Abstract
The first PIVET algorithm for individualized recombinant follicle stimulating hormone (rFSH) dosing in in vitro fertilization, reported in 2012, was based on age and antral follicle count grading with adjustments for anti-Müllerian hormone level, body mass index, day-2 FSH, and smoking history. In 2007, it was enabled by the introduction of a metered rFSH pen allowing small dosage increments of ~8.3 IU per click. In 2011, a second rFSH pen was introduced allowing more precise dosages of 12.5 IU per click, and both pens with their individual algorithms have been applied continuously at our clinic. The objective of this observational study was to validate the PIVET algorithms pertaining to the two rFSH pens with the aim of collecting ≤15 oocytes and minimizing the risk of ovarian hyperstimulation syndrome. The data set included 2,822 in vitro fertilization stimulations over a 6-year period until April 2014 applying either of the two individualized dosing algorithms and corresponding pens. The main outcome measures were mean oocytes retrieved and resultant embryos designated for transfer or cryopreservation permitted calculation of oocyte and embryo utilization rates. Ensuing pregnancies were tracked until live births, and live birth productivity rates embracing fresh and frozen transfers were calculated. Overall, the results showed that mean oocyte numbers were 10.0 for all women <40 years with 24% requiring rFSH dosages <150 IU. Applying both specific algorithms in our clinic meant that the starting dose was not altered for 79.1% of patients and for 30.1% of those receiving the very lowest rFSH dosages (≤75 IU). Only 0.3% patients were diagnosed with severe ovarian hyperstimulation syndrome, all deemed avoidable due to definable breaches from the protocols. The live birth productivity rates exceeded 50% for women <35 years and was 33.2% for the group aged 35–39 years. Routine use of both algorithms led to only 11.6% of women generating >15 oocytes, significantly lower than recently published data applying conventional dosages (38.2%; P<0.0001). When comparing both specific algorithms to each other, the outcomes were mainly comparable for pregnancy, live birth, and miscarriage rate. However, there were significant differences in relation to number of oocytes retrieved, but the mean for both the algorithms remained well below 15 oocytes. Consequently, application of both these algorithms in our in vitro fertilization clinic allows the use of both the rFSH products, with very similar results, and they can be considered validated on the basis of effectiveness and safety, clearly avoiding ovarian hyperstimulation syndrome.
Introduction
The first live births from in vitro fertilization (IVF) were achieved from single oocytes recovered from monitoring natural cycles and detecting the luteinizing hormone surge. However, higher rates of pregnancy and live births were found after ovarian stimulation followed by human chorionic gonadotrophin (HCG) trigger to recover several oocytes in a controlled fashion. Currently, ovarian stimulation is most often undertaken with recombinant follicle stimulating hormone (rFSH), and the starting dose of this gonadotrophin is important for achieving optimal numbers of oocytes in order to produce viable embryos. However, the response needs to be tempered to diminish the risk of ovarian hyperstimulation syndrome (OHSS), a potentially lethal condition, occurring at increased likelihood when oocyte numbers exceed 15. At present, most clinicians still rely on a standard dose of rFSH, typically 150–225 IU, with adjustments based on each clinician’s experience and the patient’s previous response. The earliest attempts to develop algorithms based on various biological and anthropometric parameters ensued from an academic IVF group in CopenhagenCitation1,Citation2 who constructed their risk algorithm based on antral follicle count (AFC), ovarian volume, Doppler score, age, and smoking habits. However, some of these parameters are not commonly used, measured, or not obtainable in most clinics. Howles et alCitation3 evaluated several potential variables and identified four critically important parameters: FSH level at screening, body mass index (BMI), age, and number of follicles <11 mm and found them to be the most predictive factors. A formula was calculated using the CONSORT algorithm based on these four variables. This algorithm was tested in a multicentre study on 172 normoovulatory women aged 18–34 years, and a mean of 10.3 oocytes was retrieved. However, the cancellation rate was as much as 17%, which was rather high in this potentially good-responder group.Citation4
Recently, PIVET Medical Centre published an algorithmCitation5 designed to calculate the appropriate dosage of rFSH to provide 8–12 oocytes from all women within the IVF program and to reduce the risk of severe OHSS. The algorithm covered all patients from very poor responders to hyper-responders. The rationale behind that algorithm was to target those women at higher risk for OHSS with smaller dosages of rFSH than was standard practice. This was enabled by the introduction of the Puregon Pen by Merck Sharp & Dohme (Sydney, NSW, Australia) (MSD) into Australia in 2007, which enabled a dial-up dosage of 25 IU increments, but with three subdivisional clicks assumed to be approximately 8.3 IU each (). This algorithm then allowed much lower dosages to be provided to women with high AFC ratings and higher anti-Müllerian hormone (AMH) levels, and the dose was adjusted downwards for younger women and those with low BMI ratings.
Figure 1 First PIVET rFSH algorithm designed for ~8.3 IU increments suited to the Puregon Pen.
Abbreviation: rFSH, recombinant follicle stimulating hormone.
