Abstract
Objective
Ocular hypertension is an important risk factor for glaucoma. The purpose of this study was to investigate the gliotoxic effects of α-aminoadipic acid (AAA) in a rat model of AOH and its underlying mechanisms.
Materials and methods
In the rat model of acute ocular hypertension (AOH), intraocular pressure was increased to 110 mmHg for 60 minutes. Animals were divided into four groups: sham operation (Ctrl), AOH, AOH + phosphate-buffered saline (PBS), and AOH + AAA. Cell apoptosis in the ganglion cell layer was detected with the terminal deoxynucleotidyl transferase-mediated uridine 5′-triphosphate-biotin nick end labeling (TUNEL) assay, and retinal ganglion cells (RGCs) immunostained with Thy-1 were counted. Müller cell activation was detected using immunostaining with glutamine synthetase and glial fibrillary acidic protein. Tumor necrosis factor-α (TNF-α) was examined using Western blot.
Results
In the rat model of AOH, cell apoptosis was induced in the ganglion cell layer and the number of RGCs was decreased. Müller cell gliosis in the retinas of rats was induced, and retinal protein levels of TNF-α were increased. Intravitreal treatment of AAA versus PBS control attenuated these retinal abnormalities to show protective effects in the rat model of AOH.
Conclusion
In the retinas of the rat model of AOH, AAA treatment attenuated retinal apoptosis in the ganglion cell layer and preserved the number of RGCs, likely through the attenuation of Müller cell gliosis and suppression of TNF-α induction. Our observations suggest that AAA might be a potential therapeutic target in glaucoma.
Introduction
Glaucoma, a neurodegenerative disease, is characterized by gradual loss of retinal ganglion cells (RGCs) and optic nerve atrophy.Citation1 Multiple factors are related to glaucoma, such as high intraocular pressure (IOP)Citation2 and low cerebral spinal fluid pressure.Citation3 Müller cells are the major type of glial cells in the mammalian retina that can support and nourish retinal neurons, maintain extracellular ion homeostasis, glutamate recycling, and interaction in synaptic transmission.Citation4,Citation5 Therefore, Müller cells are involved in retinal function. Investigations indicate that Müller cells not only play an important physiological role, but they are also involved in multiple pathological retinal diseases such as glaucoma.Citation6–Citation8 It has been reported that reactive Müller cells can aggravate retinal damage by releasing cytokines such as tumor necrosis factor-α (TNF-α).Citation9 Growing evidence supports that increased glial production of TNF-α contributes to the neurodegeneration in glaucoma.Citation10 TNF-α is a secreted inflammatory cytokine that is responsible for apoptosis, necrosis, and inflammation.Citation11,Citation12 TNF-α is increased in the retina following ischemia or damage and other neurodegenerative disorders.Citation13–Citation17
α-Aminoadipic acid (AAA), a six-carbon homolog of glutamate, is a well-known compound that induces specific glial toxicity through blocking the glutamate uptake.Citation18–Citation20 The use of AAA to interfere with glial influence on neuronal tissues is reported.Citation21,Citation22 However, the effects of AAA inhibition on Müller cell gliosis in glaucoma are unknown. The current study aimed to determine if AAA treatment inhibits Müller cell gliosis and protects against retinal abnormalities induced in a rat model of acute ocular hypertension (AOH) mimicking glaucoma. Müller cell activation of RGCs and TNF-α induction were examined.
Materials and methods
Animals
Adult male Sprague Dawley rats (weight range, 200–250 g, 8–10 weeks) were used in this study in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and followed the People’s Republic of China animal care guidelines. This study was monitored and approved by the Animal Care Committee of the Capital Medical University, SCXK (Jing) 2012-0001. The animals were housed with a 12-hour light/12-hour dark cycle with standard chow and water. All surgical procedures were performed under anesthesia with intraperitoneal chloral hydrate (450 mg/kg) and topical 0.5% proparacaine hydrochloride eye drops (Alcon, Inc., Hünenberg, Switzerland).
