Abstract
Migraine is a common neurovascular disorder, affecting millions of people worldwide. Current guidelines recommend triptans as first-line treatment for moderate-to-severe migraine attacks. Frovatriptan is a second-generation triptan with a longer terminal elimination half-life in blood than other triptans (~26 hours). Three double-blind, randomized crossover preference studies have been recently conducted, assessing efficacy and safety of frovatriptan versus rizatriptan, zolmitriptan, and almotriptan, respectively. Frovatriptan showed favorable tolerability and sustained effect, with a significantly lower rate of relapse over 48 hours versus the other triptans. These findings were confirmed in a series of analyses of patient subsets from the three studies, including patients with menstrually related and oral contraceptive-induced migraine, hypertension, obesity, weekend migraine, as well as patients with migraine with aura. In all patient subsets analyzed, lower headache recurrence rates were observed versus the comparator triptans, indicating a more sustained pain-relieving effect on migraine symptoms. A further randomized, double-blind study demonstrated that frovatriptan given in combination with the fast-acting cyclooxygenase inhibitor dexketoprofen provided improved migraine pain-free activity at 2 hours, and gave more sustained pain-free activity at 24 hours, versus frovatriptan alone. These benefits were observed both when the combination was administered early (<1 hour after symptom onset) or late (>1 hour after onset). Different pharmacokinetic, but synergistic, properties between frovatriptan and dexketoprofen may make the combination of these agents particularly effective in migraine treatment, with rapid onset of action and sustained effect over 48 hours. These benefits, together with potential cost-effectiveness advantages versus other triptans could drive selection of the most appropriate treatment for acute migraine attacks.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Migraine is a common neurovascular disorder, affecting millions of people worldwide.Citation1 Migraine without aura is the most commonly experienced type of migraine,Citation2 with this type of migraine often having a relationship with the menstrual cycle.Citation2 Indeed, in ~50% of females suffering from migraine, there is a menstrual association.Citation3
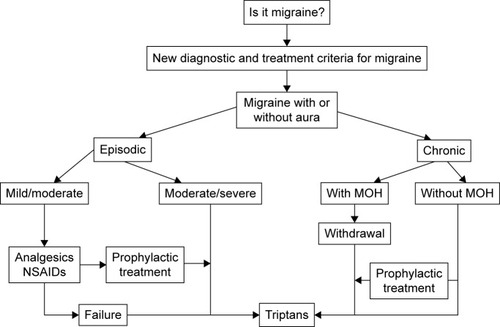
Current guidelines advocate a stratified approach to migraine treatment, with the choice of initial treatment based on the severity of the attack. Migraine-specific drugs such as triptans are recommended as first-line therapy for moderate/severe attacks.Citation4,Citation5 Triptans form a group of selective serotonin 5HT-1 receptor agonists, and are widely considered to be the most effective acute treatment for migraine.Citation6,Citation7 For mild/moderate attacks, nonsteroidal anti-inflammatory drugs (NSAIDs) and/or simple analgesics are recommended as initial treatment,Citation4,Citation5 with triptans indicated if response to such nonspecific drugs is considered inadequate. A treatment algorithm for migraine has recently been createdCitation8 ().
Figure 1 Treatment algorithm for migraine.
Abbreviations: MOH, medication-overuse headache; NSAIDs, nonsteroidal anti-inflammatory drugs.
The first triptan to be discovered was sumatriptan, following which a number of second-generation triptans were developed. Frovatriptan is one of these second-generation triptans (); it has a longer terminal elimination half-life in blood compared with other triptans (~26 hours),Citation9 and was specifically developed to provide a long duration of action and a low likelihood of side effects.Citation10 Randomized clinical studies and postmarketing surveys have demonstrated significantly improved effectiveness and tolerability with frovatriptan compared with previous acute therapies, including other triptans.Citation11,Citation12
Table 1 Overview of frovatriptan for the treatment of migraine
Three direct comparative, double-blind, randomized, crossover studies have assessed the efficacy and safety of frovatriptan (2.5 mg) and compared it with that of rizatriptan (10 mg),Citation13 zolmitriptan (2.5 mg),Citation14 and almotriptan (12.5 mg).Citation15 Recently, post hoc analyses of these three studies have compared the efficacy of frovatriptan versus rizatriptan, zolmitriptan, and almotriptan in a number of specific patient subsets, including patients with menstrually related migraine,Citation16–Citation19 oral contraceptive-induced migraine,Citation20 hypertension,Citation21 obesity,Citation22 weekend migraine,Citation23 as well as in patients with migraine with aura.Citation24,Citation25
The efficacy of the combination of frovatriptan and the NSAID dexketoprofen has also recently been investigated.Citation26–Citation28
This review aims to provide an overview of the efficacy of frovatriptan in the acute treatment of migraine, in light of recent publications.