The original PIVET algorithm enabled rFSH dosages for the Puregon Pen,Citation5 which were much lower than the usually administered 150–225 IU, yet still permitted an adequate response but with minimal risk of over-response and the associated concomitant risk of severe OHSS. According to the algorithm, such dosage could range as low as 41.7 IU daily for very young women with BMI as low as 16 kg/m2, often with typically enlarged polycystic ovaries and AMH levels >30 pmol/L. Such women had “targeted mild stimulation” but still had the benefits of sufficient oocytes to enable blastocyst culture and single embryo transfers (ETs) as well as the benefit of some embryos for cryopreservation facilitating a higher pregnancy productivity rateCitation6 from a single transvaginal oocyte aspiration (TVOA). From our perspective, this strategy is superior to the idea of minimal stimulation protocols using clomiphene citrate, where few eggs are recovered, many cases are cancelled, and minimal cases have the benefit of additional cryopreserved embryos for future use.Citation7
At the time of the algorithm study (October 2009), only the Puregon Pen enabled small dosage increments (~8.3 IU), while the competitive Gonal-f Pen (Merck Serono, Sydney, Australia) allowed only 37.5 IU increments, which in our pilot studies proved to be unsatisfactory in achieving our aims. Subsequently, Merck Serono introduced a new pen early in 2011, which enabled three clicks between the previous dosages, that is, increments of 12.5 IU/click and filled by mass so that rFSH quantity was assured. We elected to design two new algorithms, one adjusted to 12.5 increments for the Gonal-f Pen (), and a revised version of the original Puregon algorithm (), that captures the current trend in a reduction of maximal rFSH dosages from 600 to 450 IUCitation5, thus providing clinicians with the option to use either of the rFSH products with a view to targeted low-dose stimulation of those women at increased risk of OHSS.
Figure 2 Second PIVET rFSH algorithm designed for 12.5 IU increments suited to Gonal-F Pen.
Abbreviation: rFSH, recombinant follicle stimulating hormone.

The current observational study analysed IVF data following stimulation using either PIVET algorithms, and adjusted for 8.3 IU or 12.5 IU increments over a continuous 6-year period. The clinical aims were to collect eight to twelve oocytes for optimal benefit and four to eight oocytes in the very-high-risk group, while maintaining a minimum risk of overstimulation. These data showed that routine application of the PIVET algorithms respective for each drug makes these aims eminently feasible.
Materials and methods
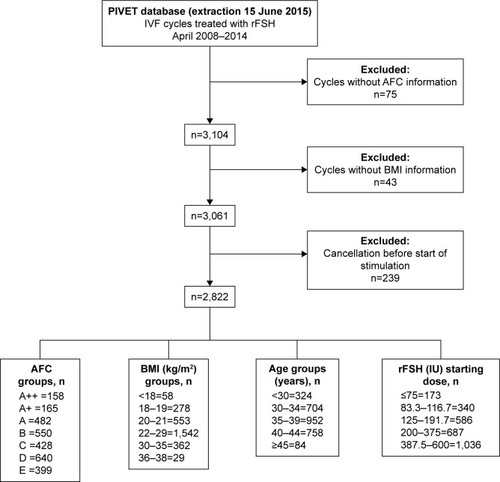
Study design
This retrospective study covers a continuous 6-year period at PIVET (April 2008 to April 2014) during which period both the algorithms were used to determine the rFSH starting dose. The data were drawn from our entire database containing all cases treated in this 6-year period (). The data period enabled all pregnancies to be tracked through to deliveries. Few patients with BMI >36 kg/m2 were treated during the entire period, and they were treated according to the algorithms as if the BMI was 35 kg/m2. The protocol was the same as applied in our previous publication,Citation5 but the new algorithm for Gonal-f was introduced in April 2011 with the launch of the new pen. Dosages were adjusted to be close to the original Puregon dosages, and both the rFSH products were used routinely based on clinicians’ choice and availability in the current study. All clinical and laboratory management of IVF or Intracytoplasmic Sperm Injection (ICSI) procedures was not altered during the entire 6-year study period, apart from trends in ETs, namely more cases committed to blastocyst culture, more single ETs, and more cases with selective cryopreservation of the better quality embryos.
Patient management
All IVF patients completed informed and written consent when undergoing an assessment cycle, which occurred following their primary consultation and within 1–2 months immediately prior to the IVF treatment cycle. This included serum day-2 FSH, AMH, and AFC measured day 5±1 along with cycle tracking, a hysterosalpingo-contrast-sonography (HyCoSy) test day 7 to day 9, periovulatory postcoital evaluation, and mid-luteal serum progesterone (P4). At this latter, mid-luteal point, the couple were reviewed for the consideration of treatment proposals. For those proceeding to IVF, the clinician decided the stimulation protocol, and the starting gonadotrophin dosage was calculated from the appropriate algorithm. However, if a further fresh cycle was planned, the essential measurement parameters were repeated in the month prior to the cycle and the rFSH dosage calculated at the day 21 pre-IVF check. Clinicians were instructed to avoid adjusting dosages on the basis of previous responses (eg, oocytes collected) but to simply calculate using the updated parameters.