Rat model of AOH
The IOP was increased to 110 mmHg (14.63 kPa) for 60 minutes by using an elevated 500 mL plastic container of sterile physiological saline connected to a 27-gauge needle placed in the anterior chamber of the eye. Sham procedure eyes were treated similarly but without the elevation of the bottle; hence, the normal IOP was maintained. Time-course examination was performed at 1 day, 3 days, and 5 days after acute IOP elevation. Animals were divided into sham operation (Ctrl) group, AOH group, AOH + phosphate-buffered saline (PBS) control group, and AOH + AAA-treated group. AAA (250 μg) was injected into vitreous humor in 5 μL at 50 g/L concentration after 3 hours of acute IOP elevation according to the literature.Citation23,Citation24
Immunohistochemistry
Tissues were fixed with 4% paraformaldehyde. Eyeballs were cryoprotected in 30% sucrose overnight at 4°C and then embedded in optimal cutting temperature compound (Sakura Finetechinical Co. Ltd., Tokyo, Japan). Sections (14 μm) were incubated in 3% bovine serum albumin and 0.3% Triton X-100 (Sigma-Aldrich Co., St Louis, MO, USA) to block nonspecific binding and then incubated with primary mouse monoclonal antibodies against Thy-1 (1:200, RGC marker; Abcam, Cambridge, UK), primary mouse monoclonal antibodies against glutamine synthetase (GS; 1:100, Müller glial cell maker; Abcam), rabbit polyclonal antibodies against glial fibrillary acidic protein (GFAP; 1:200, gliosis maker; Cell Signaling Technology Inc., Danvers, MA, USA) overnight at 4°C, followed by incubation with secondary antibodies for 1 hour at room temperature. Nuclei were stained by Hoechst 33342. Sections were mounted with Fluoromount-G (SouthernBiotech, Birmingham, AL, USA). Negative controls were incubated without primary antibodies. Slides were visualized using a confocal microscope (Leica Microsystems, Wetzlar, Germany). The intensity of immunoreactivity from photographs was analyzed using Image-Pro®Plus 6.0 (Media Cybernetics Inc., Silver Spring, MD, USA). Optical densities obtained from immunohistochemistry images were corrected by subtracting the average value of background noise from five image inputs.
Terminal deoxynucleotidyl transferase-mediated uridine 5′-triphosphate-biotin nick end labeling assay
The terminal deoxynucleotidyl transferase-mediated uridine 5′-triphosphate-biotin nick end labeling (TUNEL) assay was conducted according to the manufacturer’s protocol. Briefly, tissue sections were fixed in paraformaldehyde and were subsequently incubated in a TUNEL reaction medium. The immune-labeled RGCs were costained with Hoechst 33342 for 5 minutes. The sections were then mounted on microscope slides and examined under ultraviolet light using an epifluorescence microscope. Ten visual fields were analyzed per retina; the experiment was replicated three times.
Western blot
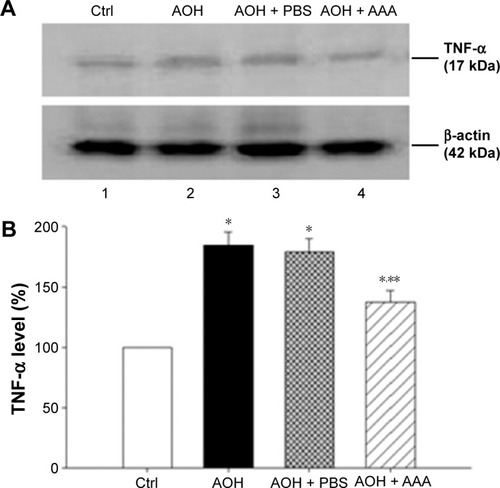
Protein extracts were generated from retinas. Retinas from sham operation group (Ctrl), AOH group, AOH + PBS control group, and AOH + AAA group were homogenized in lysis buffer and sonicated to dissolve the tissue completely. In all, 40 μg of total proteins from each sample were loaded per lane for sodium dodecyl sulfate polyacrylamide gel electrophoresis. The proteins were transferred onto the nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany) and the blocked nitrocellulose membrane was incubated with a primary rabbit polyclonal antibody against TNF-α (1:1,000; Abcam) for 3 hours (room temperature). After washing in Tris Buffered Saline, with Tween-20 (TBST), the membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit or mouse IgG prior to detection of the labeled proteins by using ECL-plus kit (PerkinElmer Inc., Waltham, MA, USA) followed by exposure of blots to X-Omat (Kodak, Rochester, NY, USA) imaging film. The images were quantified using ImageJ software (National Institutes of Health, Maryland, USA). The Gel Doc-2000 imaging system (Bio-Rad Laboratories Inc., Hercules, CA, USA) was used to perform the quantitative analysis of Western blot results. The ratio of TNF-α was determined by normalizing with the levels of endogenous β-actin. The β-actin band density was expressed as 100%, and the other group was expressed as percentage of that from the control group.