Frovatriptan in the acute treatment of migraine: evidence from randomized controlled studies
The three direct comparative, double-blind, randomized, crossover studies involved 33 centers across Italy, and shared a similar study design. The studies included patients from both sexes, aged 18–65 years, with a current history of migraine with or without aura, according to International Headache Society 2004 criteriaCitation29 and with between one and six migraine attacks per month for 6 months before commencing the study. Frovatriptan 2.5 mg was compared with rizatriptan 10 mg in the first study, zolmitriptan 2.5 mg in the second study, and almotriptan 12.5 mg in the third study. After treating three episodes of migraine in not >3 months with the first treatment, the patient switched to the other treatment. Patients were encouraged to treat one to three attacks for a maximum period of 6 months. Patients with no migraine episodes during one of the two observation periods were excluded. The primary end point of the studies was the between-treatment comparison of the direction and strength of patient preference, assessed by a questionnaire at the end of the study, and measured on a scale from 0 (no preference) to 5 (strong preference). Secondary end points included numbers of pain-free at 2 hours, pain relief at 2 hours, pain-free at 24 hours, and recurrence of headache within 48 hours. End points for each of the studies are summarized in .
Table 2 Summary of key studies and subanalyses evaluating the efficacy and safety of frovatriptan versus rizatriptan, zolmitriptan, and almotriptan
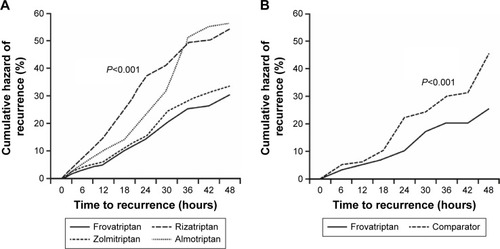
Average preference scores did not differ significantly between the triptans in the three studies (). In each individual study, similar efficacy was demonstrated between the four triptans in the immediate treatment of migraine, but there were lower recurrence rates, and thus a better sustained pain relief with frovatriptan (). A pooled analysis of the three studies was subsequently conducted.Citation30 Rates of pain-free and pain-relief episodes at 2 hours, and sustained pain-free episodes at 48 hours, were similar between frovatriptan and comparators.Citation30 However, consistent with the results of the individual studies, frovatriptan demonstrated significantly lower headache recurrence rates over 48 hours (27% versus 40% with comparators; P<0.001) ().Citation30 The lower recurrence rate with frovatriptan may be explained by its longer elimination half-life versus rizatriptan, zolmitriptan, and almotriptan (25–26 versus 2–4 hours).Citation31
Figure 2 Cumulative hazard of recurrence over 48 hours during treatment with frovatriptan or comparators (n=346).
Frovatriptan in the acute treatment of menstrual migraine
Menstrual migraine attacks are more severe, have a longer duration, and respond less well to analgesics compared to nonmenstrual attacks.Citation32 The development of diagnostic criteriaCitation2 has led to increased recognition of menstrual migraine as a frequently occurring and disabling condition warranting specific treatment. Two clinical forms of menstrual migraine exist: pure menstrual migraine and menstrually related migraine.