PIVET algorithm development
Examination of more than 5,000 IVF cycles along with tracking the destiny of more than 50,000 recovered oocytes created a profile of those protocols leading to the development of eight to twelve follicles and oocytes according to the background AFC ratings. These data were then compared with the risk charts (chance of pregnancy vs risk of OHSS) published from the Copenhagen Group.Citation1,Citation2 As the algorithms evolved, we applied data modeling and graphic smoothing techniques to create a continuously rising dosage schedule, without irregularity. As previously stated, we commenced by applying the Puregon Pen, which is filled by bioassay and provides MSD-guaranteed dosages of 25 IU rFSH. We made an assumption that each click of the pen provides approximately one-third dosage, that is, ~8.3 IU/click, although this has not been endorsed or confirmed by MSD, hence ~8.3 IU increments ().Citation5 Following the release of the Gonal-f Pen (Merck Serono) in 2011, we applied the closest dosage schedule to match the Puregon Pen algorithm, with further data modeling and graphic smoothing techniques to create the model, as shown in . Merck Serono indicates that it is this rFSH pen that is filled by mass; hence, all dosage increments of 12.5 IU are fully endorsed and quality assured by Merck Serono.
Selecting rFSH and adjusting starting dosage
The main parameters relevant to the algorithm are female, age, and current AFC rating, where all detectable follicles 2–10 mm are included. Definition of ratings are grade E, ≤4 follicles; grade D, 5–8 follicles; grade C, 9–12 follicles; grade B, 13–19 follicles; and grade A, 20–29 follicles (). Patients in the latter grade belong to the high-risk polycystic ovary group and are further classified as grade A+ (30–39 follicles) and grade A++ (≥40 follicles) . In selecting the AFC grading, the AMH level was also factored in as a modulator, adjusting the AFC grading category upwards if AMH implied a higher AFC rating (eg, if the AFC categorized to B, but AMH categorized to A, the patients would be categorized as an A grading). Clinicians are advised not to adjust the AFC rating downwards if suggested by a lower AMH reading as this implies a higher rFSH dosage, often proving inappropriate with excessive ovarian responses. Internal correlation studies at PIVET show high correlation between AMH and AFC, but there can be discordance in ~11% of cases (Keane et al; unpublished data, 2016). Moving the AFC rating upwards ensured that a lower dosage of rFSH would be selected, ensuring safety and reducing risk of overstimulation and OHSS. The current AMH levels were determined using Gen II (Beckman Coulter, Brea, CA, USA).Citation8 Other adjustments within the algorithm include BMI, smoking history, and day-2 serum FSH, the latter adjusting 1 or 2 readings to the right (ie, higher rFSH dosage; ). Patients classified as egg donors or those having egg storage (freeze all) have their rFSH dosage adjusted four readings to the right (ie, higher rFSH dosage), as they will have an antagonist stimulation cycle with agonist (eg, Lucrin, leuprolide acetate; Abbott Australasia Pharmaceuticals, Sydney, NSW, Australia) triggerCitation9 and no luteal support drugs because there is no transfer and the risk of OHSS is considered to be low. These data have been included to determine the impact of the algorithms on oocyte retrieval and utilization but excluded for calculations of pregnancy, live births, and miscarriage rates.
Ovarian stimulation regimens
Women with high AFC ratings (all A categories and some B if AFC >15 antral follicles) were treated by an antagonist regimen preferentially. Those with low categories (D and E) were treated by a flare regimen with some very poor responders treated by an agonist antagonist conversion with estrogen priming (AACEP) regimen.Citation10 Women with AFC category B <15 antral follicles and category C could be treated by either regimen or even a long downregulation protocol at clinician’s discretion. The long down-regulation protocol was also preferred for cases of underlying adenomyosis and endometriosis. This approach accords with the Nelson algorithm.Citation11
Adjusting rFSH dosage
IVF cycle management commences with day-2 parameters of estradiol (E2) <200 pmol/L, P4 <5.0 nmol/L, and luteinizing hormone <10 IU/L. FSH levels are measured and may lead to adjustment of the algorithm dosage if different from previous, for example, FSH 20 IU may cause rFSH dosage selection 2 squares to the right (8.3/12.5 to 16.7/25 IU where <300 IU; or 25–37.5 IU where ≥300 IU). Most cycles utilize the antagonist regimen, with rFSH commencing on day 3, but “agonist flare cycles” means that patients commence Lucrin 4 IU on day 2, followed by rFSH day 3. Serum E2 levels are determined at day 7, and if >500 pmol/L, the dosage of rFSH remains unchanged. Conversely, lower levels will require 12.5 IU incremental rises in dosage. The antagonist (Orgalutran: MSD or Cetrotide; Merck Serono) 0.25 mg is introduced with E2 levels >500 pmol/L. Thereafter, E2, P4, and luteinizing hormone levels are measured every 2–3 days along with follicle tracking by transvaginal ultrasound from day 9. Most cases present with two or more leading follicles, >17 or (≥18) mm on day 12 of the cycle, the most common day for giving the ovulatory trigger, and TVOA is scheduled for 37 hours posttrigger.
Trigger and luteal support
Recombinant HCG (rhCG: Ovidrel; Merck Serono) was used as ovulation trigger in doses 6,500–13,000 IU (one or two ampoules). However, in cases considered at high risk of developing OHSS (≥12 follicles or ≥12,000 pmol/L E2) and treated with an antagonist regimen, GnRHa (leuprolide acetate, Lucrin 50 IU; Abbott Australasia Pharmaceuticals, Sydney, NSW, Australia) was used as the ovulatory trigger. Luteal support was given as described earlierCitation5 and includes rhCG 500–1,000 IU days 4, 7, 10, and 13 after TVOA for those cases with <12 follicles and <12 oocytes according to our long-standing practices;Citation12,Citation13 all other cases received P4 400 mg thrice daily. Cases with >12 oocytes were given Cabergoline 1 mg nocte for 10 days and cases >20 oocytes had freeze-all except if the trigger was Lucrin.
