Abstract
Rheumatic disease is not a single disorder, but a group of more than 100 diseases that affect joints, connective tissues, and/or internal organs. Although rheumatic diseases like rheumatoid arthritis (RA), psoriatic arthritis, and ankylosing spondylitis (AS) differ in their pathogenesis and clinical presentation, the treatment of these inflammatory disorders overlaps. Non-steroid anti-inflammatory drugs are used to reduce pain and inflammation. Additional disease-modifying anti-rheumatic drugs are prescribed to slowdown disease progression, and is in RA more frequently and effectively applied than in AS. Biologicals are a relatively new class of treatments that specifically target cytokines or cells of the immune system, like tumor necrosis factor alpha inhibitors or B-cell blockers. A new kid on the block is the interleukin-17 (IL-17) inhibitor secukinumab, which has been recently approved by the US Food and Drug Administration for moderate-to-severe plaque psoriasis, psoriatic arthritis, and AS. IL-17 is a proinflammatory cytokine that has an important role in host defense, but its proinflammatory and destructive effects have also been linked to pathogenic processes in autoimmune diseases like RA and psoriasis. Animal models have greatly contributed to further insights in the potential of IL-17 blockade in autoimmune and autoinflammatory diseases, and have resulted in the development of various potential drugs targeting the IL-17 pathway. Secukinumab (AIN457) is a fully human monoclonal antibody that selectively binds to IL-17A and recently entered the market under the brand name Cosentyx®. By binding to IL-17A, secukinumab prevents it from binding to its receptor and inhibits its ability to trigger inflammatory responses that play a role in the development of various autoimmune diseases. With secukinumab being the first in class to receive Food and Drug Administration approval, this article will further focus on this new biologic agent and review the milestones in its development and marketing.
Introduction
Rheumatic diseases are characterized by pain and loss of function in one or more areas of the musculoskeletal system. It is not a single disorder, but a group of more than 100 diseases that affect joints; bones; cartilage; and connective tissues like tendons, ligaments, and muscles; they may also affect internal organs.
Arthritis (from Greek: arthro = joint, itis = inflammation) is one of the clinical manifestations of rheumatic diseases, and is characterized by pain, swelling, and stiffness of the affected synovial joints. Rheumatoid arthritis (RA) is the most common inflammation-driven rheumatic disease, which mainly affects the joints in a symmetrical manner and finally results in the destruction of cartilage and bone. This chronic autoimmune disease has been associated with genetic predisposition (eg, HLA-DR4, cytotoxic T-lymphocyte-associated antigen [CTLA]-4, and PTPN22) and environmental risk factors (eg, smoking and microorganisms), and is often accompanied by rheumatoid factor and anti-cyclic citrullinated protein antibodies as diagnostic and prognostic biomarkers for RA.Citation1–Citation3
In contrast to RA, psoriatic arthritis (PsA) and ankylosing spondylitis (AS) are considered seronegative rheumatic diseases; both PsA and AS are associated with genetic inheritance of the HLA-B27 gene.Citation4,Citation5 PsA is, like RA, also an inflammatory rheumatic disease characterized by arthritis and affects up to 30% of patients with the chronic skin condition psoriasis.Citation6 Its peripheral joint involvement may range from mild asymmetric joint inflammation to severe erosive arthritis. AS, formerly also known as Bechterew’s disease, is a rheumatic disease of the axial skeleton that mainly affects the spine and the sacroiliac joint in the pelvis. This spondyloarthropathy is characterized by erosion, sclerosis, and ossification, which may result in complete fusion and rigidity of the spine.Citation7
Despite the differences in pathogenesis and clinical presentation of RA, PsA, and AS, the treatment of these inflammatory rheumatic disorders is very overlapping. Nonsteroidal anti-inflammatory drugs are used to reduce pain and inflammation in rheumatic diseases; also, additional disease-modifying antirheumatic drugs, such as methotrexate (MTX) and sulfasalazine, are prescribed to slow down disease progression, and are more frequently and effectively applied in RA than in AS.Citation8 Biologicals form a relatively new class of treatments that specifically target specific cytokines or cells in the immune system. The most frequently applied biological agents approved for RA, PsA, and AS are tumor necrosis factor alpha (TNFα) inhibitors (including infliximab, etanercept, adalimumab, golimumab, and certolizumab pegol).Citation9,Citation10 For RA, alternative and approved biologicals are directed against CTLA-4-driven T-cells (abatacept), CD20-expressing B-cells (rituximab), or the IL-6 receptor tocilizumab, and many new drugs are still in the pipeline.Citation11–Citation14
However, alternatives for anti-TNF treatment failed to show efficacy in ASCitation15,Citation16 or are still in clinical trial for AS and PsA.Citation17–Citation19 PsA patients may also experience relief of symptoms by using the IL-12/IL-23 inhibitor ustekinumab, or by treatment with the synthetic disease-modifying antirheumatic drug phosphodiesterase-4 inhibitor apremilast, which is also being tested in other rheumatic diseases like AS.Citation20–Citation22
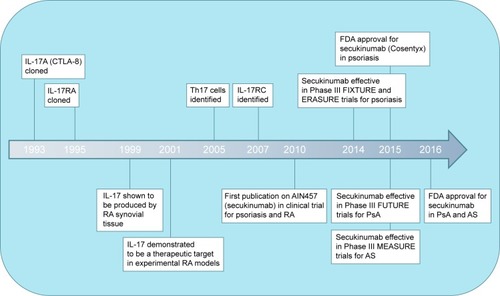
A new kid on the block is the interleukin-17 (IL-17) inhibitor secukinumab, which has been recently approved by the US Food and Drug Administration (FDA) for moderate-to-severe plaque psoriasis, PsA, and ASCitation23,Citation24 (). Secukinumab (AIN457) is a fully human monoclonal antibody that selectively binds to IL-17A and is now registered by Novartis International AG under the brand name Cosentyx®. By binding to IL-17A, secukinumab prevents it from binding to its receptor and inhibits its ability to trigger inflammatory responses that play a role in the development of various autoimmune diseases.