Pure menstrual migraine
In pure menstrual migraine, the attacks occur exclusively on day 1±2 (ie, days −2 to +3) of menstruation in at least two out of three menstrual cycles and at no other times of the cycle.Citation2
Menstrually related migraine
In menstrually related migraine, attacks occur on day 1±2 (ie, days −2 to +3) of menstruation in at least two out of three menstrual cycles and additionally at other times of the cycle.Citation2
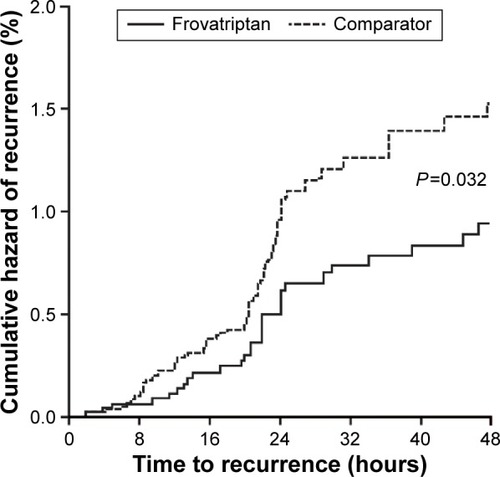
Prespecified subgroup analyses of the three randomized, crossover studies were carried out in females with menstrually related migraine,Citation16–Citation18 and a pooled analysis was then carried out on these three subanalysis studies.Citation19 Similar proportions of pain-relief and pain-free episodes were observed at 2, 4, and 24 hours. However, once again, frovatriptan demonstrated lower headache recurrence rates over 24 hours and even more so over 48 hours ().Citation19 shows the greatly reduced risk of recurrence demonstrated with frovatriptan versus other triptans.
Figure 3 Cumulative hazard of recurrence over 48 hours in females with menstrual migraine during treatment with frovatriptan versus comparators.
Oral contraceptive-induced menstrual migraine
Oral contraceptive-induced menstrual migraine is primarily triggered by estrogen withdrawal during the week of pill suspension,Citation2 although this migraine subtype has not been widely studied. An additional post hoc analysis of the three crossover studiesCitation13–Citation15 found that frovatriptan had similar efficacy to comparators in the immediate treatment of acute attacks of oral contraceptive-induced menstrual migraine.Citation20 However, relapse at 24 and 48 hours was significantly lower with frovatriptan than with the comparators (), potentially making frovatriptan more suitable for treatment of this form of migraine.
Some form of preventive drug treatment may be useful for menstrual migraine.Citation33 However, acute treatment is typically more effective,Citation34 and helps to avoid medication overuse. While the frovatriptan summary of product characteristics states that it should not be used prophylactically,Citation35 there is level A evidence indicating that frovatriptan is effective for prevention of menstrual migraine,Citation36 making it the only triptan efficacious for both acute and prophylactic migraine treatment.
Frovatriptan in combination with dexketoprofen for both early and late treatment administration
NSAIDs are indicated in the symptomatic treatment of migraine, particularly for episodic mild/moderate attacksCitation5 (). The cyclooxygenase inhibitor dexketoprofen reaches maximal plasma concentration after 30 minutes and has been shown to provide rapid reduction of pain in patients with migraine with or without aura.Citation37,Citation38
The combination of frovatriptan and dexketoprofen was investigated in a recent randomized, double-blind, parallel group study.Citation28 The rationale for combining these two agents is linked to the different intrinsic pharmacokinetic properties of each drug, and the potential to target different mechanisms involved in migraine. The primary end point was the proportion pain-free at 2 hours. Secondary end points included pain-free and pain relief at 1, 2, and 4 hours, sustained pain-free at 24 and 48 hours, and recurrence at 48 hours. The study demonstrated that the combination of frovatriptan 2.5 mg and dexketoprofen (25 or 37.5 mg) provided migraine pain-free activity at 2 hours in 51% of patients (versus 29% with frovatriptan alone; P<0.05 [primary end point]). Pain relief at 2 hours was ~80% with both combinations, while sustained pain-free activity at 24 and 48 hours was achieved with frovatriptan plus dexketoprofen 25 mg in 43% and 36% of patients, respectively (secondary end points).Citation28 This final end point is of particular importance, as it is often considered the more difficult factor to treat.