Intensive monitoring protocol
Women were classified as OHSS risk at two occasions – firstly, at trigger if E2 was ≥12,000 pmol/L or ultrasound revealed ≥12 follicles that were ≥12 mm; and secondly, at TVOA if ≥12 oocytes were recovered. Such patients were instructed to record abdominal girth at the umbilicus each day and report the measurement and wellness (especially noting breathing difficulty, urine flow, and color, as well as any nausea and/or vomiting) daily to the clinic nurse. A blood test was performed every third day and was considered for E2 >6,000 pmol/L or P4 >600 nmol/L; such cases having luteal phase support adjusted to exclude rhCG, if previously prescribed. Women exceeding safe parameters were arranged for abdominal ultrasound to check for OHSS (and need for paracentesis), along with urine sample to detect specific gravity (SG) and need for intravenous hydration if SG >1,015.
ET trends
In the late algorithm period, there were some changing trends in ET policies. Overall, there was a strong Australian drive to minimize multiple pregnancies; hence, elective single ETs were encouraged. There was also a concern that those cycles characterized by rising P4 levels prior to trigger would result in disturbances affecting embryo–endometrial synchrony, therefore PIVET policy changes encouraged cryopreservation by vitrification of the best-quality embryos, and fresh transfer of those embryos graded in the lower tier. The trend also included an increased blastocyst culture policy. Consequently, fewer embryos became available for transfer or cryopreservation.
Oocyte retrieval and pregnancy outcomes
Two utilization measures were calculated to summate the proportion of “usable” embryos transferred or vitrified arising from the total number of two pronucleate embryos generated from a single oocyte retrieval procedure (embryo utilization rate), or the proportion of “usable” oocytes transferred and vitrified arising from the total number of oocytes retrieved from a single oocyte retrieval procedure (oocyte utilization rate). The outcomes of treatment cycles are categorized for cycles initiated and fresh cycles per TVOA or per ET. They are categorized as clinical pregnancy rate (detectable foetal heartbeat at 7 weeks) or live birth rate (at least one live, surviving infant after 28 weeks). The pregnancy and live birth productivity rates include the subsequent frozen embryo transfer outcomes arising from the single TVOA procedure from an individual IVF treatment cycle. PIVET introduced this terminology to avoid confusion with the term “cumulative pregnancy rate,” which mostly relates to outcomes from more than one IVF treatment cycle.Citation5,Citation6 By using productivity rates, the outcomes of IVF treatments, where top-quality embryos are frozen for various reasons, for example, elevated late follicular P4 levels or risk of OHSS, can still be favorably reflected.Citation14
Statistical analysis
Data were analyzed using Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA) applying one-way or two-way analysis of variance for multiple comparisons of the means within groups, including AFC and age groupings. Ratios were compared using χ2 contingency tables applying Fisher’s exact test. P-values <0.05 were considered statistically significant. Multivariate statistical analysis was conducted by logistic regression using SPSS version 22 (IBM Corporation, Armonk, NY, USA).
Results
A summary of treatment cycle details along with patient age distribution is shown in . The mean age for the total women treated during the 73-month study period was 36.2 years, and ages ranged from 20 to 51 years. The most commonly applied stimulation protocols were the antagonist regimen (57.4% of cases) followed by the flare protocol (30.1% of cases). Only a small group of patients (12.5%) were treated by other protocols including the long agonist downregulation protocol and the variant AACEP regimen. The incidence of women needing admission to hospital for various diagnoses post-TVOA is shown in . In order to track all patients developing OHSS, 483 risk patients had the intensive monitoring protocol (IMP) applied during the period. Furthermore, 308 patients with >12 oocytes were treated with Cabergoline to diminish or prevent OHSS symptoms. Nine cases were categorized as severe OHSS requiring paracentesis or drainage of pleural effusion (one case), which comprised 0.3% of all cases receiving rFSH stimulation. Only one case of severe OHSS occurred during the reporting period of the first algorithm publicationCitation5 and that related to a marked violation of protocol. In this full study period, each of the nine cases had identifiable noncompliance issues in four areas of “protocol violation.” They included 1) excessive starting dosage (due to adjusting the AFC rating the wrong way once the AMH level was factored); 2) incorrect elevation of dosage (due to increasing more than 12.5 IU when hormonal or ultrasound monitoring indicated a need for elevation); 3) inappropriate trigger when E2 >10,000 pmol/L or follicle number ≥12 (usually because a flare cycle had prevented use of a GnRHa trigger); and finally, 4) wrong use of HCG during the luteal phase (sometimes a patient from a remote location self-administering Ovidrel prior to receiving approval from the clinic during IMP). Each time the OHSS was exacerbated by superimposed pregnancy (so-called late onset OHSS in all nine cases).