Figure 1 Milestones in the development of the therapeutic anti-IL-17 antibody secukinumab.
IL-17 and its biological function
IL-17A is a proinflammatory cytokine that is a part of a family of six members (IL-17A to IL-17F).Citation25 IL-17A, often referred to as IL-17, is a 17 kDa protein that is secreted as a dimer and was first described as CTLA-8 in the early nineties.Citation26 IL-17 is mainly produced by a specific T-helper subset, the Th17 cell,Citation27,Citation28 although other immune cells like γδT-cells, natural killer cells, natural killer T-cells, innate lymphoid cells, neutrophils, and mast cells have also been described to express or produce this cytokine.Citation29–Citation31 Upon binding of IL-17 to its ubiquitously expressed receptor, IL-17RA pairs with IL-17RC to induce proinflammatory responses via TRAF6, ACT1, NF-κB, C/EBP, and MAPKs.Citation32,Citation33
The existence of IL-17-producing CD4+ T-cells, Th17 cells, was only recently described after the discovery that the p40 subunit of IL-12 not only forms a heterodimer with IL-12p35, promoting interferon gamma-producing Th1 cells, but is also shared with IL-23p19.Citation34,Citation35 IL-23, together with tumor growth factor beta and IL-6, contributes to Th17 differentiation and survival under the control of transcription factor RORγT.Citation36,Citation37 Th17 cells are abundant at mucosal interfaces like the gut, where IL-17 plays an important function in the protection against extracellular bacterial and fungal infections by upregulation of cytokines and antimicrobial peptides and by recruitment of neutrophils.Citation38,Citation39 In experimental studies, IL-17 was demonstrated to be required for host defense against Klebsiella pneumoniae infection in the lungs and Citrobacter rodentium infection in the gut.Citation40 In patients suffering from the hyperimmunoglobulinemia E syndrome, an impaired IL-17 production by T-cells due to mutations in the Stat3 gene has been identified as the main cause of a devastating susceptibility for common pathogens like Staphylococcus aureus and Candida albicans, resulting in recurrent and often severe pulmonary infections, mucocutaneous candidiasis, eczema, and staphylococcal abscesses.Citation41,Citation42 These findings and the recently described effects of modulating the microbiome on T-cell biology underscore the importance of Th17 responses in mucosal homeostasis and immunity.Citation43,Citation44
Target identification: IL-17 in rheumatic diseases
Besides its role in immunity against infections, IL-17’s proinflammatory and destructive effects have also been linked to pathogenic processes in autoimmune diseases like RA, psoriasis, and PsA.Citation45 Stimulation of synovial fibroblasts with IL-17 induces the expression of IL-6, IL-8, and matrix metalloproteinases,Citation46 thereby promoting inflammation and cartilage destruction. In addition, IL-17 in synovial fluids from RA patients has been shown to induce the expression of RANKL,Citation47 an essential mediator for osteoclastogenesis and bone resorption. Interesting, IL-17 has been described to synergize with various proinflammatory cytokines, including TNFα,Citation48–Citation51 implicating an important inflammatory and catabolic function for IL-17 in disease pathogenesis ().
Figure 2 Simplified scheme of IL-17 and its effector cells in the inflamed arthritic joint.
Abbreviation: IL-17, interleukin 17.
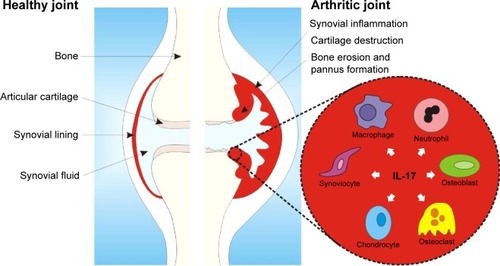
In RA patients, increased systemic and local levels of this proinflammatory cytokine can be found. RA synovial tissue was found to spontaneously produce IL-17,Citation52 and elevated levels of this cytokine have been reported in the synovial fluid of RA patients.Citation49 Also in blood, increased IL-17 expression has been observed in patients with RA; besides increased cytokine levels in serum,Citation53,Citation54 more IL-17+ CD4+ Th17 cells were found in blood from RA patients compared to healthy controls.Citation55 Recent studies reported that IL-17/Th17 levels were surprisingly increased by anti-TNF treatment.Citation56–Citation58 Interestingly, in addition to CD4+ Th17 cells, CD8+ IL-17+ cytotoxic T-cells have been described in the synovial fluid of PsA patients, and this Tc17 cell was shown to positively correlate with various markers of disease severity.Citation59 In addition to the expression of IL-17, IL-17RC was also found to be upregulated in the synovial lining in PsA patients compared to osteoarthritis patients.Citation60 For both PsA and AS, genome-wide association studies have identified polymorphisms of genes in the IL-17 pathway. PsA also has been associated with a polymorphism in the gene encoding for ACT1, a downstream signaling pathway of the IL-17 receptor.Citation61 A single-nucleotide substitution in the IL-23R gene has been demonstrated to be protective against AS.Citation62 Overall, IL-17 has been identified as a potential, interesting therapeutic target in RA, PsA, and AS, with further preclinical and clinical studies testing this hypothesis.
Preclinical development of anti-IL-17 treatment
Animal models have greatly contributed to further insights in the potential of IL-17 blockade in autoimmune and auto-inflammatory diseases. Although animal models for PsA and AS are limited, many IL-17 inhibition studies have been performed in joint inflammation models resembling RA.