In contrast to treatment with triptan monotherapy, where early timing of administration plays a key role in providing pain relief, the timing of combination treatment relative to headache onset did not appear to influence clinical benefits of the drug combination.Citation26
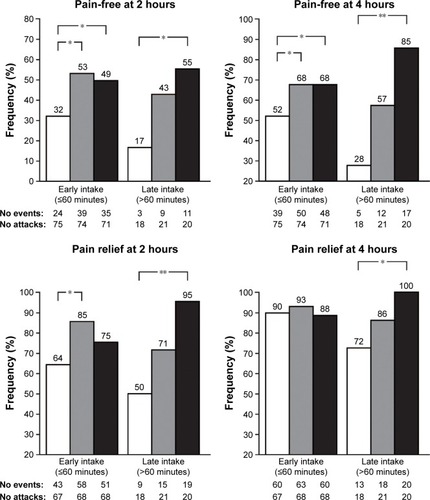
A further post hoc analysis of the study specifically evaluated the efficacy of frovatriptan plus dexketoprofen compared with monotherapy, when administered early (<1 hour after symptom onset) or late (>1 hour after onset).Citation27 Both early and late intake of the combination significantly improved pain-free and pain relief at 2 and 4 hours (), with the combination therapy similarly efficacious in treating acute migraine attack irrespective of the time of drug intake.Citation27 Furthermore, late intake of frovatriptan plus high-dose dexketoprofen proved efficient at managing the most severe and difficult attacks, decreasing the use of rescue medication, with 95% pain relief at 2 hours and 100% at 4 hours. This is noteworthy, as it lends support to the notion that the combination of triptan plus NSAIDs could potentially reduce the hazards of medication-overuse headaches and related adverse effects.Citation39
Figure 4 Proportion (%) of pain-free at 2 and 4 hours, and of pain relief at 2 and 4 hours, after administration of frovatriptan 2.5 mg, frovatriptan 2.5 mg plus dexketoprofen 25 mg, and frovatriptan 2.5 mg plus dexketoprofen 37.5 mg, by early and late drug intake.
These results suggest that the differences in pharmacokinetic properties between frovatriptan and dexketoprofen may make the combination of these two agents particularly effective in migraine treatment, with dexketoprofen providing early pain relief while the longer half-life of frovatriptan reduces the risk of recurrence within 48 hours.Citation27
Frovatriptan for the treatment of migraine with aura
Migraine with aura constitutes approximately 20%–30% of total migraine attacks,Citation40 and can be difficult to treat.Citation24 However, many patients suffer from both forms of attack.Citation24 Evers et alCitation24 performed a meta-analysis of the three previously described randomized, crossover trials,Citation13–Citation15 together with two other European studies with rizatriptan and zolmitriptan of similar trial design to the three previous studies (data on file).
Pain-free at 2 hours was significantly better with frovatriptan versus rizatriptan ().Citation24 While higher pain-free rates at 2 hours were achieved with frovatriptan versus zolmitriptan and almotriptan, these did not reach statistical significance. In concordance with the results obtained in the analysis of all migraine attacks studied in the larger trial program,Citation30 frovatriptan resulted in considerably lower relapse rate versus the comparators.Citation24 These results are particularly important as there are few data published regarding triptan efficacy during the aura phase.
On a scale where the headache intensity was graded as 0 = none; 1= mild; 2= moderate; 3= severe, the mean headache intensity was lower with frovatriptan than with the other triptans at both 2 and 4 hours (secondary end points; ). The authors speculate that the severe and long-lasting attacks experienced by patients enrolled, as confirmed by baseline characteristics and migraine disability assessment scores, may have contributed to the particular efficacy demonstrated by frovatriptan at the 2- and 4-hour timepoints.Citation24 It should be noted that, while patients were advised to take the study drug at the start of the headache phase, and not during aura, it is possible that, in some patients, the aura phase was still ongoing at the time of drug intake.
A post hoc analysis of the original randomized, double-blind, crossover studyCitation14 was carried out in patients with a diagnosis of migraine with aura, independently of whether the study drug was taken during aura or headache phase.Citation25 The analysis showed the proportion of pain-free episodes at 2 hours to be significantly greater with frovatriptan (). Sustained pain-free episodes over 48 hours were also reported significantly more frequently with frovatriptan versus zolmitriptan ().
Triptans are not approved to be taken during the aura phase of migraine with aura,Citation24 due to a current lack of data in this setting.
Frovatriptan benefits in migraine: evidence from additional subanalyses of the three randomized, controlled studies
Hypertension
Hypertension may be associated with migraine, with high blood pressure potentially enhancing the effects of migraine on the vascular wall, leading to further impairment of cerebral endothelial function, resulting in a form of migraine that may be more difficult to treat.Citation21,Citation41 Tullo et alCitation21 assessed triptan efficacy in subjects with a history of treated or untreated essential arterial hypertension versus normotensive subjects. Pain relief was achieved on significantly fewer occasions in hypertensive than normotensive subjects for frovatriptan and the comparators (). However, frovatriptan displayed a more sustained antimigraine effect in both hypertensive and normotensive subjects, with relapses at 48 hours similarly low in hypertensive and normotensive subjects following frovatriptan treatment, and significantly larger relapse rates in hypertensive and normotensive subjects with comparators ().