Table 1 Patient demographics and treatment flow for women initiating rFSH stimulation under PIVET algorithms
The cancellation rate before TVOA was 6.2% and included all cancellations after starting rFSH stimulation (ie, initiated cycle). However, from 173 patients () who received low rFSH (≤75 IU), only 15 (8.7%) patient cycles were cancelled. Most cancellations were a result from inadequate responses in the group of younger patients receiving low rFSH dosage.
As part of the Australia-wide drive to decrease the twin rate, there was an increasing rise in the transfer of single embryos during the study period, being 78.4% of all transfers with some variation among the age groups. Younger patients (<30 years) had a mean of 1.1 embryos transferred and the mean increased in the elder age groups to a maximum of 1.5 embryos in the group of 40–44 years. The overall mean (± standard deviation) number of embryos transferred with the PIVET algorithms was 1.28±0.45.
The mean number of oocytes collected through the study period was 8.7 (±5.7) oocytes and was 10.0 (±5.6) for those <40 years (). Women aged under 30 years had 11±6.2 oocytes recovered, while women 40–44 years had lower but acceptable oocyte numbers (mean of 6), and those with the poorest prognosis with age >45 years had a mean of four oocytes recovered. When the oocyte distribution was examined in relation to the AFC categories (), the highest AFC ratings showed a mean number of 12 oocytes, despite the very low rFSH starting doses. With respect to BMI groupings shown in , the mean number of oocytes recovered was equivalent among different BMI groups, ranging from 7.4±5.9 in the lowest BMI group (<18 kg/m2) to 8.6±5.4 in a higher BMI group (18–19 kg/m2), but these minimal differences across the other BMI groups were not significant.
Table 2 Oocytes recovered and embryo/oocyte utilization rates for all women having a TVOA procedure categorized by age groups
Table 3 Oocytes recovered for all women having a TVOA procedure categorized according to AFC rating
shows the pregnancy outcomes categorized according to different age groups. The data show an expected decreasing pregnancy and live birth rate per ET with increasing age groups, from 43.4% and 36.4% in the young group, respectively, decreasing to 11.7% and 7.5% in the group of 40–44 years, respectively. Some women treated toward the end of study period will not yet have had spare embryos returned in a FET cycle; however, the live birth productivity rate in the young group (<30 years) was as high as 57.1% per initiated cycle and 60.1% per TVOA (data not shown).
Table 4 Clinical pregnancy and live birth outcomes for all women having a TVOA procedure and categorized according to age group
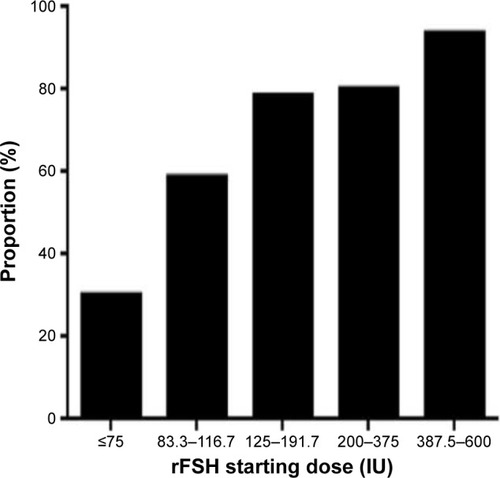
From , it can be seen that 513 women (18.2% of total) had starting doses <125 IU, and approximately half of these patients did not require any increase in dosage (). Even for those starting at ≤75 IU, one-third had no change in dosage. The overall percentage of patients continuing at their starting dosage was 79.1% during the entire study period, which demonstrated the accuracy of the combined application of both the algorithms. Examining the data for dosages outside the conventional range of 150–225 IU rFSH, 667 (23.7%) received <150 IU; while 1,343 (47.6%) received >300 IU. This means that less than one-third of women (29.7%) received conventional dosages of rFSH during the study period using the PIVET algorithms, and consequently the vast majority of patients received a “milder” form of stimulation.
Figure 4 The proportion of patients in each rFSH starting dose category, who responded adequately to the starting dose of FSH and required no adjustment (elevation) to achieve at least four follicles (for the high AFC groups) and eight to twelve follicles for the remainder.
Abbreviations: AFC, antral follicle count; rFSH, recombinant follicle stimulating hormone.
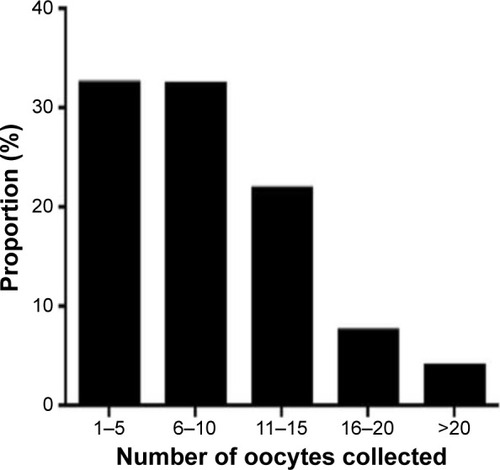
It was observed that half of the patients had from 6–10 and 11–15 oocytes retrieved (~32% and 22%, respectively), with the greatest distribution being in the 1–5 and 6–10 oocyte collection groups (). The main contribution to the first group (low oocyte numbers) came from older women. Very few women had 16–20 oocytes recovered (7.6%), and an absolute minimum of cases had >20 oocytes recovered (4.0%). Most of the latter cases were attributed to noncompliance with dosing strategy. The number of women with >15 oocytes collected at PIVET was 328/2,822 (11.6%).
