Abstract
Clausena excavata is a natural herb with both antioxidant and anti-inflammatory properties. It has been used for decades in folkloric practice for the amelioration of various ailments. In this study, the gastroprotective activity of methanolic extract of C. excavata leaves (MECE) was determined in the Sprague Dawley rat ethanol-induced gastric ulcer model. Rats were pretreated with a single dose of vehicle (5% Tween 20), 20 mg/mL omeprazole, 400 and 200 mg/mL of MECE dissolved in 5% Tween 20. Ulcer was induced with 5 mL/kg of ethanol and stomach tissue was obtained after 1 hour. Histological examination was done on hematoxylin and eosin, periodic acid-Schiff, and immunochemically stained gastric mucosal tissues. Prostaglandin E2, superoxide dismutase, catalase, glutathione peroxidase, and lipid peroxidation levels of the gastric tissue homogenates were also determined. Significantly (P<0.05) smaller ulcer areas, less intense edema, and fewer leukocytes’ infiltration were observed in MECE- and omeprazole-treated than in untreated gastric mucosa with ulcer. The gastric pH, mucus production, superoxide dismutase, catalase, and glutathione peroxidase contents increased, while the lipid peroxidation content decreased as a result of MECE treatment. Bcl-2-associated X protein was underexpressed, while heat shock protein 70 and transforming growth factor-beta protein were overexpressed in the ulcerated gastric mucosa tissues treated with omeprazole and MECE. Similarly, there was a reduction in the levels of tumor necrotic factor-alpha and interleukin-6, while the level of interleukin-10 was increased. This study showed that the gastroprotective effect of MECE is achieved through inhibition of gastric juice secretion and ulcer lesion development, stimulation of mucus secretion, elevation of gastric pH, reduction of reactive oxygen species production, inhibition of apoptosis in the gastric mucosa, and modulation of inflammatory cytokines.
Introduction
Peptic ulcer disease (PUD), a protracted and multifactorial disease, is characterized by the presence of ulcerated lesions in the gastric or duodenal mucosa. PUD occurs in approximately 20% of the world population.Citation1 Development and progression of PUD is governed by the acidity of the stomach’s gastric juice. Thus, modulation of the stomach acidity is fundamental to the prevention and healing of gastric ulcers.Citation2 Although several antiulcer medications such as H2 receptor antagonists (ranitidine, famotidine), antacid, and proton-pump inhibitors (omeprazole) are readily available for the treatment of ulcers, these therapeutic agents have side effects that include impaired calcium, iron, magnesium, and vitamin B12 absorption in the intestine. Hence, there is a great need to develop more efficacious drugs for the treatment of the disease.Citation3
Ethanol has been shown to induce oxidative stress that manifests as an increase in malondialdehyde and decrease in catalase (CAT) and glutathione activities. Oral application of ethanol causes gastric mucosal damage with hemorrhage and ulceration. This deleterious effect of ethanol is often used in laboratory animals to develop gastric ulcer models.Citation4–Citation6
The antiulcerogenic effects of many natural compounds have been attributed to their antioxidant properties and regulation of oxidative stress enzymes and protein expression. The leaves of Clausena excavata (Rutaceae) are traditionally used in the treatment of malaria, stomachache, abdominal pain, cold, headache, pulmonary tuberculosis, wound, dysentery, snakebite, poisoning, and diarrhea.Citation5,Citation6 C. excavata has also been shown to have antioxidant, immune-modulatory, analgesic, anti-inflammatory, antiviral, anticancer, antioxidant, antimycobacterial, and antifungal properties.Citation7–Citation11 However, there is no report on the antiulcer effect of the plant. Thus, this study was undertaken to investigate the prophylactic potential of methanolic extract of C. excavata leaf extract in a rat ulcer model.
Materials and methods
Preparation of C. excavata extract
The C. excavata plant was obtained from Pendang, Kedah (5°59′N, 100°28′E), Malaysia, and identified and authenticated by Dr Shamsul Khamis, a resident botanist at the Biodiversity Unit, Institute of Bioscience, Universiti Putra Malaysia, Malaysia. Plant samples are naturally grown in the fields and are not classified as endangered species. Therefore, no specific permission was required for sample collection. Fresh C. excavata leaves were cleaned, shade-dried for 2 weeks, powdered, and soaked in methanol for 3 days. The solvent was removed by rotary evaporation and the extract stored at 4°C. Methanolic extract of C. excavata (MECE) leaves was chosen for the study because it was shown to have significant antioxidant activities.Citation8
In vitro assay
Cell maintenance
J774A.1 macrophage cell line (American Type Culture Collection [ATCC], Manassas, VA, USA) was cultured in Dulbecco’s Modified Eagle’s Medium, supplemented with 10% fetal bovine serum, and incubated at 37°C in a 5% CO2 humidified incubator. Cells that reached 80% confluence were detached from the culture flasks by addition of trypsin-ethylenediaminetetraacetic acid (EDTA), centrifuged at 2,000× g for 10 minutes, stained with trypan blue, and counted in a Neubauer chamber.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
Stock MECE was dissolved in 0.1% dimethyl sulfoxide. The J774A.1 cells were seeded at a density of 5×104 cells/well/100 μL in a 96-well plate, treated with 25, 50, 100, 200, and 400 μg/mL MECE in Dulbecco’s Modified Eagle’s Medium with 2% fetal bovine serum, and incubated at 37°C under 5% CO2 for 24 hours. Cell proliferation was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Twenty microliters of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide reagent (5 mg/mL) was added to each well and the plate incubated for 3 hours. The purple formazan formed was solubilized with 100 μL dimethyl sulfoxide. The plate was swirled gently to mix and kept in the dark at room temperature for approximately 20 minutes. The absorbance was determined using a microplate reader (Tecan, Grödig, Austria) at 570 nm with reference at 630 nm. Each concentration was tested in triplicate.