Obesity
There have been observations of an association between migraine and obesity, which are supported by potential biological mechanisms.Citation42 While adult weight-based dose adjustment is not currently recommended in the product information for the available triptans, the need for increased doses in obese patients remains unclear,Citation43 with anecdotal evidence suggesting that obese migraineurs may take higher numbers of tablets of their antimigraine medication due to their condition.
Saracco et alCitation22 compared efficacy and safety of frovatriptan against the three comparators in nonobese versus overweight and obese subjects. The proportion pain-free at 2 hours did not significantly differ between frovatriptan and the comparators in either nonobese or obese subjects. However, the proportion pain-free at 2 hours was significantly larger in nonobese than in obese subjects irrespective of the type of triptan. Recurrence at 48 hours occurred in significantly fewer episodes treated with frovatriptan versus comparators in both nonobese and obese subjects ().
Of note, the rate of relapse in 48 hours was similar in nonobese and obese subjects with frovatriptan, while it was significantly higher in obese subjects with the comparators. These results suggest that the long half-life of frovatriptan may ensure a low rate of headache recurrence in obese as well as normal-weight individuals, which may be important given that obesity is common among individuals with migraine.Citation22 The results confirm that dose adjustment of frovatriptan is not required in obese patients and therefore implies a lower need of tablets to be taken to relieve pain.
Weekend migraine
Migraine often occurs during weekends. However, evidence regarding efficacy of triptans in weekend migraine is limited.Citation44 A retrospective analysis was performed in patients enrolled in the three head-to-head studies who had migraine attacks on weekends as well as workdays.Citation23 The average number of migraine attacks was greater (P<0.0001) during weekends (285±45 attacks) compared with workdays (256±37 attacks). The proportion pain-free at 2 hours did not significantly differ between weekend and workday attacks for either frovatriptan (26% versus 27%) or comparators (34% versus 32%). However, the relapse rate within 48 hours for weekend compared with workday attacks was significantly lower with frovatriptan, but not with comparators ().
Therefore, among the different triptans, frovatriptan appears to offer the advantage of a lower risk of recurrence and more sustained antimigraine effect during weekend attacks. These results are an important addition to the literature given the paucity of data on this topic.
Sex
The importance of sex-specific medicine has been emerging worldwide over recent decades. In migraine, specific anatomical, hormonal, and behavioral components make the pathophysiology different between males and females.Citation45 As migraine is considerably more common in females than in males, Franconi et alCitation46 performed a retrospective analysis of the three head-to-head studies to address possible sex differences in response to antimigraine therapy. The proportion pain-free at 2 hours did not differ significantly between frovatriptan and comparators in either males or females, and pain relief was also similar between treatments for both sexes. However, the rate of relapse was significantly lower with frovatriptan than with the comparators in both males and female ().Citation46
Although migraine tends to present in a more severe form in females than in males, frovatriptan was shown to be efficacious in both sexes, with a favorable sustained antimigraine effect compared with the comparators.Citation46 These results represent an important addition to the currently sparse literature on the subject of sex in migraine.
Safety
Pooled analysis of the three randomized, controlled studies showed frovatriptan to be associated with significantly fewer adverse drug reactions, particularly cardiovascular symptoms.Citation30 Tolerability of frovatriptan, used for immediate or repeated sustained use, also compares favorably against sumatriptanCitation47 and other acute therapies.Citation11
Cost-effectiveness of frovatriptan in the treatment of migraine
Migraine has a large economic impact on society, with two-thirds of the financial burden linked to indirect costs, such as effect on productivity at work.Citation48,Citation49 An economical advantage of frovatriptan among the oral triptans has been suggested, with model analysis based on the human capital approach showing frovatriptan to be significantly more cost-effective than rizatriptan in particular.Citation49 This outcome is likely due to lower acquisition cost of frovatriptan, the need for fewer doses, and loss of fewer working hours.Citation49
Treatment of migraine in pediatric patients
Although the present review focuses on frovatriptan treatment in the acute treatment of migraine in adults, the subject of migraine treatment in pediatric patients is a particularly challenging issue and also worthy of note here.