The complete absence of IL-17 in mice during collagen-induced arthritis development markedly suppressed disease onset as well as arthritis severity,Citation63 and the effect of IL-17 deficiency was even more pronounced in the spontaneous arthritis development in mice deficient of the IL-1 receptor antagonist, where disease was completely prevented.Citation64 Another approach, knocking out the IL-17RA subunit followed by chronic reactivated streptococcal cell wall-induced arthritis, demonstrated the requirement of IL-17R signaling for sustained and destructive joint inflammation.Citation65
In contrast to complete absence of IL-17 signaling in transgenic mice, more refined inhibition of the IL-17 pathway in in vivo models has been applied using neutralizing antibodies directed against this cytokine, or with soluble IL-17 receptors fused to an immunoglobulin fragment crystallizable (Fc)-domain to increase its circulation time. Blocking of endogenous IL-17 in the autoimmune collagen-induced arthritis model significantly reduced arthritis progression without affecting antigen-specific T- and B-cell responses,Citation66 and was even effective in established disease in IL-1RA-deficient mice.Citation67 In reactivation of the murine antigen-induced arthritis model with local exposure to a small amount of methylated bovine serum albumin as antigen, flare of the joint inflammation and subsequent enhanced destruction could be completely prevented using anti-IL-17 treatment.Citation68 Interestingly, the additive or synergistic effects of IL-17 with other cytokines were proven in murine collagen-induced arthritis, where blocking of IL-17 in combination with TNF or granulocyte macrophage colony stimulating factor more effectively reduced joint pathology than either treatment alone.Citation69–Citation71
Although collagen-induced arthritis is considered one of the classical models for RA, the SKG mouse model may also present with features of spondyloarthritides, including AS and PsA. Upon systemic injection with beta-glucan, autoimmune-prone SKG mice with mutated ZAP-70 develop peripheral and axial arthritis, ileitis, and psoriasis-like skin inflammation.Citation72 Reduced arthritis, spondylitis, and enthesitis in the absence of IL-17 or after treatment with IL-23 inhibitorsCitation73 also support the concept of IL-17 as a therapeutic target in these rheumatic diseases. This has finally led to the development of various IL-17 inhibitors: secukinumab and ixekizumab targeting IL-17 cytokines and brodalumab targeting IL-17RA. With secukinumab being the first in class to receive FDA approval, this article will further focus on this new biologic agent and review the milestones in its development and marketing ().
Safety profile of secukinumab
The safety profile of secukinumab in various Phase II/III clinical trials on patients with rheumatic diseases was consistent with that observed with other biological therapies like etanercept.Citation74 Most observed adverse events were mild to moderate in severity. Infections were more frequent with secukinumab than with placebo; nasopharyngitis or infections of the upper respiratory tract were most often reported.Citation75–Citation79 Some cases of mild-to-moderate candidiasis were reported, which resolved spontaneously or with oral therapy.Citation77 Grade 2–3 neutropenia was reported in a couple of patients, although this was not associated with increased risk of infection.Citation80 The occurrence of reported serious adverse events could not be directly linked to secukinumab treatment, and no deaths occurred.Citation74–Citation80 Overall, after several Phase II and III clinical trials in psoriasis and various rheumatic diseases with no unexpected safety signals and no specific organ-related toxicities, secukinumab is in general considered safe and well tolerated.
One important safety issue, however, should be mentioned here that is not directly associated with secukinumab but with the IL-17R-inhibitor brodalumab, which showed significant clinical improvements in patients with moderate-to-severe psoriasis in the AMAGINE-2 and AMAGINE-3 trials.Citation81 Mid 2015, Amgen Inc. unexpectedly announced that it was discontinuing its co-development of brodalumab with AstraZeneca plc based on events of suicidal ideation and behavior in the brodalumab program.Citation82 AstraZeneca plc claimed the observations of suicidal ideation and behavior are unlikely to be causally related to brodalumab therapy and is continuing full analysis of the AMAGINE studies. Without the exact details of these deaths in relation to their treatment, speculation on other confounding factors like a national surge in suicidality during the recent economic crisis,Citation83 particularly affecting the study population in these trials, will not save the bad reputation of this IL-17R inhibitor. Despite all these, in January 2016, a Biologics License Application was submitted to the FDA by AstraZeneca plc in partnership with Valeant Pharmaceuticals, and a response is due by the end of the year.
Although secukinumab is far ahead of brodalumab in its clinical marketing and development, and suicidality is not a reported issue during its clinical trials, the concerns raised by the brodalumab program may influence ongoing secukinumab programs and may put mental health of future secukinumab users under the microscope.
Therapeutic value of secukinumab in psoriasis
Before discussing the therapeutic value of secukinumab in rheumatic diseases, the impressive results of this anti-IL-17 antibody in the treatment of plaque psoriasis need to be mentioned here. Plaque psoriasis is a chronic inflammatory skin disease characterized by raised areas of inflamed skin covered with silvery-white scaly skin. After the first-in-man study with secukinumab already demonstrating highly significant effects on psoriasis severity,Citation84 a Phase II, randomized, double-blind, placebo-controlled trial was performed on 125 patients with moderate-to-severe plaque psoriasis.Citation85 Subcutaneous injections with secukinumab at 4-week intervals resulted in significantly higher PASI75 scores, indicating at least 75% improvement from baseline in the Psoriasis Area and Severity Index score: 82% in the 150 mg arm (P<0.001) and 57% in the 75 mg arm (P=0.002) versus 9% in the placebo group (Papp, BRD2013).Citation85 This study was followed by several Phase III trials, which further confirmed and proved the clinical efficacy of this anti-IL-17 antibody in psoriasis. The ERASURE, FIXTURE, and FEATURE trials achieved their primary efficacy endpoint of PASI75 at week 12 with over 75% of the patients on 300 mg reaching PASI75 compared to <5% in the placebo controls (3REF),Citation74,Citation86 resulting in rapid FDA approval for secukinumab for its first indication: moderate-to-severe plaque psoriasisCitation23 (). Key trials with secukinumab in psoriasis and rheumatic diseases are summarized in , providing a quick overview of the study design and primary endpoints in various Phase II/III clinical trials.