Statistical analysis
The normality of the data was tested using the Shapiro–Wilk method. Analysis of variance was used to determine the difference between TNF-α and fluorescence intensity among three or more independent (unrelated) groups, followed by multiple comparisons using the Student–Newman–Keuls test. The Kruskal–Wallis test was used to determine the difference between TUNEL-positive cells and Thy-1-positive RGCs among three or more independent (unrelated) groups, followed by multiple comparisons using the Nemenyi test. Statistical analysis was performed using SAS 9.2 (SAS Institute Inc., Cary, NC, USA). Values were reported as mean ± SD. A value of P<0.05 was considered as statistically significant.
Results
AAA attenuates AOH-induced cell apoptosis in RGCs
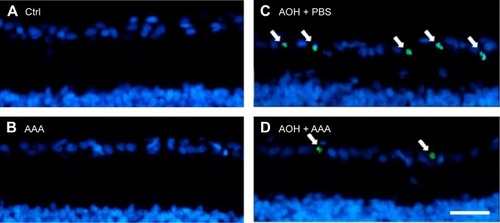
To determine the effects of AAA on RGCs in the AOH model, AOH was induced in SD rats and rats were intravitreally treated with PBS, AAA, AOH + PBS, and AOH + AAA. Significantly reduced retinal thickness in the inner plexiform layer (IPL) and inner nuclear layer was observed after AOH induction versus control (). TUNEL assay was used to examine the cell apoptosis in the retinal sections. The mean values of TUNEL-positive cells per visual field were 0.1±0.3 in Control (), 0.2±0.4 in AAA(), 4.6±0.8 in AOH + PBS, and 1.7±0.7 in AOH + AAA. AOH induced the apoptotic death of cells in the ganglion cell layer(GCL; ). In the AOH model, intravitreal treatment with AAA attenuated the TUNEL-positive RGCs more significantly than the intravitreal treatment with PBS (P<0.05; ), indicating that AAA treatment rescued RGCs from the cell apoptosis induced in the AOH model.
Figure 1 AAA attenuates AOH-induced retinal apoptosis.
Abbreviations: AAA, α-aminoadipic acid; AOH, acute ocular hypertension; TUNEL, terminal deoxynucleotidyl transferase-mediated uridine 5′-triphosphate-biotin nick end labeling; Ctrl, control.
AAA preserves the AOH-reduced number of RGCs
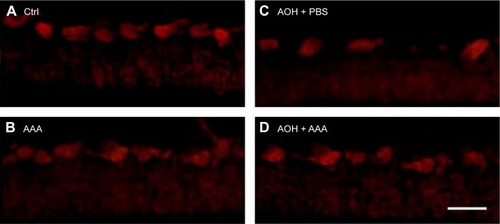
In addition, retinal sections were immunostained with antibody against Thy-1, a marker for RGCs.Citation25 The mean values of Thy-1-positive RGCs per visual field in Ctrl, AAA, AOH + PBS, and AOH + AAA groups were 8.2±0.9, 8.1±0.8, 3.9±0.7, and 6±0.6, respectively. AAA treatment did not change the number of RGCs in normal conditions (). When compared with the Ctrl group (), the number of Thy-1-positive RGCs was decreased in the AOH + PBS group (), and AAA treatment significantly rescued Thy-1-positive RGCs in AOH (P<0.05; ), corresponding to AAA-attenuated apoptosis of RGCs in AOH (AOH + PBS versus AOH + AAA, P<0.05; ).
Figure 2 AAA rescued AOH-reduced number of RGCs.