Management of pediatric migraine includes a comprehensive approach, using both pharmacologic as well as nonpharmacologic therapies. Children and adolescents often respond to over-the-counter medication, including NSAIDs or combination analgesics.Citation50 Magnesium has been shown to increase the efficacy of ibuprofen and acetaminophen (paracetamol) in children.Citation51 There is a paucity of data from randomized, controlled trials regarding triptan use in children and adolescents, and more studies are needed in this patient population. Intranasal sumatriptan and zolmitriptan are approved for use in adolescents in Europe;Citation52 however, other triptans and formulations for children and adolescents are currently off-label in Europe. Results of a double-blind, placebo-controlled study by Elkind et alCitation53 showed the pharmacokinetic profile of frovatriptan in adolescents (12–17 years) to be similar to that seen in adults, suggesting that dosing adjustments would be unlikely to be required. The use of frovatriptan in adolescent patients may therefore be possible in the future, although further studies are needed.
Studies have shown beneficial effects of the herbal preparations ginkgolide B and Griffonia simplicifolia extract in children.Citation54,Citation55 Weight loss is associated with improvement in headache data in obese adolescent migraineurs.Citation56,Citation57
Conclusion
Triptans remain the preferred treatment option of the majority of patients and physicians for the acute treatment of adult migraine.Citation8 A similar extent of initial migraine pain relief is afforded by frovatriptan and other second-generation triptan comparators rizatriptan, zolmitriptan, and almotriptan. Importantly, however, the longer duration of action of frovatriptan, which correlates with its pharmacokinetic profile, provides sustained efficacy and reduced relapse rate, compared with other triptans, offering a benefit to patients in the acute treatment of migraine. These results are consistent across a variety of patient profiles.
These benefits, together with potential advantages of cost-effectiveness versus other triptans, could drive selection of the most appropriate treatment for acute migraine attacks. In all cases, treatment should be tailored to the need of the individual patient.
Acknowledgments
Medical writing support was provided by Melanie More at Prime Medica Ltd (Knutsford, Cheshire, UK) during the preparation of this manuscript, funded by Menarini. The authors participated in the development and reviewing of the manuscript and the opinions and conclusions are those of the authors.
Disclosure
Both authors have occasionally served as scientific consultants for manufacturers of frovatriptan. The authors report no other conflicts of interest in this work.
References
- HazardEMunakataJBigalMERupnowMFLiptonRBThe burden of migraine in the United States: current and emerging perspectives on disease management and economic analysisValue Health2009121556418671771
- Headache Classification Committee of the International Headache Society (IHS)The International Classification of Headache Disorders, 3rd edition (beta version)Cephalalgia201333962980823771276
- MartinVTLiptonRBEpidemiology and biology of menstrual migraineHeadache200848Suppl 3S124S13019076658
- LiptonRBStewartWFStoneAMLainezMJSawyerJPDisability in Strategies of Care Group. Stratified care vs step care strategies for migraine: the Disability in Strategies of Care (DISC) Study: 4. A randomized trialJAMA2000284202599260511086366
- SarchielliPGranellaFPrudenzanoMPItalian guidelines for primary headaches: 2012 revised versionJ Headache Pain201213Suppl 2S31S7022581120
- JohnstonMMRapoportAMTriptans for the management of migraineDrugs201070121505151820687618
- LoderETriptan therapy in migraineN Engl J Med20103631637020592298
- EversSLisottoCAn algorithm of migraine treatmentEur Neurol Rev201382149152
- BalbisiEAFrovatriptan: a review of pharmacology, pharmacokinetics and clinical potential in the treatment of menstrual migraineTher Clin Risk Manag20062330330818360605
- BalbisiEAFrovatriptan succinate, a 5-HT1B/1D receptor agonist for migraineInt J Clin Pract200458769570515311727
