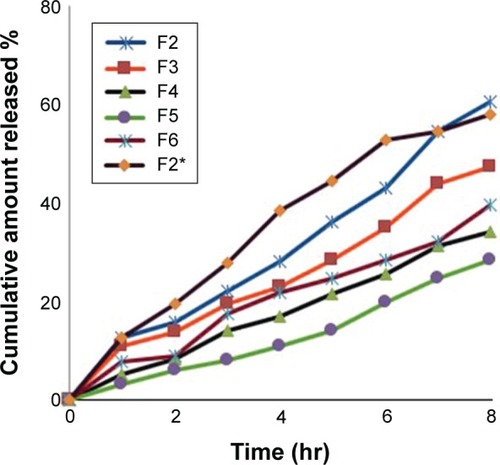
Moreover, a comparative in vitro drug release profile between F2 (20% pluronic) and F3 (30% pluronic) showed 12.5% and 8% after the initial 60 minutes, respectively. However, at the end of 8 hours, the drug release was found to b ~60% and 36% respectively. It was clear that increasing the ratio of pluronic led to more sustained drug release. These results could be due to two different reasons: formation of a network-like structure and high viscosity of the formulation.Citation26,Citation28
The conclusion which can be drawn from this study is that the most influential factor on the release rate of silymarin from the developed PLO was its viscosity. The results obtained from the in vitro test seem to emphasize the theory already described by some authors, that an inverse correlation exists between the release rate and the viscosity.Citation22,Citation26 In addition, there was no statistically significant difference between the percentage release of silymarin from F2 (using 30% ethyl alcohol) and F2* (using 3% DMSO). The percentage silymarin released after 8 hours was ~60% for each formulation.
Release kinetics illustrated that all the developed formulations followed zero order kinetics with correlation coefficient values ranging from 0.9758 to 0.9976.
In vitro permeability study
shows the ex vivo permeability for PLOs, F2 and F2*, using excised rabbit skin, compared with their in vitro release study. It was found that the permeability study was in line with the in vitro release study. These results confirm that the prepared PLO has the ability to penetrate the skin. Moreover, there was no statistically significant difference between PLOs F2 and F2*, indicating that using ethyl alcohol as a cosolvent for silymarin was successful. Ethyl alcohol was found to have the same effect as DMSO on silymarin penetration and solubilization. Therefore, PLO was successful as a novel topical formulation for silymarin.
Clinical evaluation
The severity of AD varied in different patients according to the SCORAD index. Clinical phase, severity, and distribution of lesions are tabulated in . Four cases (26.7%) were mild, six cases (40%) were moderate, and five cases (33.3%) were severe.
Table 3 Clinical phase, severity, and distribution of AD lesions among patients
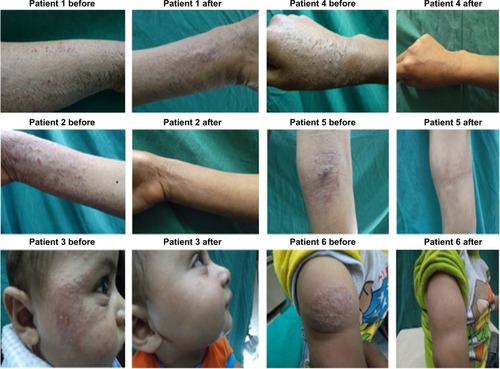
Photography
shows the photographs of the lesions of some patients before and 3 months after starting treatment. It was clear that using silymarin-based PLO gel significantly improved AD in terms of redness, swelling, lichenification, and overall severity, as compared to placebo. Moreover, there was a positive improvement effect on the control side using free drug PLO gel in some patients.
Patient follow-up
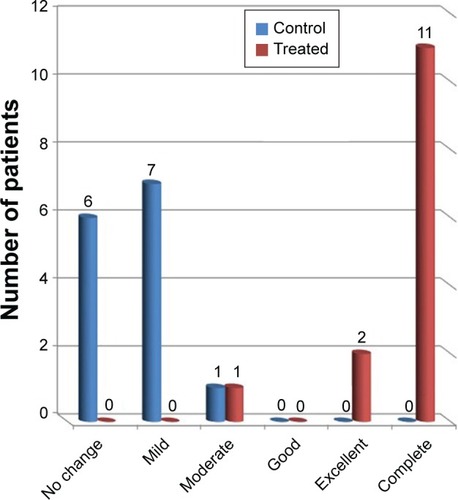
Patients’ signs and symptoms regarding itching were assessed using an improvement index (SCORAD) ().
shows the comparison between control side and treated side regarding itching symptoms. As shown for the treated side, moderate, complete, and excellent improvement in itching was observed in 7.1%, 78.5%, and 14.2% of patients, respectively. On the other hand, positive improvement effects were observed in 57.1% ranging from mild (50%) to moderate effects (7.1%) after using drug-free PLO gel, while no change was observed in other patients (42.8%). The difference between the two sides was statistically significant (P=0.001).