Abbreviations: AAA, α-aminoadipic acid; AOH, acute ocular hypertension; RGCs, retinal ganglion cells; Ctrl, control; PBS, phosphate-buffered saline.
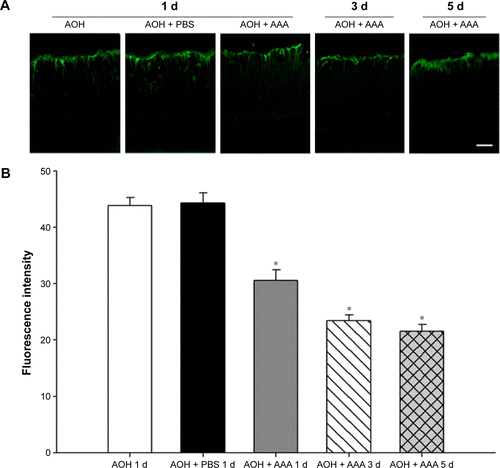
AAA attenuates AOH-induced Müller cell gliosis
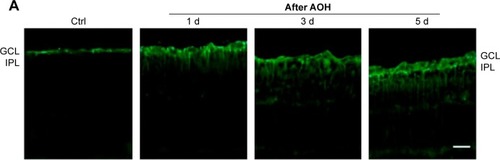
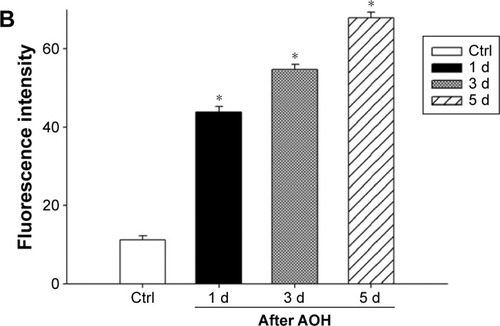
In the rat model of AOH, AAA rescued RGCs. To determine if AAA protects against AOH-induced RGC death through modulating Müller cell gliosis, immonohistochemistry for GFAP (a marker for activated glial cells) and GS (Müller cell-specific marker) was conducted.Citation26 Müller cells cover the whole retina from the nerve fiber layer to the photoreceptor layer,Citation27 and astrocytes mainly locate along GCL. GFAP was mainly expressed along nerve fiber layer and GCL and was less or absent at other layers in the control group (). In the rat model of AOH, colocalization of GFAP and GS was observed across IPL (), indicating Müller cell activation. After 1 day, 3 days, and 5 days of AOH induction in rat, GFAP expression was also observed across IPL (), indicating Müller cell gliosis. AAA treatment versus PBS control markedly reduced GFAP immunoreactivity across IPL (), indicating attenuation in Müller cell gliosis.
Figure 3 AAA attenuated induced Müller gliosis in the rat model of AOH.
Abbreviations: AAA, α-aminoadipic acid; AOH, acute ocular hypertension; GFAP, glial fibrillary acidic protein; d, day; Ctrl, control; GCL, ganglion cell layer; IPL, inner plexiform layer.
AAA decreases AOH-induced TNF-α production
As mentioned earlier, AAA rescued RGCs likely through the inhibition of Müller cells gliosis in the rat model with AOH. Activation of Müller cells may induce TNF-α production, leading to neurodegeneration in glaucoma.Citation10 To determine if AAA modulates TNF-α production, Western blot was performed for the investigation of retinal protein levels of TNF-α. Significantly upregulated protein levels of retinal TNF-α were observed in AOH versus sham operation (Ctrl) group (P<0.05; ). Importantly, AAA treatment versus PBS control significantly attenuated TNF-α levels in the retinas of the rat model of AOH (P<0.05; ).
Figure 4 AAA treatment reduced the induction of TNF-α in AOH retinas.
Abbreviations: AAA, α-aminoadipic acid; TNF-α, tumor necrosis factor-α; d, days; AOH, acute ocular hypertension; Ctrl, control; PBS, phosphate-buffered saline.