- CadyRKBanksJJonesBACampbellJPostmarketing migraine survey of frovatriptan: effectiveness and tolerability vs previous triptans, NSAIDs or a combinationCurr Med Res Opin200925112711272119778164
- PoolsupNLeelasangalukVJittangtrongJRithlamlertCRatanapantamaneeNKhanthongMEfficacy and tolerability of frovatriptan in acute migraine treatment: systematic review of randomized controlled trialsJ Clin Pharm Ther200530652153216336284
- SaviLOmboniSLisottoCA double-blind, randomized, multicenter, Italian study of frovatriptan versus rizatriptan for the acute treatment of migraineJ Headache Pain201112221922620686810
- TulloVAllaisGFerrariMDFrovatriptan versus zolmitriptan for the acute treatment of migraine: a double-blind, randomized, multicenter, Italian studyNeurol Sci201031Suppl 1S51S5420464583
- BartoliniMGiamberardinoMALisottoCA double-blind, randomized, multicenter, Italian study of frovatriptan versus almotriptan for the acute treatment of migraineJ Headache Pain201112336136821437714
- SaviLOmboniSLisottoCEfficacy of frovatriptan in the acute treatment of menstrually related migraine: analysis of a double-blind, randomized, cross-over, multicenter, Italian, comparative study versus rizatriptanJ Headache Pain201112660961521842274
- AllaisGTulloVBenedettoCZavaDOmboniSBussoneGEfficacy of frovatriptan in the acute treatment of menstrually related migraine: analysis of a double-blind, randomized, multicenter, Italian, comparative study versus zolmitriptanNeurol Sci201132Suppl 1S99S10421533723
- BartoliniMGiamberardinoMALisottoCFrovatriptan versus almotriptan for acute treatment of menstrual migraine: analysis of a double-blind, randomized, cross-over, multicenter, Italian, comparative studyJ Headache Pain201213540140622592864
- AllaisGTulloVOmboniSEfficacy of frovatriptan versus other triptans in the acute treatment of menstrual migraine: pooled analysis of three double-blind, randomized, crossover, multicenter studiesNeurol Sci201233Suppl 1S65S6922644174
- AllaisGTulloVOmboniSFrovatriptan vs other triptans for the acute treatment of oral contraceptive-induced menstrual migraine: pooled analysis of three double-blind, randomized, crossover, multicenter studiesNeurol Sci201334Suppl 1S83S8623695052
- TulloVBussoneGOmboniSEfficacy of frovatriptan and other triptans in the treatment of acute migraine of hypertensive and normotensive subjects: a review of randomized studiesNeurol Sci201334Suppl 1S87S9123695053
- SaraccoMGAllaisGTulloVEfficacy of frovatriptan and other triptans in the treatment of acute migraine of normal weight and obese subjects: a review of randomized studiesNeurol Sci201435Suppl 111511924867847
- LisottoCSaviLPinessiLGuidottiMOmboniSZanchinGEfficacy of frovatriptan vs other triptans in weekend migraine: Pooled analysis of three double-blind, randomized, crossover, multicenter studiesBrain Disord Ther20143128
- EversSSaviLOmboniSLisottoCZanchinGPinessiLEfficacy of frovatriptan as compared to other triptans in migraine with auraJ Headache Pain20151651425916333
- TulloVAllaisGCuroneMFrovatriptan versus zolmitriptan for the acute treatment of migraine with aura: a double-blind, randomized, multicenter, Italian studyNeurol Sci201233Suppl 1S61S6422644173
- AllaisGTulloVCortelliPEfficacy of early vs late use of frovatriptan combined with dexketoprofen vs frovatriptan alone in the acute treatment of migraine attacks with or without auraNeurol Sci201435Suppl 110711324867846
- AllaisGBussoneGTulloVEarly (</=1-h) vs late (>1-h) administration of frovatriptan plus dexketoprofen combination vs frovatriptan monotherapy in the acute treatment of migraine attacks with or without aura: a post hoc analysis of a double-blind, randomized, parallel group studyNeurol Sci201536Suppl 1161167
- TulloVValguarneraFBarbantiPComparison of frovatriptan plus dexketoprofen (25 mg or 37.5 mg) with frovatriptan alone in the treatment of migraine attacks with or without aura: a randomized studyCephalalgia201434643444524363238
- Headache Classification Subcommittee of the International Headache SocietyThe International Classification of Headache Disorders: 2nd editionCephalalgia200424Suppl 1916014979299