Table 1 Overview of the key randomized, double-blind, placebo-controlled clinical trials with secukinumab in psoriasis and rheumatic diseases
Therapeutic value of secukinumab in RA
Phase I
In 2010, the combined results of three clinical trials on the safety and possible efficacy of secukinumab in RA, psoriasis, and chronic noninfectious uveitis were published.Citation84 In the RA trial, 52 patients were enrolled (26 AIN457- and 26 placebo-treated patients) for a 16-week study with two infusions of AIN457 (10 mg/kg) at an interval of 3 weeks. Although the primary efficacy endpoint was not achieved, this study provided the first indications of clinical responses to secukinumab. The American College of Rheumatology 20% response (ACR20) rate expressed as area under the response-time curve was significantly higher in the secukinumab group than in the placebo control group, and this was also found for the 28-joint disease activity score (DAS28) and the serological inflammation marker C-reactive protein (CRP).Citation84
Phase II
In contrast to the impressive effects of anti-IL-17 treatment in psoriasis, published Phase II/III randomized controlled trials failed to demonstrate convincing data that secukinumab is effective in RA. The efficacy and safety of secukinumab were investigated in a double-blind, randomized, placebo-controlled Phase II study including 237 RA patients with inadequate response to MTX, who were randomly assigned to monthly subcutaneous injections of 25, 75, 150, or 300 mg secukinumab or placebo.Citation75 At week 16, this resulted in ACR20 responses of 34.0%, 46.9%, 46.5%, and 53.7%, respectively, for the doses of secukinumab, compared to 36.0% in the placebo arm. However, these differences did not reach statistical significance, thereby failing to show clinical efficacy for secukinumab in RA. Although this primary efficacy endpoint of significant ACR20 responses at 16 weeks was not achieved, beneficial effects of the secukinumab doses 75, 150, and 300 mg were observed on DAS28–CRP scores.Citation75 When evaluating the long-term safety and efficacy in a follow-up study till 52 weeks, RA patients responding to secukinumab in the first 16 weeks showed a sustained clinical response, or even further improvement in their response rates up to week 52.Citation87 A recent Phase II biomarker study investigating the association of HLA-DRB1 alleles with clinical response to secukinumab was actually the first and only study to demonstrate the clinical efficacy of secukinumab over a placebo arm in RA with significantly better ACR20 response rates (87.1% in the secukinumab group vs 25.0% in the placebo group) and reduced DAS28–CRP at week 12,Citation80 although no association was found between the HLA-DRB1*04 allele and response to secukinumab treatment. Current data from a limited set of Phase II trials are not convincing, and evidence for the efficacy of secukinumab in RA from a proper Phase III trial is still lacking. Preclinical research using the human RA synovium SCID mouse model demonstrated that anti-IL-17 was only effective when the synovial tissue was rich in CD3+ T-cells,Citation88 suggesting that determining the T-cell dominance in RA patients using a set of biomarkers might help to predict the responsiveness to secukinumab treatment.
Phase III
Various Phase III studies on secukinumab in RA have recently been completed (eg, NCT01350804 and NCT01640938) or are still in progress (NCT01901900 and NCT01770379).Citation89,Citation90 More information will be obtained in the next few years on the clinical efficacy of this anti-IL-17 inhibitor in RA patients on MTX or with an inadequate response to anti-TNF agents. These results will yield a final verdict on the efficacy of secukinumab in RA and will further define the potential clinical utility of this treatment in patients with RA.
Application of secukinumab in PsA
Compared to RA, clinical trials on secukinumab for the treatment of PsA are much further in progress, and Novartis International AG filed a regulatory application for secukinumab as a PsA drug in the US and EU in Q2 2015. A Phase II proof-of-concept trial in 42 patients with active PsA did not meet its primary endpoint of ACR20 response at week 6, with ACR20 response in 39% of patients for secukinumab versus 23% for placebo (P=0.27), although significant improvement in the acute-phase proteins CRP and erythrocyte sedimentation rate (ESR) and quality of life as secondary endpoints suggested some clinical benefit from secukinumab treatment in PsA.Citation91 Recent results from the randomized, placebo-controlled Phase III FUTURE 1 and 2 trials (NCT01392326 and NCT01752634) indicate that secukinumab is indeed an efficacious treatment for patients with PsA.Citation76,Citation77 FUTURE 1 included 606 PsA patients randomly assigned to intravenous secukinumab followed by subcutaneous treatment with this IL-17 blocker at two different doses (150 or 300 mg), or placebo, and resulted in impressive and significant improvement in ACR20 and the American College of Rheumatology 50% response (ACR50), as well as two psoriasis area and severity indexes (PASI75 and PASI90) as secondary outcome parameters.Citation76 FUTURE 2 had a slightly different design with 397 PsA patients receiving subcutaneous injections of either placebo or secukinumab 75, 150, or 300 mg once a week for 4 weeks, continued every 4 weeks.Citation77 Also in this Phase III trial, the primary efficacy endpoint was met for all the secukinumab doses, as secukinumab treatment clearly improved the signs and symptoms of PsA; with secukinumab 300 mg, 54% of the patients achieved ACR20 at week 24 versus 51% with 150 mg, 29% with 75 mg, and 15% with placebo.Citation77 In line with this impressive effect on the primary endpoint, for the doses 75 and 150 mg, significant effects were reported on various secondary endpoints, including PASI75 and PASI90, DAS28–CRP, and ACR50. Interestingly, clinical response rates were generally higher in anti-TNF-naive patients than in anti-TNF inadequate responders, with the low-dose (75 mg) secukinumab group failing to show significant ACR20 and ACR50 responses to secukinumab treatment upon stratification.Citation77 Overall, these FUTURE trials have build an impressive record on the efficacy of secukinumab in PsA, resulting in the European and US FDA approval for secukinumab for PsA, together with the approval for AS, in November 2015 and January 2016, respectively.Citation24,Citation92
Treatment of AS
As already mentioned, the European Medicines Agency and FDA have approved secukinumab for the treatment of AS since multiple clinical trials have demonstrated its positive findings in AS. To test the safety and efficacy of secukinumab in AS, a randomized, double-blind, placebo-controlled Phase II trial was performed among 30 patients with moderate-to-severe AS.Citation93 Secukinumab administered intravenously 3 weeks apart was well tolerated and clinically efficacious, as convincingly demonstrated by improved 20% Assessment of SpondyloArthritis international Society response rates at week 6 (59.2% with secukinumab vs 24.5% with placebo), with Bayesian analysis indicating a probability of 99.8% of secukinumab inducing greater response rates than placebo.Citation93 A follow-up study demonstrated a sustained clinical response in the majority of these AS patients when secukinumab was continued at 3 mg/kg every 4 weeks until week 94, which was accompanied by regression of inflammatory spinal lesions as assessed by magnetic resonance imaging.Citation94 Recent results of the Phase III MEASURE 1 and 2 trials (NCT01358175 and NCT01649375) presented at the ACR conferences in Boston (2014)Citation78 and San Francisco (2015)Citation79,Citation95 and published by Baeten et alCitation96 confirmed that secukinumab resulted in rapid and significant improvement of signs and symptoms in patients with active AS,Citation78,Citation79 with ~61% of the patients in the 150 mg secukinumab arms showing 20% Assessment of SpondyloArthritis international Society response at week 16 compared to 28% in the placebo arms. Data from both the MEASURE trials also indicated that secukinumab was associated with a reduction of spinal inflammation as assessed by magnetic resonance imaging, as in ~80% of the patients treated with secukinumab for 104 weeks no radiographic progression was observed.Citation95 Other Phase III trials are ongoing to provide even more evidence on long-term safety and efficacy of secukinumab in AS (NTC02008916, NCT01863732, and NCT02159053), with results expected in 2017/2018.