Histological study
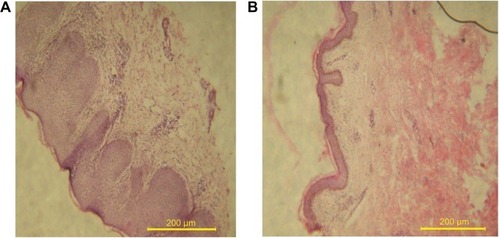
Microscopic examination of skin biopsies of AD patients before () and after treatment () with silymarin loaded PLO gel are shown in . AD patients’ skin histology was characterized by thickness of the epithelial layer of the skin, presence of vacuoles or holes, and increase in the inflammatory cells in the dermis layer (), indicating skin lichenification and inflammation of the skin due to itching. On the contrary, the treated patients’ skin showed a decrease in the thickness of the epidermis and a decrease in the inflammatory cells infiltrating the dermis layer (). These improvements can be explained according to the results obtained from a previous animal study which indicated that silymarin induced anti-oxidative, anti-inflammatory, immune modulating actions, and has been shown to support dermal regeneration.Citation29–Citation31 It can further be stated that the effectiveness of the topical formulation of silymarin-based PLO gel results from enhanced drug molecules’ penetration despite skin lichenification. Local occlusive factor, warmth, and hydration produced by the PLO gel base are among the factors that can increase silymarin skin penetration.
Figure 7 Patient’s skin histology.
Abbreviation: PLO, pluronic-lecithin organogel.
Some patients in the present study who received a silymarin-free base showed mild, moderate, and complete improvement in AD manifestation. This could be explained by the fact that first line treatment of AD is hydration of skin by using an emollient, and this hydration effect was provided by the base. These results were consistent with a previous study which reported that a PLO gel base provided desired hydration to the skin, thus was effective in the improvement of eczema or psoriasis.Citation32,Citation33 Therefore, the PLO base could be considered as an adjuvant therapy for AD patients.
Becker-Schiebe et al used silymarin-based cream (Leviaderm®) for the treatment of radiodermatitis patients.Citation19 However, cream dosage form is the most suitable vehicle for the moist and subacute weeping lesions. Moreover, emulsifiers must be added to stabilize cream, thus having the potential to sensitize and cause allergic reactions. On the other hand, dry, fissured, and lichenified skin diseases need an occlusive base due to its moisturizing effect.
Conclusion
Silymarin loaded PLO gel was successfully prepared. The high penetrating ability and hydration effect of the base provided a significant improvement in the signs and symptoms of AD patients.
Therefore, it could be concluded that silymarin loaded PLO gel may be introduced as a novel topical formulation of silymarin.
Disclosure
The authors have no conflicts of interest to disclose in this work.
References
- DarsowURaapUStänderSRCarstensEAkiyamaTItch: Mechanisms and TreatmentBoca RatonCRC Press2014832836
- RingJPrzybillaBRuzickaTHandbook of atopic eczema2nd edNew YorkSpringer-Verlag Berlin Heidelberg2006
- FriedmmanPSHoldenCAAtopic dermatitisBurnsTBreathnachSCoxNGriffithsCRook’s Textbook of Dermatology7th edOxford, EnglandBlackwell Sciences2008755786
- CharmanCClinical evidence: atopic eczemaBMJ199931871981600160410364122
- SudilovskyAMuirJGBocoboFCA comparison of single and multiple applications of halcinonide creamInt J Dermatol19812096096137030988
- DarsowULübbeJTaïebAPosition paper on diagnosis and treatment of atopic dermatitisJ Eur Acad Dermatol Venereol200519328629515857453
- HaeckIMRouwenTJMikLTBruin-WellerMSBruijnzeel-KoomenCATopical corticosteroids in atopic dermatitis and the risk of glaucoma and cataractsJ Am Acad Dermatol201164227528121122943
- HajarTLeshemYAHanifinJMA systematic review of topical corticosteroid withdrawal (“steroid addiction”) in patients with atopic dermatitis and other dermatosesJ Am Acad Dermatol2015723541e549e.e225592622
- O’HaraMKieferDFarrellKKemperKA review of 12 commonly used medicinal herbsArch Fam Med1998765235369821826