Animals
Fifty-four disease-free, adult male Sprague Dawley rats (weighing 220–240 g) aged 6 weeks were purchased from the Animal Resource Centre, Faculty of Veterinary Medicine, Universiti Putra Malaysia. The rats were housed in groups of three per cage and allowed to acclimatize, with free access to commercial feed and water for 1 week prior to experimentation. The experiment was conducted under a constant ambient temperature of 22°C and 12-hour light/dark cycle.
Ethics statement
This study was conducted in strict compliance with the guidelines set by the Institutional Animal Care and Use Committee (IACUC), University of Malaya. All experimental studies conducted were approved by the institutional IACUC with approval no: ISN/22/007/2013/1111/SFA. The animals were handled and treated humanely, according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals.Citation12
Selection of dose of extract
Determination of acute toxicity of C. excavata was done in our earlier study as per the guidelines of the Organization for Economic Cooperation and Development using MECE at doses of 2,000 and 5,000 mg/kg body weight in both male and female rats.Citation8 The rats were observed for 48 hours for development of signs of pain, distress, or mortality and euthanized under CO2 at day14 posttreatment. Based on that study, we adopted the doses of 200 and 400 mg/kg body weight for use in this study.
Antisecretory effect
The antisecretory effect of MECE was determined in rats according to the method of ShayCitation13 with slight modifications. Briefly, 24 rats, assigned to four equal groups, were fasted for 24 hours with free access to water. The rats were then pretreated once by oral gavage as follows:
Group 1: 5% Tween 20 v/v (negative control)
Group 2: 20 mg/kg body weight omeprazole (positive control)
Group 3: 200 mg/kg body weight MECE dissolved in 5% Tween 20 v/v
Group 4: 400 mg/kg body weight MECE dissolved in 5% Tween 20 v/v.
After 1 hour, the rats were anesthetized through intramuscular route with a combination of ketamine (50 mg/kg) and xylazine (5 mg/kg). The abdomen was opened by making a small midline incision below the xiphoid process. The pyloric portion of the stomach was slightly lifted and ligated, avoiding traction to the pylorus or damage to blood vessels. The stomach was then carefully put back into the abdominal cavity and the abdominal wall was sutured. After 4 hours, all rats were euthanized under CO2, their stomachs immediately removed, and the volume and pH of gastric content determined. Total acidity of the gastric content was determined by titrating with 0.01 N sodium hydroxide (NaOH) solution using phenolphthalein as an indicator, and calculated using the formula: total acidity (mEq/L) = volume of NaOH solution ×0.01×36.45×1,000.Citation13,Citation14
Rat gastric ulcer model
Thirty male rats were randomly divided into five groups of six rats each in wire-bottomed cages, fasted for 24 hours, and deprived of water for 2 hours. The rats were orally pretreated as follows:
Group 1: vehicle (5% Tween 20% v/v, 5 mL/kg body weight) (normal control)
Group 2: absolute ethanol (5 mL/kg body weight) (ulcer control)
Group 3: 20 mg/kg body weight omeprazole in 5% Tween 20 v/v (positive control)
Group 4: 200 mg/kg body weight of MECE dissolved in 5% Tween 20 v/v
Group 5: 400 mg/kg body weight of MECE dissolved in 5% Tween 20 v/v.
One hour after pretreatment, all rats, except the normal controls, were orally gavaged with 5 mL/kg body weight absolute ethanol, anesthetized by intramuscular route with a combination of ketamine (50 mg/kg) and xylazine (5 mg/kg), and their stomachs immediately removed.Citation15,Citation16
Evaluation of gross lesions on the gastric mucosa
Gastric ulcers appeared as elongated bands of hemorrhagic lesions on the stomach mucosal surface. The ulcer area was determined by counting 2×2 mm2 areas under a grid-fitted dissecting microscope at ×1.8 magnification. The total lesion area for each stomach was calculated from the following formula: ulcer area (mm2) = (sum of 2×2 mm2 squares counted) ×4×1.8. The inhibition percentage was then calculated as described earlier.Citation17,Citation18
Gastric mucus content and juice acidity
The gastric mucosa was gently scrapped using a glass slide to obtain mucus and gastric mucosal tissue, which were weighed. Gastric juice was collected and centrifuged at 4,000× g, at room temperature for 10 minutes. The acidity of the supernatant was determined using a digital pH meter.
Histopathology and lesion evaluation
A small piece of the ulcerated gastric mucosal tissue from each group was fixed with 10% buffered formalin solution and dehydrated with alcohol and finally embedded in paraffin wax. Tissue sections of 5 μm were cut and stained with hematoxylin and eosin for light microscopic examination.Citation19 Lesions such as hemorrhage, submucosal edema, epithelial erosion, and inflammatory cell infiltrates were determined in one unit of microscopic focus field, equivalent to 2×2 mm2 area of the tissue having a lesion. Tissue sections were also stained with periodic acid-Schiff stain for evaluation of mucus production.Citation20
Immunohistochemical staining for Bax, heat shock protein 70, and transforming growth factor-beta
Tissue sections were deparaffinized for 15 minutes at 58°C before immersing in decreasing concentrations of 100%, 80%, 70%, and 50% alcohol for 5 minutes each, followed by hydration in phosphate-buffered saline (PBS) twice for 5 minutes. The sections were then heated in 10 mM tris buffer (pH 9.0) for 15 minutes in order to retrieve the antigens. The slides were allowed to cool to room temperature and endogenous peroxidase was blocked by incubating the sections in 3% H2O2 for 10 minutes at room temperature. The slides were then washed twice with PBS and incubated with rabbit primary anti-heat shock protein (anti-HSP70), anti-Bcl-2-associated X protein (anti-Bax), or transforming growth factor-beta (TGF-β) antibodies (Abcam, Cambridge, UK) at dilutions of 1:500, 1:200, and 1:800, respectively. The slides were incubated in a humidified dark chamber at room temperature for 30 minutes before immersion in 0.1% Tween 20 dissolved in PBS twice for 5 minutes each. Horseradish peroxidase-conjugated secondary antibodies (Envision Systems, South Melbourne, Australia) were added to the sections and the slides were incubated at room temperature for 30 minutes. Furthermore, the sections were incubated with a substrate, 3,3′-diaminobenzidine tetrahydrochloride (Dako, Carpintaria, CA, USA), allowed to stand at room temperature for 5 minutes, counterstained with Harris hematoxylin, dehydrated with 50%, 70%, 80%, and 100% alcohol, and finally cleared in xylene. Air-dried sections were mounted with DPX mountant for light microscopic examination.