Other rheumatic diseases: Behçet’s disease
A 24-week, randomized, double-blinded, placebo-controlled Phase III trial was conducted in 118 Behçet’s patients with posterior uveitis or panuveitis to assess the difference in the rate of recurrent exacerbations when treated with secukinumab versus placebo adjunctive to standard-of-care immunosuppressive therapy. This trial (NCT00995709) failed to demonstrate clinical improvement and showed a similar rate of recurrent ocular exacerbations during 24 weeks of treatment. Additional trials investigating the efficacy of secukinumab in (Behçet’s) uveitis were halted (eg, NCT01093846, NCT01032915, NCT01090310, and NCT01103024) or withdrawn (NCT01327664).
Secukinumab in Crohn’s disease
Expectations were high when anti-IL-17 treatment was recently tested in Crohn’s disease (CD), but it results in dramatic failure of both secukinumab as well as AMG 827 for this indication.Citation97,Citation98 The multicenter Phase II trial on secukinumab in 59 patients with established moderate-to-severe CD was prematurely terminated. Secukinumab did not help in improving CD; moreover, worsening of the disease was reported as reflected in the high rate of serious adverse events as well as fungal infections.Citation97 It seems that the role of IL-17 in diseases like CD has to be revisited, as more and more studies have uncovered the protective effects of IL-17 at mucosal sides, important for host defense and immune homeostasis together with other Th17 cytokines like IL-22.Citation99
Secukinumab in autoinflammatory syndromes
In contrast to autoimmune diseases with a dysregulated adaptive immune system, autoinflammatory syndromes like familial Mediterranean fever and TNF receptor-associated periodic syndrome are caused by an exaggerated innate immune system response resulting in episodes of spontaneous inflammation affecting multiple organs. This major difference in pathogenesis also affects the type of treatments applied; rather than anti-TNF, CTLA-4-Ig, or B-cell-based biologicals, corticosteroids or anti-IL-1 treatment are most frequently applied. Many autoinflammatory diseases are linked to a dysfunctional caspase-1 activity and increased secretion of IL-1β by monocytes/macrophages, and the blocking of IL-1β has resulted in dramatic improvement in various autoinflammatory diseases.Citation100 As the role of T-cells like Th17 cells in the pathogenesis of this type of inflammations is minimal or absent, the potential of secukinumab treatment in autoinflammatory disease will be only based on IL-17 production by innate immune cells and is therefore expected to be very limited.
Conclusion: potential place in therapy
After demonstrating great clinical efficacy in psoriasis,Citation74 secukinumab received its first global approval in Japan on Boxing Day 2014 for the treatment of psoriasis and PsA. The secukinumab US regulatory application for moderate-to-severe plaque psoriasis was filed in October 2013, with subsequent FDA recommendation in October 2014 and final FDA approval granted on January 21, 2015. Regulatory approval for secukinumab for the indications of AS and PsA was recently obtained in Europe and the US at the end of 2015/in early 2016.Citation24,Citation92 Depending on the outcome of Phase III trials on secukinumab in RA, this may be followed by filing for RA in the coming years, although it must be stressed that convincing clinical efficacy data for secukinumab in RA are still lacking and most likely will not show similar efficacy as demonstrated in other rheumatic diseases such as PsA and AS. With secukinumab entering the market, international treatment guidelines for various rheumatic diseasesCitation101–Citation104 may need to be updated, giving this IL-17 inhibitor as first in a novel class of drugs a position and ranking between the current available treatments for patients with various inflammatory rheumatic diseases.
Acknowledgments
This work was sponsored by the Innovative Medicines Initiative Joint Undertaking funded project BTCure (grant number: agreement 115142-2).
Disclosure
The authors report no conflicts of interest in this work.