Discussion
Glaucoma is a leading cause of irreversible vision loss characterized by progressive death of RGCs, and elevated IOP is a major risk factor.Citation28 A rodent model of AOH is well established for acute angle closure glaucoma, and it has been widely used to investigate the pathogenesis of death of RGCs.Citation29 In the current rat model of AOH, we found induced cell apoptosis and decreased number of RGCs with IOP induction versus sham operation control. Importantly, treatment of AAA versus PBS control significantly attenuated RGC apoptosis and rescued the reduced number of RGCs, demonstrating protective effects on AOH retinas ().
Figure 5 A schematic flow chart of AAA protection in the rat model of AOH.
Abbreviations: AAA, α-aminoadipic acid; AOH, acute ocular hypertension; IOP, intraocular pressure; RGC, retinal ganglion cells, TNF-α, tumor necrosis factor-α.
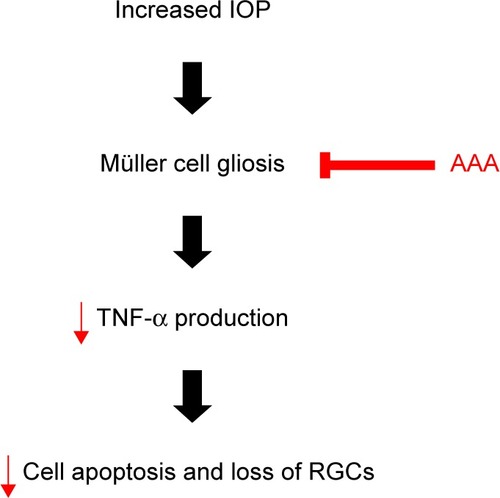
Activated Müller cells contribute to the progression of glaucoma.Citation30 Müller cells perform a multitude of important regulatory and supportive roles, including secretion of trophic factors, removal of metabolic waste, and neurotransmitter recycling.Citation31,Citation32 We found that Müller cells were activated with induced GFAP immunoreactivity in the rat model of AOH versus control retinas. GFAP, an intermediate filament protein, is considered as a marker of reactive Müller cell gliosis,Citation27 which is not or less expressed in Müller cells in normal retinas and expressed highly at ischemic,Citation33 light-induced retinal degeneration,Citation34 and retinal detachment.Citation35 Activation of Müller cells so far was demonstrated to have both protective and detrimental effects.Citation36–Citation38 Especially early after injury, Müller cell gliosis is believed to be neuroprotective and promotes the repair of neurons in response to injury,Citation36 with GFAP upregulation to provide additional structural integrity to the retina at the site of injury.Citation39 However, activated Müller cells also express TNF-α,Citation40 monocyte chemoattractant protein,Citation41 and nitric oxideCitation42 to induce death of RGCs. TNF-α induction contributes to inflammation, apoptosis, and necrosis, leading to cell death. We observed that AAA treatment attenuated GFAP immunoreactivity in Müller cell processes across IPL and reduced retinal TNF-α levels in the rat model of AOH. These findings suggested that AAA promoted survival of RGCs in the rat model of AOH, likely through the inhibition of AOH-induced Müller cell gliosis and in turn downregulation of TNF-α protein production.
Conclusion
We found that AAA could effectively protect retina against loss of RGCs and apoptosis in AOH retinas through attenuating Müller cell gliosis and downregulating TNF-α production. These observations suggested that AAA might be a potential therapeutic target in the treatment of neurodegeneration in glaucoma.
Acknowledgments
This study was funded by Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (No XM201305) and Clinical-Basic Cooperation Program from Capital Medical University (Nos 14JL32 and 2015YKSJ02).
Supplementary materials
Figure S1 (A) Reprehensive results of Hoechst staining in rat retina after 1 d, 3 d, and 5 d of AOH; (B) Quantitative analysis of the retinal thickness.
Abbreviations: AOH, acute ocular hypertension; IPL, inner plexiform layer; INL, inner nuclear layer; Ctrl, control; d, day; GCL, ganglion cell layer; ONL, outer nuclear layer.
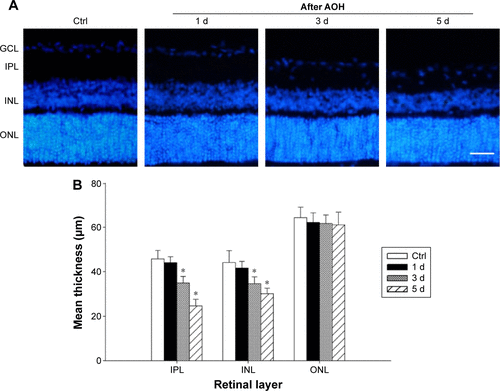
Figure S2 Immunohistochemistry costained with antibodies against GS (green) and GFAP (red).