Evaluation of immunopositive gastric tissue sections
Brown-colored cells, signifying positive staining, were selected and estimated as a proportion of the total focal slide area using the ImageJ software (National Institutes of Health, Bethesda, MD, USA) (http://imagej.nih.gov/ij/). Six microscopic focal fields at ×200 magnification were captured from each slide and used for the ImageJ analysis. Briefly, images were opened with the ImageJ software using the file menu and RGB stack was created from the image menu. An image montage was created from the image stack and the best montage was used to adjust the threshold in order to select the most precise stained areas. Measurement parameters such as area, mean, standard deviation, area fraction, limit to threshold, and display label were selected and measurement values were obtained. Each replicate from the same group was evaluated and mean ± standard error of the mean of the stained areas (μm2) determined.Citation21
Antioxidant activity
Superoxide dismutase assay
Gastric mucosal tissues in 20 mM 4-(2-hydroxyethyl)-1-piper-azineethanesulfonic acid (HEPES) buffer (pH 7.2), containing 1 mM EDTA, 210 mM mannitol, and 70 mM sucrose, were homogenized on ice and centrifuged at 1,500× g, at 4°C for 10 minutes. The supernatant was harvested and used for the determination of superoxide dismutase (SOD) activity using the Cayman SOD assay kit (Cayman, Ann Arbor, MI, USA).
Catalase activity assay
Gastric mucosal tissues in cold 50 mM potassium phosphate buffer (pH 7.0), containing 1 mM EDTA, were homogenized on ice and centrifuged at 10,000× g, at 4°C for 15 minutes. The supernatant was harvested and used for the determination of CAT activity using the Cayman CAT assay kit (Cayman).
Glutathione peroxidase assay
Gastric mucosal tissues in 50 mM Tris-HCl (pH 7.5), containing 5 mM EDTA and 1 mM dithiothreitol, were homogenized and centrifuged at 10,000× g, at 4°C for 15 minutes. The supernatant was harvested and used for the determination of glutathione peroxidase (GPx) activity using the Cayman GPx assay kit (Cayman).
Lipid peroxidation assay
Gastric mucosal tissues were sonicated in PBS and chloroform. Hydroperoxide was extracted from the mixture using an extraction buffer. The mixture was centrifuged at 1,500× g, at 0°C for 10 minutes. The chloroform layer at the bottom of the tube was aspirated, mixed with a chromogenic reagent, and allowed to stand at room temperature for 5 minutes. Lipid peroxidation (LPO) activity was determined from the mixture using the LPO assay kit (Cayman).
Prostaglandin E2 assay
Gastric mucosal tissues were weighed and homogenized in PBS buffer at 4°C. The homogenates were then centrifuged at 13,400 ×g for 10 minutes and the supernatants used for the determination of prostaglandin E2 (PGE2) using the PGE2 Express Enzyme Immunoassay Kit (Cayman), according to the manufacturer’s instruction. The PGE2 concentrations were expressed as pg/mL.
Cytokine activity assay
Determination of the levels of gastric mucosal tumor necrosis factor alpha (TNF-α), interleukin (IL)-6, and IL-10 was done using the gastric mucosal homogenates. Briefly, tissue homogenate was centrifuged at 3,000× g for 10 minutes and the supernatant was used for the detection of cytokine levels using commercial enzyme-linked immunosorbent assay kits for TNF-α (http://www.cusabio.com/ELISA-Kit/Rat-TNF-%CE%B1-ELISA-kit-109331.html), IL-6 (http://www.cusabio.com/ELISA-Kit/Rat-Interleukin-6IL-6-ELISA-KIT-84597.html), and IL-10 (http://www.cusabio.com/ELISA-Kit/Rat-Interleukin-10IL-10-ELISA-KIT-84236.html) (Cusabio Biotech Co. Ltd, Cusabio Biotech Co Ltd, Hubei, People’s Republic of China).
Statistical analysis
All values are reported as mean ± standard error of the mean. Statistical differences between groups were determined by one-way analysis of variance using Kruskal–Wallis test for comparisons of histological and immunohistochemical data, while a one-way analysis of variance with Tukey’s post hoc test was employed for other parameters. GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA) was used for the analysis and significant differences between mean values were determined at P<0.05.
Results
In vitro cytotoxicity
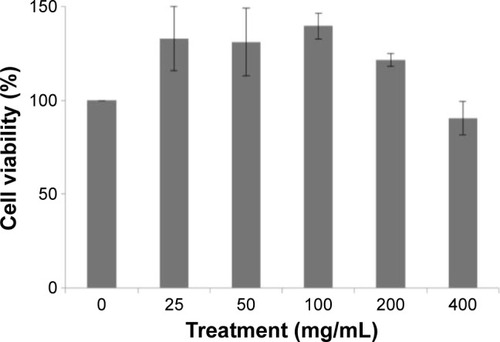
The cytotoxic effect of MECE on J774A.1 cells is shown in . At 200 and 400 μg/mL, MECE was not toxic to J774A.1 cells. This suggests that MECE is safe to be used in these cells.
Dosage selection
Since no adverse effects were observed following acute toxicity studies and the extract proved nontoxic to J774A.1 cells in vitro, MECE doses of 200 and 400 mg/kg body weight were chosen for the in vivo experiments.