- ZhaoJLahiri-ChatterjeeMSharmaYAgarwalRInhibitory effect of a flavonoid antioxidant silymarin on benzoyl peroxide-induced tumor promotion, oxidative stress and inflammatory responses in SENCAR mouse skinCarcinogenesis200021481181610753220
- GazakRWalterovaDKrenVSilybin and silymarin – new and emerging applications in medicineCurr Med Chem200714331533817305535
- SinghRPAgarwalRCosmeceuticals and SilibininClin Dermatol200927547948419695480
- DehmlowCErhardJde GrootHInhibition of Kupffer cell functions as an explanation for the hepatoprotective properties of silibininHepatology19962347497548666328
- RasulANaveedAAliAAssessment of anti erythmic and skin whitening effects of milk thistle extractAfrican Journal of Pharmacy and Pharmacology20115202023062309
- SvobodováAWalterováDPsotováJInfluence of silymarin and its flavonolignans on H(2)O(2)-induced oxidative stress in human keratinocytes and mouse fibroblastsBurns200632897397917011711
- TokluHZTunalı-AkbayTErkanlGYukselMErcanFSenerGSilymarin, the antioxidant component of Silybum marianum, protects against burn-induced oxidative skin injuryBurns200733790891617521818
- Ashkani-EsfahaniSEmamiYEsmaeilzadehESilymarin enhanced fibroblast proliferation and tissue regeneration in full thickness skin wounds in rat models; a stereological studyJournal of the Saudi Society of Dermatology & Dermatologic Surgery2013171712
- HanMHYoonWKLeeHTopical application of silymarin reduces chemical-induced irritant contact dermatitis in BALB/c miceInt Immunopharmacol20077131651167817996674
- Becker-SchiebeMMengsUSchaeferMBulittaMHoffmannWTopical use of a silymarin-based preparation to prevent radiodermatitis: results of a prospective study in breast cancer patientsStrahlenther Onkol2011187848549121786113
- GilletALecomteFHubertPDucatEEvrardBPielGSkin penetration behaviour of liposomes as a function of their compositionEur J Pharm and Biopharm2011791435321272638
- JosefEZilbermanMBianco-PeledHComposite alginate hydrogels: An innovative approach for the controlled release of hydrophobic drugsActa Biomater20106124642464920601237
- SpadaGGaviniECossuMRassuGCartaAGiunchediPEvaluation of the effect of hydroxypropyl-β-cyclodextrin on topical administration of milk thistle extractCarbohydr Polym2013921404723218263
- KumarRKatareOPLecithin organogels as a potential phospholipid structured system for topical drug delivery: a reviewAAPS Pharm-SciTech200562E298E310
- ParsaeeSSarboloukiMNParniapourMIn-vitro release of diclofenac dethylammnium from lipid-based formulationsInt J Pharm2002241118519012086734
- PeinzesTCsoikaIErosIRheological analysis of the structural properties effecting the percutaneous absorption and stability in pharmaceutical organogelsRheol Acta200443457463
- BalataGEl NahasHMRadwanSPropolis organogel as a novel topical delivery system for treating woundsDrug Deliv2014211556124295500
- SurjyanarayanMSnigdhaSMKrutikaKSLecithin stabilized organogel: design and development for topical application of clobetasol propionateInt J Pharm Tech Res20102211331138
- StationwalaRPatidarAMainPTransdermal delivery of Iornoxicam from pluronic lecithin organogelInt J Chem Pharm Sci2011223237
- PiscitelliSCFormentiniEBursteinAHAlfaroRJagannathaSFalloonJEffect of milk thistle on the pharmacokinetics of indinavir in healthy volunteersPharmacotherapy200222555155612013352
- JohnsonVJHeQOsuchowskiMFSharmaRPPhysiological responses of a natural antioxidant flavonoid mixture, silymarin, in BALB/c mice: III. Silymarin inhibits T-lymphocyte function at low doses but stimulates inflammatory processes at high dosesPlanta Med2003691444912567278
- KangJSYoonWKHanMHInhibition of atopic dermatitis by topical application of silymarin in NC/Nga miceInt Immunopharmacol20088101475148018593606
- AlmeidaHAmaralMHLobaoPLoboJMPluronic® F-127 and Pluronic Lecithin Organogel (PLO): main features and their applications in topical and transdermal administration of drugsJ Pharm Pharm Sci201215459260523106961
- BhatiaASinghBRazaKWadhwaSKatareOTamoxifen-loaded lecithin organogel (LO) for topical application: Development, optimization and characterizationInt J Pharm20134441–2475923353077