References
- ScottDLWolfeFHuizingaTWRheumatoid arthritisLancet201037697461094110820870100
- McInnesIBSchettGThe pathogenesis of rheumatoid arthritisN Engl J Med2011365232205221922150039
- KlareskogLAmaraKMalmströmVAdaptive immunity in rheumatoid arthritis: anticitrulline and other antibodies in the pathogenesis of rheumatoid arthritisCurr Opin Rheumatol2014261727924257366
- EderLChandranVPelletFHuman leucocyte antigen risk alleles for psoriatic arthritis among patients with psoriasisAnn Rheum Dis2012711505521900282
- BrownMAKennaTWordsworthBPGenetics of ankylosing spondylitis–insights into pathogenesisNat Rev Rheumatol2016122819126439405
- KaneDStaffordLBresnihanBFitzGeraldOA prospective, clinical and radiological study of early psoriatic arthritis: an early synovitis clinic experienceRheumatology (Oxford)200342121460146814523223
- van der HeijdeDBellamyNCalinADougadosMKhanMAvan der LindenSPreliminary core sets for endpoints in ankylosing spondylitis. Assessments in Ankylosing Spondylitis Working GroupJ Rheumatol19972411222522299375888
- ColebatchANMarksJLEdwardsCJSafety of non-steroidal anti-inflammatory drugs, including aspirin and paracetamol (acetaminophen) in people receiving methotrexate for inflammatory arthritis (rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, other spondyloarthritis)Cochrane Database Syst Rev201111CD00887222071858
- van der HeijdeDBrebanMHalterDMaintenance of improvement in spinal mobility, physical function and quality of life in patients with ankylosing spondylitis after 5 years in a clinical trial of adalimumabRheumatology (Oxford)20155471210121925541333
- ScirèCACaporaliRSarzi-PuttiniPMonitornet projectDrug survival of the first course of anti-TNF agents in patients with rheumatoid arthritis and seronegative spondyloarthritis: analysis from the MonitorNet databaseClin Exp Rheumatol201331685786323981363
- O’DellJRMikulsTRTaylorTHCSP 551 RACAT InvestigatorsTherapies for active rheumatoid arthritis after methotrexate failureN Engl J Med2013369430731823755969
- SmolenJSLandewéRBreedveldFCEULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 updateAnn Rheum Dis201473349250924161836
- NamJLRamiroSGaujoux-VialaCEfficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2013 update of the EULAR recommendations for the management of rheumatoid arthritisAnn Rheum Dis201473351652824399231
- KoendersMIvan den BergWBNovel therapeutic targets in rheumatoid arthritisTrends Pharmacol Sci201536418919525732812
- SieperJPorter-BrownBThompsonLHarariODougadosMAssessment of short-term symptomatic efficacy of tocilizumab in ankylosing spondylitis: results of randomised, placebo-controlled trialsAnn Rheum Dis20147319510023765873
- SongIHHeldmannFRudwaleitMTreatment of active ankylosing spondylitis with abatacept: an open-label, 24-week pilot studyAnn Rheum Dis20117061108111021415053
- SongIHHeldmannFRudwaleitMOne-year follow-up of ankylosing spondylitis patients responding to rituximab treatment and re-treated in case of a flareAnn Rheum Dis201372230530622887847
- Jimenez-BojEStammTASadlonovaMRituximab in psoriatic arthritis: an exploratory evaluationAnn Rheum Dis201271111868187122833373
- MeasePGenoveseMCGladsteinGAbatacept in the treatment of patients with psoriatic arthritis: results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trialArthritis Rheum201163493994821128258
- McInnesIBKavanaughAGottliebABPSUMMIT 1 Study GroupEfficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trialLancet2013382989478078923769296
- GossecLSmolenJSRamiroSEuropean League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 updateAnn Rheum Dis201675349951026644232
- Celgene CorporationStudy of Apremilast to Treat Subjects With Active Ankylosing Spondylitis (POSTURE) Available from: https://clinicaltrials.gov/ct2/show/NCT01583374
- U.S. Food and Drug AdministrationFDA approves new psoriasis drug Cosentyx Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm430969.htm
- NovartisNovartis receives two new FDA approvals for Cosentyx to treat patients with ankylosing spondylitis and psoriatic arthritis in the US Available from: https://www.novartis.com/news/media-releases/novartis-receives-two-new-fda-approvals-cosentyx-treat-patients-ankylosing
- AggarwalSGurneyALIL-17: prototype member of an emerging cytokine familyJ Leukoc Biol20027111811781375
- RouvierELucianiMFMattéiMGDenizotFGolsteinPCTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri geneJ Immunol199315012544554568390535
- HarringtonLEHattonRDManganPRInterleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineagesNat Immunol20056111123113216200070
- ParkHLiZYangXOA distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17Nat Immunol20056111133114116200068
- GladiatorAWanglerNTrautwein-WeidnerKLeibundGut-LandmannSCutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infectionJ Immunol2013190252152523255360
- FerrettiSBonneauODuboisGRJonesCETrifilieffAIL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible triggerJ Immunol200317042106211212574382
- BucklandJNew role for mast cells as IL-17-expressing effector cells in established RANat Rev Rheumatol20106524320440895
- SchwandnerRYamaguchiKCaoZRequirement of tumor necrosis factor receptor-associated factor (TRAF) 6 in interleukin 17 signal transductionJ Exp Med200019171233124010748240
- GaffenSLStructure and signalling in the IL-17 receptor familyNat Rev Immunol20099855656719575028
- MurphyCALangrishCLChenYDivergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammationJ Exp Med2003198121951195714662908
- CuaDJSherlockJChenYInterleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brainNature2003421692474474812610626
- IvanovIIMcKenzieBSZhouLThe orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cellsCell200612661121113316990136
- ManelNUnutmazDLittmanDRThe differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammatNat Immunol20089664164918454151
- YePRodriguezFHKanalySRequirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defenseJ Exp Med2001194451952711514607
- HuangWNaLFidelPLSchwarzenbergerPRequirement of interleukin-17A for systemic anti-Candida albicans host defense in miceJ Infect Dis2004190362463115243941