Gastric acid secretion
The MECE and omeprazole pretreatment significantly (P<0.05) reduced the volume and total acidity of gastric juice at 4 hours posttreatment (). While the omeprazole group and MECE 200 mg/kg group had comparable volumes of gastric juice, the omeprazole group had a higher (P<0.05) gastric juice pH than MECE at 200 and 400 mg/kg. However, total acidity was comparable between the omeprazole group and MECE 200 mg/kg group, while it was lower (P<0.05) in the MECE 400 mg/kg group.
Table 1 Effect of methanolic Clausena excavata leaf extract treatment on gastric secretory effects of rats
Gastric mucosa
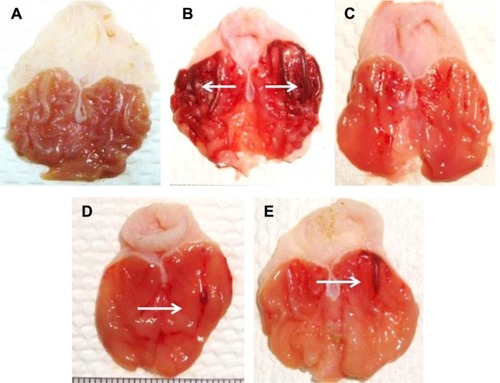
At 200 and 400 mg/kg body weight, MECE significantly (P<0.05) inhibited ulcer formation by 92.6% and 87.8%, respectively (; ). There was gross evidence of flattening of gastric mucosa folds and reduction in mucosal damage in the rats upon pretreatment with MECE and omeprazole. The result showed that MECE is protective toward the development of gastric ulcers.
Table 2 Effect of methanolic Clausena excavata leaf extract treatment on pH of gastric content, gastric ulcer area, and ulcer inhibition in rats
Figure 2 Protective effect of methanolic extract of Clausena excavata leaf on ethanol-induced gastric mucosal lesions in rats.
Gastric content, pH, and mucus weight
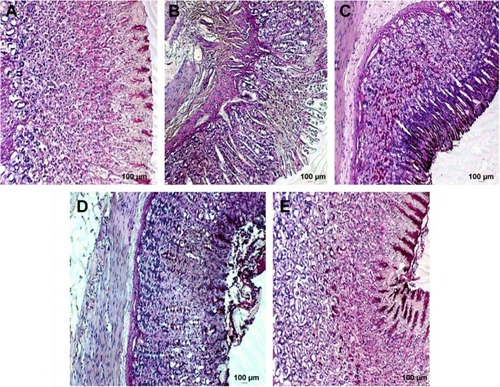
Periodic acid-Schiff–stained gastric mucosal tissues showing mucus production are presented in . Pretreatment with MECE and omeprazole increased mucus content of the gastric mucosa, with the effect being more pronounced (P<0.05) in 400 mg/kg body weight MECE than other treatments. The pH of the gastric content of rats treated with MECE was significantly (P<0.05) higher than that of the untreated rats with ulcer ().
Figure 3 Rat gastric tissue stained showing mucus production.
Gastric tissue lesion scoring
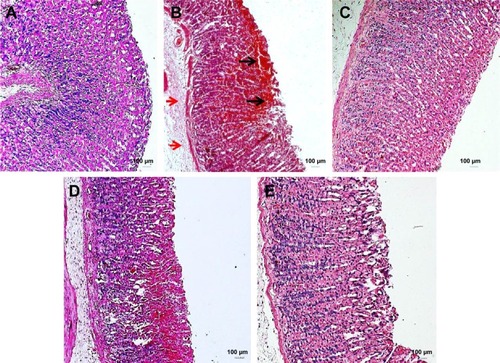
Histopathologically, rats with untreated ulcers showed extensive gastric lesions with submucosal edema and leukocyte infiltration. The ulcer control rats had the highest lesion scores among groups. Pretreatment with MECE significantly (P<0.05) reduced ulceration in the gastric mucosa, as evident by the decrease in ulcer area, reduction or complete absence of edema and leucocyte infiltration (; ). Rats treated with MECE 400 mg/kg showed the least distribution of lesions (P<0.05), when compared with the omeprazole and MECE 200 mg/kg groups, had comparable (P>0.05) lesion distribution.
Table 3 Gastric tissue lesion score in rat gastric tissue with ulcer treated with methanolic Clausena excavata leaf extract
Figure 4 Untreated gastric ulcer with extensive hemorrhages (black arrows), and sub-mucosal oedema (red arrows).
Abbreviation: H&E, hematoxylin and eosin.
Gastric mucus
MECE and omeprazole pretreatments resulted in significantly (P<0.05) enhanced mucus secretion over the entire lining of rat gastric mucosa with ulcer. However, untreated ulcers did not show evidence of mucus secretion on the gastric mucosa ().
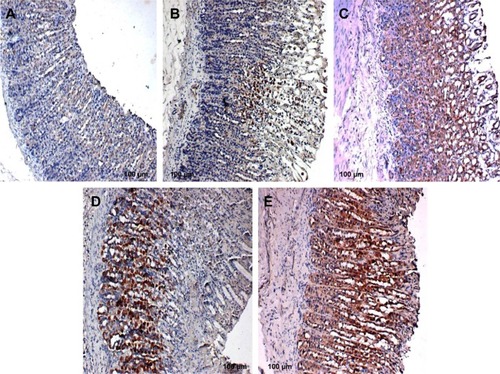
Bax, HSP70, and TGF-β expression
Unlike in untreated ulcerated gastric mucosa which showed upregulation of Bax protein, the MECE-treated mucosa showed a downregulation of this protein (; ). On the other hand, the HSP70 expression was upregulated in both omeprazole- and MECE-treated groups. The effect of MECE on HSP70 expression was dose-dependent, with greater expression at the higher dose of the extract (; ). TGF-B was upregulated in all treatment groups, but higher (P<0.05) in the omeprazole group. (, ).