Figure 5 The distribution of oocytes recovered, indicating that almost 90% of women had fewer than 16 oocytes and under 4% had more than 20 recovered; most resulting from breaches of the described protocols.
Although not the main objective of the study, a direct comparison of the two rFSH products and consequently the two algorithms for the main outcome measures – efficacy (oocyte and embryo utilization rates and pregnancy and live birth rates) as well as safety (proportion of cases with >15 oocytes and OHSS cases) was performed and showed some significant differences (–). Overall, the data demonstrated that the Puregon group were slightly but significantly older (mean of 0.5 years) with a slightly greater BMI (0.5 kg/m2) (). While there were significantly more oocytes and two pronuclei embryos retrieved for the Gonal-f group, there were no significant differences in relation to clinical outcomes such as pregnancy, live births, and miscarriage rates (). When younger women (<40 years) with lower AMH (<25 pmol/L) were analyzed, only the numbers of oocytes retrieved and two pronuclei embryos were significantly altered, whereas all other clinical outcomes and oocyte and embryo utilization rates were similar (). Finally, we investigated the impact of the different rFSH products on the outcomes in the two main treatment regimens, antagonist and flare agonist cycles (). No significant difference was observed in the flare cycles, and clinical outcomes (pregnancy, live births, and miscarriage rates) were not altered in the antagonist group. However, in the latter group, the patients receiving Puregon were significantly older and heavier and had a lower oocyte yield and embryo utilization ().
Discussion
PIVET is developing unique algorithms to assist clinicians to select appropriate rFSH dosages with a view to accommodate modern trends of milder stimulation, while maintaining high pregnancy rates, but minimizing or even completely removing the risk of OHSS. Since the first PIVET algorithm was published,Citation5 the assisted reproductive technology (ART) world has continued to evolve with several new trends, including the complete removal of OHSS risk by administering antagonist stimulation with GnRH agonist trigger and freeze-all procedures. In addition, there is a strong push, led by Australia and New Zealand,Citation15 to commit to an elective single ET policy. Along with that concept, the idea of blastocyst culture with a better embryo selection policy has developed and goes hand-in-glove with the emergent idea of advanced genetic screening of embryos.Citation16,Citation17 Consequently, the pregnancy and live birth productivity rates show persisting high levels, in keeping with the oocyte retrieval numbers.Citation6 Although it has been shown that 15 oocytes collected per TVOA may be the optimal number for the highest pregnancy and live birth rates from fresh ETsCitation18,Citation19 as well as for pregnancy and live birth productivity rates,Citation6 the risks for severe OHSS rise drastically after the collection of twelve or more oocytes. Furthermore, attempts to schedule for 12–15 oocytes retrieval means that many cases will unintentionally result in ≥20 oocytes collected and an exponential rise in OHSS risk. Here, using the PIVET algorithms, only 4% of cases returned 20 or more oocytes recovered, but these were due to specific protocol violations. The proportion of women with >15 oocytes retrieved per egg collection was 11.6%. This was significantly lower than those receiving conventional rFSH dosages (112.5–300 IU) recently reportedCitation20 (38.2%) (P<0.0001) and demonstrated our milder stimulation.
During the entire study period, OHSS prevention has been the main focus, and other preventive initiatives were also undertaken, including the management of more cycles by an antagonist regimen, which provided the clinician with an opportunity to trigger with a GnRH-agonist and decrease the risk of OHSS.Citation21 Furthermore, patients were offered increased monitoring to ensure all patients with OHSS symptoms were detected at the earliest possible stage and that relevant expedient steps could be implemented, such as intravenous fluids to counteract hypovolemia, which were often enough to prevent the complete disorder from developing. The high number of patients on increased monitoring did not necessarily reflect many patients at risk of developing OHSS, but only as a relevant service to detect all cases and expedite early management. In addition, the freeze-all strategy to prevent OHSS in high-risk scenariosCitation22 was used throughout the study period. However, despite various preventive strategies, OHSS cases were encountered during the study (0.3%), but each and every case was shown retrospectively to be related to noncompliance with the PIVET protocols and regimens as defined in the methodology section. All the cases of severe OHSS were late onset, associated with pregnancy, but also had one of four identifiable noncompliance issues with the advised procedure as defined earlier. We are aware of the controversy over the use of HCG as luteal support, but have data supporting its use to improve the chance of pregnancyCitation23 and believe it can be used safely as defined in our policies. Its use is clearly safe in women with lower AFC and AMH levels, for example, categories C, D, and E of the algorithms; perhaps further restrictions need to be considered for the A groups as well as some group B cases. We have now adjusted the protocols to avoid luteal phase HCG in all cases with either AFC rating ≥15 antral follicles or AMH levels ≥15pmol/L, in addition to the existing restriction of recovery of ≥12 oocytes at TVOA.