Abbreviations: GS, glutamine synthetase; GFAP, glial fibrillary acidic protein; AOH, acute ocular hypertension; Ctrl, control; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; ONL, outer nuclear layer; d, days.
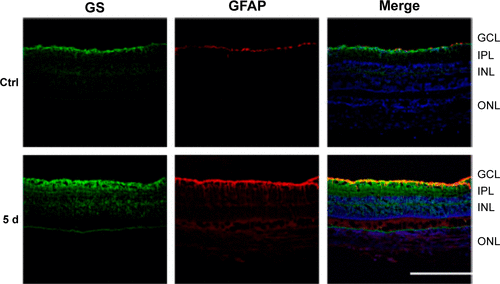
Figure S3 Increased GFAP immunoreactivity after AOH induction in rat retinas.
Abbreviations: GFAP, glial fibrillary acidic protein; AOH, acute ocular hypertension; IPL, inner plexiform layer; d, day; PBS, phosphate-buffered saline; AAA, α-aminoadipic acid.
Disclosure
The authors report no conflicts of interest in this work.
References
- GugletaKTurkseverCPoluninaAOrgulSEffect of ageing on the retinal vascular responsiveness to flicker light in glaucoma patients and in ocular hypertensionBr J Ophthalmol201397784885123624271
- HusainSAbdulYCrossonCEPreservation of retina ganglion cell function by morphine in a chronic ocular-hypertensive rat modelInvest Ophthalmol Vis Sci20125374289429822661469
- RenRJonasJBTianGCerebrospinal fluid pressure in glaucoma: a prospective studyOphthalmology2010117225926619969367
- ChungSHShenWJayawardanaKDifferential gene expression profiling after conditional Muller-cell ablation in a novel transgenic modelInvest Ophthalmol Vis Sci20135432142215223449719
- CooreyNJShenWChungSHZhuLGilliesMCThe role of glia in retinal vascular diseaseClin Exp Optom201295326628122519424
- TezelGFourth ARVO/Pfizer Ophthalmics Research Institute Conference Working GroupThe role of glia, mitochondria, and the immune system in glaucomaInvest Ophthalmol Vis Sci20095031001101219244206
- JohnsonECMorrisonJCFriend or foe? Resolving the impact of glial responses in glaucomaJ Glaucoma200918534135319525723
- BringmannAWiedemannPMuller glial cells in retinal diseaseOphthalmologica20122271119
- WangXNgYKTaySSFactors contributing to neuronal degeneration in retinas of experimental glaucomatous ratsJ Neurosci Res200582567468916273539
- TezelGTNF-alpha signaling in glaucomatous neurodegenerationProg Brain Res200817340942118929124
- GuptaSMolecular steps of tumor necrosis factor receptor-mediated apoptosisCurr Mol Med20011331732411899080
- GessleinBHakanssonGGustafssonLEkstromPMalmsjoMTumor necrosis factor and its receptors in the neuroretina and retinal vasculature after ischemia-reperfusion injury in the pig retinaMol Vis2010162317232721152396
- TezelGLiLYPatilRVWaxMBTNF-alpha and TNF-alpha receptor-1 in the retina of normal and glaucomatous eyesInvest Ophthalmol Vis Sci20014281787179411431443
- GustavssonCAgardhCDHagertPAgardhEInflammatory markers in nondiabetic and diabetic rat retinas exposed to ischemia followed by reperfusionRetina200828464565218398369
- NakazawaTNakazawaCMatsubaraATumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucomaJ Neurosci20062649126331264117151265
- CheongCUChangCPChaoCMChengBCYangCZChioCCEtanercept attenuates traumatic brain injury in rats by reducing brain TNF-alpha contents and by stimulating newly formed neurogenesisMediators Inflamm2013201362083723710117
- MaddahiAKruseLSChenQWEdvinssonLThe role of tumor necrosis factor-alpha and TNF-alpha receptors in cerebral arteries following cerebral ischemia in ratJ Neuroinflammation2011810721871121
- PannickeTStabelJHeinemannUReicheltWalpha-Aminoadipic acid blocks the Na(+)-dependent glutamate transport into acutely isolated Muller glial cells from guinea pig retinaPflugers Arch199442911401427708474