Table 4 Area of rat gastric mucosal tissue positive for Bax protein staining after treatment with methanolic Clausena excavata leaf extract
Table 5 Area of rat gastric mucosal tissue positive for HSP70 protein immunoperoxidase staining after treatment with methanolic Clausena excavata leaf extract
Table 6 Area of rat gastric mucosal tissue positive for TGF-β protein immunoperoxidase staining after treatment with methanolic Clausena excavata leaf extract
Figure 5 Rat gastric tissues showing Bax protein expression.
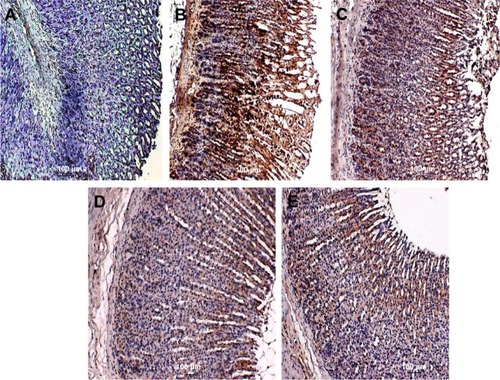
Figure 6 Rat gastric tissue showing HSP70 expression.
Abbreviation: HSP70, heat shock protein 70.
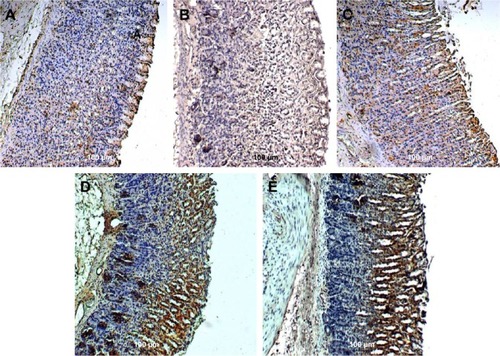
Figure 7 Rat gastric tissues showing TGF-β protein expression.
Abbreviation: TGF-β, transforming growth factor-beta.
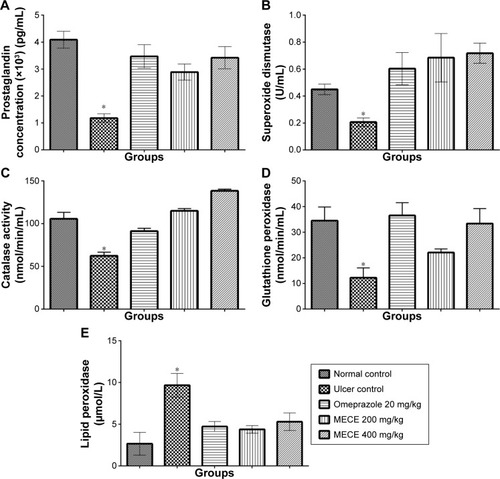
PGE2 and antioxidant activity
As shown in , the SOD, CAT, and GPx activities and PGE2 concentration were significantly (P<0.05) higher in the ulcerated gastric tissue treated with MECE and omeprazole than in the untreated tissue. In contrast, the extract produced a significantly (P<0.05) lower LPO activity in all pretreated than untreated gastric tissues with ulcer. The results show that the antioxidant activity exhibited by MECE in ulcerated gastric tissue is greater than that of tissues pretreated with omeprazole.
Figure 8 Expression of prostaglandin and antioxidant enzyme parameters in rat gastric tissues with ulcer after Clausena excavata leaf extract treatment.
Abbreviations: Bwt, body weight; LPO, lipid peroxidation; MECE, methanolic extract of C. excavata leaves.
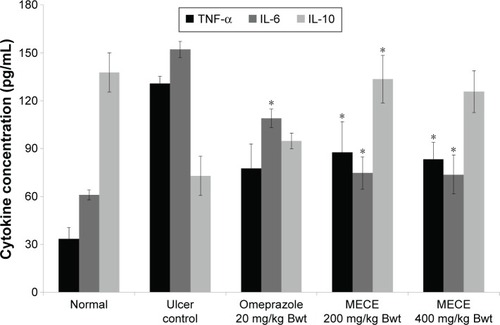
TNF-α, IL-6, and IL-10 enzyme activities
MECE demonstrated an immune-modulatory effect in the gastric tissue by reducing the levels of TNF-α and IL-6, while the level of IL-10 was increased ().
Figure 9 Effect of MECE on TNF-α, IL-6, and IL-10 levels in gastric tissue of ethanol-induced ulcerated rats.
Abbreviations: Bwt, body weight; IL, interleukin; MECE, methanolic extract of Clausena excavata leaves; SEM, standard error of the mean; TNF-α, tumor necrosis factor alpha.
Discussion
Gastric ulcers are caused by an imbalance between the intrinsic protective mechanism and disease-causing endogenous and exogenous factors in the environment of the stomach.Citation22 Gastritis and gastric ulcers can be effectively treated with several drugs available in the market. However, there are several natural plant products purported to be also effective in the treatment of gastric ulcers, among which are products from the C. excavata plant. The advantage of using natural products is that they have fewer side effects than the commercial drugs. Currently, the antiulcer property of C. excavata is yet to be corroborated.
In our recent study, MECE was found to have significant antioxidant activities, which was attributed to its rich phenolic/flavonoid content.Citation8 The MECE was also found to be nontoxic to the J774A.1 macrophage cell line, even at high doses. Since macrophages produce macrophage colony-stimulating factor, which plays a significant role in healing of gastric ulcers through promotion of angiogenesis, treatment of gastric ulcers with MECE should not inhibit healing.Citation23
Histopathological evaluation showed that MECE inhibits leukocyte infiltration, hemorrhage, edema, and epithelial cell loss in rat gastric tissues with ethanol-induced gastric ulcer. These changes suggest that the extract reduces development of pathological lesions associated with gastric ulcers, a finding that concurs with those of earlier studies. In fact, the healing and lesion-inhibition effects of MECE were suggested to be through its antioxidant and anti-inflammatory activities.Citation8,Citation24,Citation25
Current therapeutic strategies in peptic ulcer treatment are aimed at either suppression of gastric acid secretion or enhancement of effect of gastroprotective factors.Citation26 To investigate the gastroprotective effect of MECE, this study used the rat pylorus ligature model. Ligation of the pylorus causes stasis of gastric acid resulting in gastric ulcer development.Citation27 Using this model, MECE was found to reduce the volume and total acidity of the gastric juice, suggesting that the extract inhibited production and secretion of gastric juice.