High cancellation rates prior to oocyte retrieval can be explained by insufficient follicle development as a result of an inappropriately low rFSH starting dose. In our study, the cancellation rate before TVOA was 6.2% and included all cancellations after starting rFSH stimulation (ie, initiated cycles). This compared favorably with the overall cancellation rate of 24 of 161 cases (14.9%) in the CONSORT algorithm (P<0.001).Citation4 Furthermore, in our study, only 15 of 173 (8.7%) patients were cancelled in the low FSH group (≤75 IU), and this was significantly lower than the 25% (four of twelve cases) cancellation rate for the CONSORT algorithm, in a potentially good-responder group, requiring low rFSH.Citation4 Most cancellations in both series occurred from inadequate responses in the group of younger patients receiving low rFSH dosage.
Moreover, young patients with high AFC/AMH often expressed disappointment as they were part of the distribution grouping of one to five oocytes. However, their pregnancy rates were actually high, in keeping with our earlier published data that <5 oocytes from younger high-responders generate embryos that have a higher implantation rate than those from higher order oocyte collections.Citation6 While those <30 years and patients 40–44 years had a mean number of 1.1 and 1.5 embryos transferred, respectively, the overall mean was 1.28±0.45, which was significantly lower (P<0.001) than that demonstrated in another study (1.94±0.24), which was also described as an “optimum oocyte retrieval and transfer strategy.”Citation20 Furthermore, we noted a trend toward higher embryo utilization rates over oocyte utilization rates in all cases, but both of these rates were increased in older patients. The higher embryo utilization rate reflects the higher number of usable, mature, and fertilizable M-II eggs and is a useful index signifying the proportion of eggs that are actually mature. However, the higher rates in older women may reflect the request by older women to undertake an ET despite lower graded embryos.
Overall both the dosing algorithms, adjusted for ~8.3 or 12.5 IU increments, have proven effective in meeting the aims of collecting eight to twelve oocytes from the majority of women undergoing ovarian stimulation for IVF procedures. Specifically, the average number of oocytes collected for Gonal-f and Puregon was 9.1 and 8.3, respectively, and 10.1 and 8.0, respectively, for younger women (<40 years) with lower AMH. Although in the overall data set, women receiving Puregon were slightly but significantly older (0.5 years), the reduced oocyte retrieval using Puregon was probably more related to the lower dosage derived from the algorithm, as in the younger group with no significant difference in mean age, the reduced trend in oocyte retrieval was also observed. Additionally, the approximate 8.3 IU subdoses of Puregon are not guaranteed by the manufacturer and could also play a role in these observed differences. Importantly, there was no significant difference in clinical pregnancy, live births, and miscarriage rates between the two rFSH products for all patients collectively or in a subset of younger women (<40 years).
In relation to the impact of specific rFSH stimulation protocols, we did not observe any influence of the rFSH product in flare agonist stimulation, while clinical parameters (pregnancy/livebirths/miscarriage) were also not altered in the antagonist stimulation. However, similar to above, oocyte retrievals were significantly enhanced in the Gonal-f group. The cause of this increase is not known at present but is again probably related to the higher concentrations of Gonal-f used.
Interestingly, the number of cases with >15 oocytes was kept at a low of 11.4% using both of the algorithms, significantly fewer compared to 38.2% in another recent studyCitation20 and very few patients exceeded 20 or more oocytes in our study. This is comparable with modern trends of milder stimulation with a view of absolute minimization of the risk of OHSS, with patient safety being of paramount importance. It is our view that such risk can be reduced under 0.01%, but this will require a more determined clinical approach, with stronger adherence to the protocols described here, along with appropriately restricted use of HCG for triggering in tandem with its careful use in the luteal phase.
Finally, although PIVET has published data showing excellent pregnancy rates from FET cycles, particularly where these are undertaken under hormone replacement therapy control,Citation24 we are not supportive of the view that fresh ETs should be avoided.Citation22 We believe that the application of the PIVET algorithms provides a rational approach enabling fresh and frozen ETs leading to optimal pregnancy productivity rates, along with the potentially complete avoidance of severe OHSS cases, all being derived from the same TVOA.
Ethical considerations
General approval for retrospective data analysis was provided from Curtin University (HREC) number RD-25-10 (2015), and PIVET Medical Centre is accredited with both the Australian Reproductive Technology Committee (RTAC) and the Reproductive Technology Council (RTC) of Western Australia that functions under statutory regulation. Both the authorities conduct annual accreditation reviews for licencing of ART clinics, with a focus on multiple pregnancies and OHSS rates being of concern if elevated beyond currently adjusted standards.
Acknowledgments
We wish to acknowledge the relevant contribution of PIVET’s previous Scientific Director James D Stanger who helped JLY and PMH develop the first algorithm, which proved a successful idea. The Curtin Health Innovation Research Institute is acknowledged for providing excellent facilities. All participating patients and PIVET staff are acknowledged with thanks. PIVET has self-funded all the facilities, equipment, and manpower for this project.
Supplementary materials
Table S1 Clinical outcomes for all patients undergoing rFSH stimulation using either Gonal-f or Puregon
Table S2 Clinical outcomes in younger women (<40 years), with AMH values <25 pmol/L, receiving rFSH stimulation using either Gonal-f or Puregon
Table S3 Clinical outcomes for all patients undergoing rFSH stimulation using either Gonal-f or Puregon and analyzed according to treatment cycle type
Disclosure
Although the data presented in this paper concern products from two commercial pharmaceutical companies, we attest that no specific funding was provided for these studies. Along with other companies, MSD and Merck Serono have provided unrelated educational support funding of a limited degree over many years for JLY and PIVET support.