- OuyangWKollsJKZhengYThe biological functions of T helper 17 cell effector cytokines in inflammationImmunity200828445446718400188
- MilnerJDBrenchleyJMLaurenceAImpaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndromeNature2008452718877377618337720
- MaCSChewGYSimpsonNDeficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3J Exp Med200820571551155718591410
- BurkettPRMeyer zu HorsteGKuchrooVKPouring fuel on the fire: Th17 cells, the environment, and autoimmunityJ Clin Invest201512562211221925961452
- WuHJIvanovIIDarceJGut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cellsImmunity201032681582720620945
- KirkhamBWKavanaughAReichKInterleukin-17A: a unique pathway in immune-mediated diseases: psoriasis, psoriatic arthritis and rheumatoid arthritisImmunology2014141213314223819583
- FossiezFDjossouOChomaratPT cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokinesJ Exp Med19961836259326038676080
- KotakeSUdagawaNTakahashiNIL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesisJ Clin Invest199910391345135210225978
- ChabaudMMiossecPThe combination of tumor necrosis factor alpha blockade with interleukin-1 and interleukin-17 blockade is more effective for controlling synovial inflammation and bone resorption in an ex vivo modelArthritis Rheum20014461293130311407688
- LeGrandAFermorBFinkCInterleukin-1, tumor necrosis factor alpha, and interleukin-17 synergistically up-regulate nitric oxide and prostaglandin E2 production in explants of human osteoarthritic knee menisciArthritis Rheum20014492078208311592370
- KoshyPJHendersonNLoganCLifePFCawstonTERowanADInterleukin 17 induces cartilage collagen breakdown: novel synergistic effects in combination with proinflammatory cytokinesAnn Rheum Dis200261870471312117676
- Van BezooijenRLVan Der Wee-PalsLPapapoulosSELöwikCWInterleukin 17 synergises with tumour necrosis factor alpha to induce cartilage destruction in vitroAnn Rheum Dis2002611087087612228154
- ChabaudMDurandJMBuchsNHuman interleukin-17: A T cell-derived proinflammatory cytokine produced by the rheumatoid synoviumArthritis Rheum199942596397010323452
- ZiolkowskaMKocALuszczykiewiczGHigh levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanismJ Immunol200016452832283810679127
- GullickNJAbozaidHSJayarajDMEnhanced and persistent levels of interleukin (IL)-17+ CD4+ T cells and serum IL-17 in patients with early inflammatory arthritisClin Exp Immunol2013174229230123815507
- ShenHXiaLLuJXiaoWInfliximab reduces the frequency of interleukin 17-producing cells and the amounts of interleukin 17 in patients with rheumatoid arthritisJ Investig Med2010587905908
- ChenDYChenYMChenHHHsiehCWLinCCLanJLIncreasing levels of circulating Th17 cells and interleukin-17 in rheumatoid arthritis patients with an inadequate response to anti-TNF-α therapyArthritis Res Ther2011134R12621801431
- AlzabinSAbrahamSMTaherTEIncomplete response of inflammatory arthritis to TNFα blockade is associated with the Th17 pathwayAnn Rheum Dis201271101741174822550316
- HullDNWilliamsROPathanEAlzabinSAbrahamSTaylorPCAnti-tumour necrosis factor treatment increases circulating T helper type 17 cells similarly in different types of inflammatory arthritisClin Exp Immunol2015181340140625766640
- MenonBGullickNJWalterGJInterleukin-17+ CD8+ T cells are enriched in the joints of patients with psoriatic arthritis and correlate with disease activity and joint damage progressionArthritis Rheumatol20146651272128124470327
- Van BaarsenLGMLebreMCvan der CoelenDSnoekBCGerlagDMTakPPIL-17 levels in synovium of patients with rheumatoid arthritis, psoriatic arthritis and osteoarthritis: target validation in various forms of arthritisAnn Rheum Dis201170A79
- HüffmeierUUebeSEkiciABCommon variants at TRAF3IP2 are associated with susceptibility to psoriatic arthritis and psoriasisNat Genet2010421199699920953186
- RuedaBOrozcoGRayaEThe IL23R Arg381Gln non-synonymous polymorphism confers susceptibility to ankylosing spondylitisAnn Rheum Dis200867101451145418199597
- NakaeSNambuASudoKIwakuraYSuppression of immune induction of collagen-induced arthritis in IL-17-deficient miceJ Immunol2003171116173617714634133
- NakaeSSaijoSHoraiRSudoKMoriSIwakuraYIL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonistProc Natl Acad Sci U S A2003100105986599012721360
- KoendersMIKollsJKOppers-WalgreenBInterleukin-17 receptor deficiency results in impaired synovial expression of interleukin-1 and matrix metalloproteinases 3, 9, and 13 and prevents cartilage destruction during chronic reactivated streptococcal cell wall-induced arthritisArthritis Rheum200552103239324716200598
- LubbertsEKoendersMIOppers-WalgreenBTreatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosionArthritis Rheum200450265065914872510
- KoendersMIDevesaIMarijnissenRJInterleukin-1 drives pathogenic Th17 cells during spontaneous arthritis in interleukin-1 receptor antagonist-deficient miceArthritis Rheum200858113461347018975337
- KoendersMILubbertsEOppers-WalgreenBBlocking of interleukin-17 during reactivation of experimental arthritis prevents joint inflammation and bone erosion by decreasing RANKL and interleukin-1Am J Pathol2005167114114915972960
- KoendersMIMarijnissenRJDevesaITumor necrosis factor-interleukin-17 interplay induces S100A8, interleukin-1β, and matrix metalloproteinases, and drives irreversible cartilage destruction in murine arthritis: rationale for combination treatment during arthritisArthritis Rheum20116382329233921520013
- Plater-ZyberkCJoostenLAHelsenMMKoendersMIBaeuerlePAvan den BergWBCombined blockade of granulocyte-macrophage colony stimulating factor and interleukin 17 pathways potently suppresses chronic destructive arthritis in a tumour necrosis factor alpha-independent mouse modelAnn Rheum Dis200968572172818495731
- van NieuwenhuijzeAEvan de LooFAWalgreenBComplementary action of granulocyte macrophage colony-stimulating factor and interleukin-17A induces interleukin-23, receptor activator of nuclear factor-κB ligand, and matrix metalloproteinases and drives bone and cartilage pathology in experimental arthritis: rationale for combination therapy in rheumatoid arthritisArthritis Res Ther20151716326081345