Ethanol causes development of necrotic lesions in the gastric mucosa by decreasing mucus and bicarbonate and increasing hydroperoxide and superoxide anion production, thereby inducing gastric ulcer when given orally to the rat.Citation27,Citation28 Using the ethanol-induced rat gastric ulcer model, MECE was found to protect the gastric mucosa from development of ulcers; this effect may be attributable to the free radical scavenging properties of the extract.Citation8,Citation29 Reactive oxygen species (ROS) are generated at a low rate in normal cellular metabolism and these oxidative radicals are scavenged by the antioxidant defense system of the body, namely, SOD, GPx, and CAT. Since the accumulation of ROS increases LPO, the LPO content in the gastric mucosa can be used as a biomarker for development of ROS-mediated gastric mucosal lesions.Citation30–Citation32 In our study, MECE increased SOD, GPx, and CAT and decreased LPO contents in the gastric mucosa, further confirming that the extract has gastroprotective properties against the development of ethanol-induced ulcers. Other plant extracts have been shown to produce similar antioxidant effects in ethanol-induced gastric ulcer models by decreasing LPO and increasing CAT, SOD and GPX activities.Citation4,Citation5,Citation33
The gastric mucosa serves as a physical barrier against external and internal ulcerative agents through several mechanisms of protection.Citation34 One of these mechanisms is the production of mucus lining the mucosa. Decreased mucus and acid production by the stomach are among the factors contributing to the development of gastric ulcers and inhibition of healing.Citation2 In our study, MECE was found to increase the production of gastric mucus as well as the pH of gastric juice, which prevented the development of gastric ulcers. Prostaglandins control gastric acid secretion and enhance gastric mucus and bicarbonate production, thus playing a significant protective role by conferring the gastric mucosa the ability to resist injury caused by noxious compounds.Citation35,Citation36 The ulcerated gastric mucosa showed a higher level of PGE2 after MECE treatment than in untreated rats. This suggests that enhancement of PGE2 activity is another mechanism through which MECE reduces ulceration in the gastric mucosa. Thus, it can be said that the gastroprotective effect of MECE may be partly due to the coumarin in the extract, which stimulates gastric prostaglandin and mucus production.Citation8,Citation37
The susceptibility of cells to apoptosis depends on the balance between apoptosis-promoting and apoptosis-suppressing factors.Citation38 One of the mechanisms in gastric ulceration is induction of apoptosis of the gastric mucosal cells.Citation39 Thus, another means of inhibition of gastric ulceration is by the prevention of premature mucosal cell apoptosis.
Previous studies showed that the Bax protein, a proapoptotic protein, was upregulated in untreated rats with induced gastric ulcer.Citation40–Citation42 The MECE caused a downregulation of Bax protein in the rat gastric ulcer. This suggests that MECE inhibits premature cell death in the gastric mucosa, an effect that is strongly associated with the gastroprotective property of the extract. HSP70 protein from the heat shock protein family is ubiquitously present in mammalian cells and this protein serves to defend cells from oxidative stress damage. In the cell, HSP70 preserves the functional structure of normal proteins while repairing or removing damaged proteins. In the gastric mucosa, ROS generation by ethanol impedes expression of HSP70, making the mucosal layer susceptible to ulcerative damage.Citation43 However, treatment with MECE increased the expression of HSP70 in the gastric mucosa with ethanol-induced ulcer, which suggests it to be one of the many facets associated with the gastroprotective properties of MECE. Similar findings have been reported by other authors using different compounds and plant extracts.Citation44–Citation47 TGF-β is one of the most important growth factors that have a healing effect on gastric ulcer through induction of cell migration and increased vascular proliferation, thereby enhancing deposition of extracellular matrix. In this study, we observed an increased expression of TGF-β in the omeprazole-treated and MECE-treated groups. These findings were similar to those reported following administration of chalcone 1-(4-hydroxy-phenyl)-3-m-tolyl-propenone (HPTP) to rats having indomethacin-induced ulcer.Citation48 The immunoreactivity effect of TGF-β enhances the proliferation of epithelial cells in gastric glands.Citation49,Citation50
It has been shown by previous researchers that ethanol induces an inflammatory response that initiates a dynamic chain of immune responses associated with the release of vast amounts of inflammatory cytokines such as TNF-α and IL-6, which in turn produce increased quantity of ROS.Citation51 TNF-α, being a major proinflammatory cytokine produced by macrophages, attracts neutrophils to the site of gastric mucosal injury.Citation52,Citation53 IL-6 is another important proinflammatory cytokine shown to mediate immune response and acute inflammation. IL-6 activates granulocytes and agranulocytes, which in turn trigger a stress response in injured tissue.Citation54,Citation55 Sabat et alCitation55 suggested that IL-10 has the ability to suppress inflammatory response and inhibit TNF-α production. Previous reports showed that ethanol was able to increase proinflammatory cytokines and decrease anti-inflammatory cytokines in gastric tissue.Citation56,Citation57 Our results were in agreement with these observations, where exposure to ethanol showed elevated TNF-α and IL-6 and decrease in IL-10 levels, compared to normal controls. However, MECE pretreatment inhibited depletion of IL-10 and elevation of TNF-α and IL-6 levels, which shows its anti-inflammatory effect on ethanol-induced gastric ulcer in the rat. This also concurs with the earlier histological finding where less inflammatory responses were observed in MECE-treated rats with gastric ulcer.