Author contribution
JLY and PMH developed the PIVET dosing algorithms. KNK, BA, PMH, JLC and JLY conceived and designed the project. BA, KNK, and PMH extracted the data and undertook the primary analysis. Secondary analysis was performed by KNK, JLC and JLY. BA wrote the first draft, which was revised by KNK, PMH, JLC, and JLY. All authors approve the final version and agree to be accountable for all aspects of the work.
References
- Popovic-TodorovicBLoftABredkjæerHEBangsbøllSNielsenIKAndersenANA prospective randomized clinical trial comparing an individual dose of recombinant FSH based on predictive factors versus a ‘standard’ dose of 150 IU/day in ‘standard’ patients undergoing IVF/ICSI treatmentHuman Reprod2003181122752282
- Popovic-TodorovicBLoftALindhardABangsbøllSAnderssonAMAndersenANA prospective study of predictive factors of ovarian response in ‘standard’ IVF/ICSI patients treated with recombinant FSH. A suggestion for a recombinant FSH dosage normogramHuman Reprod2003184781787
- HowlesCMSaundersHAlamVEngrandPPredictive factors and a corresponding treatment algorithm for controlled ovarian stimulation in patients treated with recombinant human follicle stimulating hormone (Follitropin alfa) during assisted reproduction technology (ART) procedures. An analysis of 1378 patientsCurr Med Res Opin200622590791816709312
- OlivennesFHowlesCMBoriniAIndividualizing FSH dose for assisted reproduction using a novel algorithm: the CONSORT studyReprod Biomed Online200918219520419192339
- YovichJStangerJHinchliffePTargeted gonadotrophin stimulation using the PIVET algorithm markedly reduces the risk of OHSSReprod Biomed Online201224328129222296972
- StangerJDYovichJLFollicle recruitment determines IVF productivity rate via the number of embryos frozen and subsequent transfersReprod Biomed Online201327328629623886680
- BodriDKawachiyaSBruckerMDCumulative success rates following mild IVF in unselected infertile patients: a 3-year, single-centre cohort studyReprod Biomed Online201428557258124631167
- NelsonSMIliodromitiSFlemingRAndersonRMcConnachieAMessowC-MReference range for the antimüllerian hormone Generation II assay: a population study of 10,984 women, with comparison to the established Diagnostics Systems Laboratory nomogramFertil Steril20141012523529.e52124220704
- HumaidanPEjdrup BredkjærHBungumLGnRH agonist (buserelin) or hCG for ovulation induction in GnRH antagonist IVF/ICSI cycles: a prospective randomized studyHuman Reprod200520512131220
- FischJDKeskintepeLSherGGonadotropin-releasing hormone agonist/antagonist conversion with estrogen priming in low responders with prior in vitro fertilization failureFertil Steril200889234234717562336
- NelsonSMBiomarkers of ovarian response: current and future applicationsFertil Steril201399496396923312225
- YovichJLEdirisingheWRCumminsJMEvaluation of luteal support therapy in a randomized controlled study within a GIFT programFertil Steril19915511311391986952
- YovichJLTreatments to enhance implantationChapmanMGrudzinskasJGChardTImplantation: Biological and Clinical AspectsLondonSpringer-Verlag1989239254
- YovichJLStangerJDKeaneKNCumulative live birth rate: an outmoded termJFIV Reprod Med Genet20164165
- MacaldowieAWandYAChughtaiAAChambersGMAssisted Reproductive Technologies in Australia and New Zealand 2012SydneyNational Perinatal Epidemiology and Statistics Unit, the University of New South Wales2014
- FiorentinoFBonoSBiricikAApplication of next-generation sequencing technology for comprehensive aneuploidy screening of blastocysts in clinical preimplantation genetic screening cyclesHuman Reprod2014291228022813
- YovichJLConceicaoJHinchliffePKeaneKWhich blastocysts should be considered for genetic screening?Human Reprod201530717431744
- JiJLiuYTongXHLuoLMaJChenZThe optimum number of oocytes in IVF treatment: an analysis of 2455 cycles in ChinaHuman Reprod2013281027282734
- SunkaraSKRittenbergVRaine-FenningNBhattacharyaSZamoraJCoomarasamyAAssociation between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cyclesHuman Reprod201126717681774
- ChenY-hXuX-hWangQOptimum oocyte retrieved and transfer strategy in young women with normal ovarian reserve undergoing a long treatment protocol: a retrospective cohort studyJ Assist Reprod Genet201532101459146726384107
- HumaidanPKolSPapanikolaouEGnRH agonist for triggering of final oocyte maturation: time for a change of practice?Human Reprod Update2011174510524
- DevroeyPPolyzosNPBlockeelCAn OHSS-free clinic by segmentation of IVF treatmentHuman Reprod2011261025932597
- YovichJLStangerJDYovichJMTuvikAIHormonal profiles in the follicular phase, luteal phase and first trimester of pregnancies arising from in-vitro fertilizationBr J Obstet Gynaecol19859223743842580551
- YovichJLConceicaoJLStangerJDHinchliffePMKeaneKNMid-luteal serum progesterone concentrations govern implantation rates for cryopreserved embryo transfers conducted under hormone replacementReprod Biomed Online201531218019126099447