- IshikawaYMineSAminoadipic acid toxic effects on retinal glial cellsJpn J Ophthalmol19832711071186855004
- KhurgelMKooACIvyGOSelective ablation of astrocytes by intracerebral injections of alpha-aminoadipateGlia19961643513588721675
- LinserPJMosconaAAInduction of glutamine synthetase in embryonic neural retina: its suppression by the gliatoxic agent alpha-aminoadipic acidBrain Res198122711031196110468
- HuckSGrassFHattenMEGliotoxic effects of alpha-aminoadipic acid on monolayer cultures of dissociated postnatal mouse cerebellumNeuroscience19841237837916472620
- RichKAFigueroaSLZhanYBlanksJCEffects of Muller cell disruption on mouse photoreceptor cell developmentExp Eye Res19956122352487556487
- GaneshBSChintalaSKInhibition of reactive gliosis attenuates excitotoxicity-mediated death of retinal ganglion cellsPLoS One201163e1830521483783
- BarnstableCJDragerUCThy-1 antigen: a ganglion cell specific marker in rodent retinaNeuroscience19841148478556146113
- ChenHWeberAJExpression of glial fibrillary acidic protein and glutamine synthetase by Muller cells after optic nerve damage and intravitreal application of brain-derived neurotrophic factorGlia200238211512511948805
- BringmannAIandievIPannickeTCellular signaling and factors involved in Muller cell gliosis: neuroprotective and detrimental effectsProg Retin Eye Res200928642345119660572
- GuptaNYucelYHGlaucoma as a neurodegenerative diseaseCurr Opin Ophthalmol200718211011417301611
- MiXSFengQLoACProtection of retinal ganglion cells and retinal vasculature by Lycium barbarum polysaccharides in a mouse model of acute ocular hypertensionPLoS One2012710e4546923094016
- ChongRSMartinKRGlial cell interactions and glaucomaCurr Opin Ophthalmol2015262737725490529
- BringmannAPannickeTGroscheJMuller cells in the healthy and diseased retinaProg Retin Eye Res200625439742416839797
- ReichenbachABringmannANew functions of Muller cellsGlia201361565167823440929
- WurmAIandievIUhlmannSEffects of ischemia-reperfusion on physiological properties of Muller glial cells in the porcine retinaInvest Ophthalmol Vis Sci20115263360336721345997
- TorbidoniVIribarneMSuburoAMEndothelin receptors in light-induced retinal degenerationExp Biol Med (Maywood)200623161095110016741056
- GhoshFJohanssonKNeuronal and glial alterations in complex long-term rhegmatogenous retinal detachmentCurr Eye Res201237870471122578195
- KimJHKimJHParkJABlood-neural barrier: intercellular communication at glio-vascular interfaceJ Biochem Mol Biol200639433934516889675
- BringmannAPannickeTBiedermannBRole of retinal glial cells in neurotransmitter uptake and metabolismNeurochem Int2009543–414316019114072
- MartinKRLevkovitch-VerbinHValentaDBaumrindLPeaseMEQuigleyHARetinal glutamate transporter changes in experimental glaucoma and after optic nerve transection in the ratInvest Ophthalmol Vis Sci20024372236224312091422
- DyerMACepkoCLControl of Muller glial cell proliferation and activation following retinal injuryNat Neurosci20003987388010966617
- LeiXZhangJShenJEPO attenuates inflammatory cytokines by Muller cells in diabetic retinopathyFront Biosci (Elite Ed)2011320121121196299
- NakazawaTHisatomiTNakazawaCMonocyte chemoattractant protein 1 mediates retinal detachment-induced photoreceptor apoptosisProc Natl Acad Sci U S A200710472425243017284607
- ZengKXuHChenKEffects of taurine on glutamate uptake and degradation in Muller cells under diabetic conditions via antioxidant mechanismMol Cell Neurosci201045219219920599618