Conclusion
This study shows the potential antiulcerogenic effects of MECE against ulcerative damage caused by ethanol. Furthermore, the present study shows that the gastroprotective properties of MECE may involve a number of mechanisms that include inhibition of gastric juice production, enhancement of gastric mucus secretion, increase in gastric juice pH, stimulation of antioxidant enzyme activities, downregulation of Bax, and upregulation of HSP70 and TGF-β protein expression in the gastric mucosa, as well as elevated TNF-α and IL-6 and decrease in IL-10 levels. Based on these findings, MECE can be further explored as a potential therapeutic compound for the treatment of gastric ulcers.
Authors contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
This study was financially supported by the University of Malaya PPP Grant (no PG059-2013A).
Disclosure
The authors report no conflicts of interest in this work.
References
- ObidikeICEmejeMOMicroencapsulation enhances the anti-ulcerogenic properties of Entada africana leaf extractJ Ethnopharmacol2011137155356121704690
- BrzozowskiTKonturekPCKonturekSJRole of gastric acid secretion in progression of acute gastric erosions induced by ischemia–reperfusion into gastric ulcersEur J Pharmacol2000398114715810856459
- KumarASinghVChaudharyAKGastric antisecretory and antiulcer activities of Cedrus deodara (Roxb.) Loud in Wistar ratsJ Ethnopharmacol2011134229429721182918
- AwaadASEl-MeligyRMSolimanGANatural products in treatment of ulcerative colitis and peptic ulcerJ Saud Chem Soc2013171101124
- RepettoMGLlesuySFAntioxidant properties of natural compounds used in popular medicine for gastric ulcersBraz J Med Biol Res200235552353412011936
- AdinorteyMBAnsahCGalyuonINyarkoAIn vivo models used for evaluation of potential antigastroduodenal ulcer agentsUlcers20132013796405
- ZhengXLWeiJHSunWLiRTLiuSBDaiHFEthnobotanical study on medicinal plants around Limu Mountains of Hainan Island, ChinaJ Ethnopharmacol2013148396497423751393
- AlbaayitSFAAbbaYAbdullahRAbdullahNEvaluation of antioxidant activity and acute toxicity of Clausena excavata leaves extractEvid Based Complement Alternat Med2014201497545025610488
- KumarRSahaASahaDA new antifungal coumarin from Clausena excavataFitoterapia201283123023322088496
- ManosroiASaraphanchotiwitthayaAManosroiJImmunomodulatory activities of fractions from hot aqueous extract of wood from Clausena excavataFitoterapia200475330230815158986
- RahmanMTAlimuzzamanMShilpiJAHossainMFAntinociceptive activity of Clausena excavata leavesFitoterapia200273770170312490234
- Academy of Science, USAGuide for the Care and Use of Laboratory Animals Available from: http://www.grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdfAccessed on January 24, 2016
- ShayHA simple method for the uniform production of gastric ulceration in the ratGastroenterology194554361
- TanPVNyasseBDimoTMezuiCGastric cytoprotective anti-ulcer effects of the leaf methanol extract of Ocimum suave (Lamiaceae) in ratsJ Ethnopharmacol2002822697412241979
- SrivastavaVViswanathaswamyAHMohanGDetermination of the antiulcer properties of sodium cromoglycate in pylorus-ligated albino ratsIndian J Pharmacol201042318518820871772
- PotrichFBAllemandADa SilvaLMAntiulcerogenic activity of hydroalcoholic extract of Achillea millefolium L.: involvement of the antioxidant systemJ Ethnopharmacol20101301859220420892
- SidahmedHMHashimNMAbdullaMAAntisecretory, gastroprotective, antioxidant and anti-Helicobacter pylori activity of zerumbone from Zingiber Zerumbet (L.) SmithPLoS One201510e012106025798602
- SalgaMSAliHMAbdullaMAAbdelwahabSIGastroprotective activity and mechanism of novel dichlorido-zinc (II)-4-(2-(5-methoxybenzylideneamino) ethyl) piperazin-1-iumphenolate complex on ethanol-induced gastric ulcerationChem Biol Interact2012195214415322178775
- Al-AminMSultanaGNNHossainCFAntiulcer principle from Zingiber montanumJ Ethnopharmacol20121411576022366683
- VaccaLLLaboratory Manual of HistochemistryNew York, NYRaven Press1985
- LunaLGManual of Histologic Staining Methods of the Armed Forces Institute of Pathology3rd edNew York, NYMcGraw-Hill1968
- CollinsTJImageJ for microscopyBiotechniques2007431 Suppl253017936939
- KawaharaYNakaseYIsomotoYRole of macrophage colony stimulating factor (M-CSF)-dependent macrophages in gastric ulcer healing in miceJ Physiol Pharmacol201162444122100845
- DemirSYilmazMKoseogluMAkalinNAslanDAydinARole of free radicals in peptic ulcer and gastritisTurk J Gastroenterol2003141394314593536
- AlbaayitSFAAbbaYAbdullahRAbdullahNEffect of Clausena excavata Burm. f (Rutaceae) leaf extract on wound healing and antioxidant activity in ratsDrug Des Devel Ther2015935073518
- MejiaAKraftWKAcid peptic diseases: pharmacological approach to treatmentExpert Rev Clin Pharmacol20092329531421822447
- SantinJRLemosMJúniorLCKNieroRde AndradeSFAntiulcer effects of Achyrocline satureioides (Lam.) DC (Asteraceae)(Marcela), a folk medicine plant, in different experimental modelsJ Ethnopharmacol2010130233433920546870
- GuptaMMazumderUKManikandanLBhattacharyaSSenthilkumarGPSureshRAnti-ulcer activity of ethanol extract of Terminaliapallida Brandis. in Swiss albino ratsJ Ethnopharmacol200597240540815707782