- RuutuMThomasGSteckRβ-glucan triggers spondyloarthritis and Crohn’s disease-like ileitis in SKG miceArthritis Rheum20126472211222222328069
- BenhamHRehaumeLMHasnainSZInterleukin-23 mediates the intestinal response to microbial β-1,3-glucan and the development of spondyloarthritis pathology in SKG miceArthritis Rheumatol20146671755176724664521
- LangleyRGElewskiBELebwohlMERASURE Study GroupFIXTURE Study GroupSecukinumab in plaque psoriasis – results of two phase 3 trialsN Engl J Med2014371432633825007392
- GenoveseMCDurezPRichardsHBEfficacy and safety of secukinumab in patients with rheumatoid arthritis: a phase II, dose-finding, double-blind, randomised, placebo controlled studyAnn Rheum Dis201372686386922730366
- MeasePJMcInnesIBKirkhamBFUTURE 1 Study GroupSecukinumab inhibition of interleukin-17A in patients with psoriatic arthritisN Engl J Med2015373141329133926422723
- McInnesIBMeasePJKirkhamBFUTURE 2 Study GroupSecukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trialLancet201538699991137114626135703
- BaetenDLBraunJBaraliakosXSecukinumab, a monoclonal antibody to interleukin-17A, significantly improves signs and symptoms of active ankylosing spondylitis: results of a 52-week phase 3 randomized placebo-controlled trial with intravenous loading and subcutaneous maintenance dosingArthritis Rheumatol201466S10S360
- BraunJDeodharAASieperJSecukinumab significantly improves signs and symptoms of active ankylosing spondylitis: 52-week results from a randomized, double-blind, placebo-controlled phase 3 trial with subcutaneous loading and maintenance dosing [abstract]Arthritis Rheumatol201567suppl 10
- BurmesterGRDurezPShestakovaGAssociation of HLA-DRB1 alleles with clinical responses to the anti-interleukin-17A monoclonal antibody secukinumab in active rheumatoid arthritisRheumatology (Oxford)2016551495526268815
- LebwohlMStroberBMenterAPhase 3 studies comparing brodalumab with ustekinumab in psoriasisN Engl J Med2015373141318132826422722
- AmgenAmgen to Terminate Participation in Co-development and Commercialization of Brodalumab [press release]Thousand Oaks, CAPRNewswire2015522 Available from: http://investors.amgen.com/phoenix.zhtml?c=61656&p=irol-newsArticle&ID=2052862
- DaneshMJKimballABBrodalumab and suicidal ideation in the context of a recent economic crisis in the United StatesJ Am Acad Dermatol201674119019226702804
- HueberWPatelDDDryjaTEffects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitisSci Transl Med201025252ra72
- PappKALangleyRGSigurgeirssonBEfficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging studyBr J Dermatol2013168241242123106107
- BlauveltAPrinzJCGottliebABFEATURE Study GroupSecukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE)Br J Dermatol2015172248449325132411
- GenoveseMCDurezPRichardsHBOne-year efficacy and safety results of secukinumab in patients with rheumatoid arthritis: phase II, dose-finding, double-blind, randomized, placebo-controlled studyJ Rheumatol201441341442124429175
- KoendersMIMarijnissenRJJoostenLAT cell lessons from the rheumatoid arthritis synovium SCID mouse model: CD3-rich synovium lacks response to CTLA-4Ig but is successfully treated by interleukin-17 neutralizationArthritis Rheum20126461762177022213107
- Novartis PharmaceuticalsEfficacy at 24 Weeks and Safety, Tolerability and Long Term Efficacy up to 2 Years of Secukinumab (AIN457) in Patients With Active Rheumatoid Arthritis and an Inadequate Response to Anti-TNFα Agents (REASSURE) Available from: https://clinicaltrials.gov/ct2/show/NCT01377012
- Novartis PharmaceuticalsSafety and Efficacy of Extended Treatment With Secukinumab in Anti-TNF Inadequate Responders in RA. (REASSURE-E) Available from: https://clinicaltrials.gov/ct2/show/NCT01901900
- McInnesIBSieperJBraunJEfficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trialAnn Rheum Dis201473234935623361084
- NovartisNovartis receives two landmark European approvals for Cosentyx to treat patients with ankylosing spondylitis and psoriatic arthritis Available from: https://www.novartis.com/news/media-releases/novartis-receives-two-landmark-european-approvals-cosentyx-treat-patients
- BaetenDBaraliakosXBraunJAnti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trialLancet201338299061705171324035250
- BaraliakosXBorahBBraunJLong-term effects of secukinumab on MRI findings in relation to clinical efficacy in subjects with active ankylosing spondylitis: an observational studyAnn Rheum Dis201675240841226248638
- BaraliakosXDeodharAABraunJEffect of interleukin-17A inhibition on spinal radiographic changes through 2 years in patients with active ankylosing spondylitis: results of a phase 3 study with secukinumab [abstract]Arthritis Rheumatol201567suppl 10
- BaetenDSieperJBraunJMEASURE 1 Study GroupMEASURE 2 Study GroupSecukinumab, an interleukin-17A Inhibitor, in ankylosing spondylitisN Engl J Med2015373262534254826699169
- HueberWSandsBELewitzkySSecukinumab in Crohn’s Disease Study GroupSecukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trialGut201261121693170022595313
- TarganSRFeaganBGVermeireSA randomized, double-blind, placebo-controlled study to evaluate the safety, tolerability, and efficacy of AMG 827 in subjects with moderate to severe Crohn’s diseaseGastroenterology2012143E26
- O’ConnorWJrZenewiczLAFlavellRAThe dual nature of T(H)17 cells: shifting the focus to functionNat Immunol201011647147620485275
- DinarelloCASimonAvan der MeerJWTreating inflammation by blocking interleukin-1 in a broad spectrum of diseasesNat Rev Drug Discov201211863365222850787
- BraunJvan den BergRBaraliakosX2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitisAnn Rheum Dis201170689690421540199
- SmolenJSLandewéRBreedveldFCEULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 updateAnn Rheum Dis201473349250924161836
- SmolenJSBraunJDougadosMTreating spondyloarthritis, including ankylosing spondylitis and psoriatic arthritis, to target: recommendations of an international task forceAnn Rheum Dis201473161623749611
- GossecLSmolenJSRamiroSEuropean League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 updateAnn Rheum Dis201675349951026644232