- UmamaheswariMAsokkumarKRathideviRSivashanmugamATSubhadradeviVRaviTKAntiulcer and in vitro antioxidant activities of Jasminum grandiflorum LJ Ethnopharmacol2007110346447017125945
- IshidaKKojimaRTsuboiMTsudaYItoMEffects of artichoke leaf extract on acute gastric mucosal injury in ratsBiol Pharm Bull201033222322920118544
- Hiruma-LimaCACalvoTRRodriguesCMAndradeFDPVilegasWBritoARMSAntiulcerogenic activity of Alchornea castaneaefolia: effects on somatostatin, gastrin and prostaglandinJ Ethnopharmacol2006104121522416253451
- TandonRKhannaRDDorababuMGoelRKOxidative stress and antioxidants status in peptic ulcer and gastric carcinomaIndian J Physiol Pharmacol200448111511815270379
- Alvarez-SuarezJMDekanskiDRisticSStrawberry polyphenols attenuate ethanol-induced gastric lesions in rats by activation of antioxidant enzymes and attenuation of MDA increasePLoS One2011610e2587822016781
- KuroseIHiguchiHMiuraSOxidative stress-mediated apoptosis of hepatocytes exposed to acute ethanol intoxicationHepatology19972523683789021949
- KwiecienSBrzozowskiTKonturekSJEffects of reactive oxygen species action on gastric mucosa in various models of mucosal injuryJ Physiol Pharmacol2002531395011939718
- Da SilvaLMAllemandAMendesDAGEthanolic extract of roots from Arctium lappa L. accelerates the healing of acetic acid-induced gastric ulcer in rats: Involvement of the antioxidant systemFood Chem Toxicol20135117918723036453
- ShineVLathaPShyamalSGastric antisecretory and anti-ulcer activities of Cyclea peltata (Lam.) Hook. f. & Thoms. in ratsJ Ethnopharmacol2009125235035519397987
- PihanGMajzoubiDHaudenschildCTrierJSSzaboSEarly microcirculatory stasis in acute gastric mucosal injury in the rat and prevention by 16, 16-dimethyl prostaglandin E2 or sodium thiosulfateGastroenterology1986916141514262945748
- GoelRKMaitiRNManickamMRayABAntiulcer activity of naturally occurring pyrano-coumarin and isocoumarins and their effect on prostanoid synthesis using human colonic mucosaIndian J Exp Biol19973510108010839475044
- RudinMThompsonBApoptosis and disease: regulation and clinical relevance of programmed cell deathAnnu Rev Med19974812672819046961
- KonturekPCBrzozowskiTKonturekSJApoptosis in gastric mucosa with stress-induced gastric ulcersJ Physiol Pharmacol199950221122510424718
- ChengEHWeiMCWeilerSBCL-2, BCL-X L sequesters BH3 domain-only molecules preventing BAX-and BAK-mediated mitochondrial apoptosisMol Cell20018370571111583631
- ChenSHLiangYCChaoJCProtective effects of Ginkgo biloba extract on the ethanol-induced gastric ulcer in ratsWorld J Gastroenterol200511243746375015968732
- ShichijoKIharaMMatsuuMItoMOkumuraYSekineIOverexpression of heat shock protein 70 in stomach of stress-induced gastric ulcer-resistant ratsDig Dis Sci20038234034812643613
- Al BatranRAl-BayatyFAbdullaMAGastroprotective effects of Corchorus olitorius leaf extract against ethanol-induced gastric mucosal hemorrhagic lesions in ratsJ Gastroenterol Hepatol20132881321132923611708
- Al BatranRAl-BayatyFJamil Al-ObaidiMMIn vivo antioxidant and antiulcer activity of Parkia speciose ethanolic leaf extract against ethanol-induced gastric ulcer in ratsPLoS One201385e6475123724090
- DhiyaaldeenSMAminZADarvishPHProtective effects of the chalcone 1-(4-hydroxy-phenyl)-3-m-tolyl-propenone against indomethacin-induced gastric erosive damage in ratsBMC Vet Res201410196125551777
- TanigawaTPaiRArakawaTHiguchiKTarnawskiASTGF-beta signaling pathway: its role in gastrointestinal pathophysiology and modulation of ulcer healingJ Physiol Pharmacol200556131315795471
- WalshMFAmpasalaDRHatfieldJTransforming growth factor-β stimulates intestinal epithelial focal adhesion kinase synthesis via Smad-and p38-dependent mechanismsAm J Pathol2008173238539918583311
- AntonisamyPDuraipandiyanVAravinthanAProtective effects of friedelin isolated from Azima tetracantha Lam. against ethanol-induced gastric ulcer in rats and possible underlying mechanismsEur J Pharmacol201575016717525617794
- WeiXMHeywoodGJDiGirolamoNThomasPSNicorandil inhibits the release of TNF-alpha from a lymphocyte cell line and peripheral blood lymphocytesInt Immunopharmacol200331581158814555283
- MartinGRWallaceJLGastrointestinal inflammation: a central component of mucosal defense and repairExp Biol Med2006231130137
- KishimotoTInterleukin-6: from basic science to medicine – 40 years in immunologyAnnu Rev Immunol20052312115771564
- MeiXXuDXuSZhengYXuSNovel role of Zn (II)-curcuminin enhancing cell proliferation and adjusting pro inflammatory cytokine-mediated oxidative damage of ethanol-induced acute gastric ulcersChem Biol Interact201219713922410117
- SabatRGrutzGWarszawskaKBiology of interleukin-10Cytokine Growth Factor Rev20102133134421115385
- ParkSWOhTYKimYSArtemisiaasiatica extracts protect against ethanol-induced injury in gastric mucosa of ratsJ Gastroenterol Hepatol20082397698418444990
- AntonisamyPSubash-BabuPAlshatwiAAGastroprotective effect of nymphayol isolated from Nymphaea stellata (Willd.) flowers: contribution of antioxidant, anti-inflammatory and anti-apoptotic activitiesChem Biol Interact201422415716325289771