- CortelliPAllaisGTulloVFrovatriptan versus other triptans in the acute treatment of migraine: pooled analysis of three double-blind, randomized, cross-over, multicenter, Italian studiesNeurol Sci201132Suppl 1S95S9821533722
- AllaisGBenedettoCA review of the use of frovatriptan in the treatment of menstrually related migraineTher Adv Neurol Disord201362556723483096
- AllaisGCastagnoli GabellariIDe LorenzoCManaOBenedettoCMenstrual migraine: clinical and therapeutical aspectsExpert Rev Neurother2007791105112017868010
- RecoberAGewekeLOMenstrual migraineCurr Neurol Neurosci Rep200552939815743545
- MacGregorEAPrevention and treatment of menstrual migraineDrugs201070141799181820836574
- Migard SPC 2014Migard SPC 2014 Available from: https://www.medicines.org.uk/emc/medicine/15216Accessed November 1, 2015
- SilbersteinSDHollandSFreitagFDodickDWArgoffCAshmanEEvidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache SocietyNeurology201278171337134522529202
- MainardiFMaggioniFPezzolaDZavaDZanchinGDexketoprofen trometamol in the acute treatment of migraine attack: a phase II, randomized, double-blind, crossover, placebo-controlled, dose optimization studyJ Pain201415438839424412801
- AllaisGDe LorenzoCAirolaGPeanoSBenedettoCDexketoprofen trometamol in the treatment of acute migraine attackMinerva Med2000917–815315911155464
- D’AmicoDMoschianoFBussoneGEarly treatment of migraine attacks with triptans: a strategy to enhance outcomes and patient satisfaction?Expert Rev Neurother2006671087109716831121
- HautSDifferentiating migraine from epilepsyAdv Stud Med200556ES658S665
- BarbantiPAuriliaCEgeoGFofiLHypertension as a risk factor for migraine chronificationNeurol Sci201031Suppl 1S41S4320464581
- BigalMELiptonRBHollandPRGoadsbyPJObesity, migraine, and chronic migraine: possible mechanisms of interactionNeurology200768211851186117515549
- PeresMFLerarioDDGarridoABZukermanEPrimary headaches in obese patientsArq Neuropsiquiatr200563493193316400407
- GuidottiMBarrilaCLevaSDePCOmboniSSymptomatic or prophylactic treatment of weekend migraine: an open-label, nonrandomized, comparison study of frovatriptan versus naproxen sodium versus no therapyNeuropsychiatr Dis Treat20139818523355779
- PeterlinBLGuptaSWardTNMacgregorASex matters: evaluating sex and gender in migraine and headache researchHeadache201151683984221631471
- FranconiFFinocchiCAllaisGGender and triptan efficacy: a pooled analysis of three double-blind, randomized, crossover, multicenter, Italian studies comparing frovatriptan vs other triptansNeurol Sci201435Suppl 199105
- GeraudGSpieringsELKeywoodCTolerability and safety of frovatriptan with short- and long-term use for treatment of migraine and in comparison with sumatriptanHeadache200242Suppl 2S93S9912028325
- EdmeadsJMackellJAThe economic impact of migraine: an analysis of direct and indirect costsHeadache200242650150912167138
- LisottoCGuidottiMZavaDSaviLFrovatriptan and rizatriptan economic EVAluation: the FREEVA studyJ Headache Pain2013149624330707
- Sonal SekharMSasidharanSJosephSKumarAMigraine management: How do the adult and paediatric migraines differ?Saudi Pharm J20122011723960771
- GallelliLAvenosoTFalconeDEffects of acetaminophen and ibuprofen in children with migraine receiving preventive treatment with magnesiumHeadache201454231332423808884
- TepperSJClinical implications for breath-powered powder sumatriptan intranasal treatmentHeadache20135381341134923809006
- ElkindAHWadeAIshkanianGPharmacokinetics of frovatriptan in adolescent migraineursJ Clin Pharmacol200444101158116515342617
- EspositoMCarotenutoMGinkgolide B complex efficacy for brief prophylaxis of migraine in school-aged children: an open-label studyNeurol Sci2011321798120872034
- EspositoMRubertoMPascottoACarotenutoMNutraceutical preparations in childhood migraine prophylaxis: effects on headache outcomes including disability and behaviourNeurol Sci20123361365136822437495
- VerrottiAAgostinelliSD’EgidioCImpact of a weight loss program on migraine in obese adolescentsEur J Neurol201320239439722642299
- VerrottiACarotenutoMAltieriLMigraine and obesity: metabolic parameters and response to a weight loss programmePediatr Obes201510322022524990114
- ComerMBPharmacology of the selective 5-HT(1B/1D) agonist frovatriptanHeadache200242Suppl 2S47S5312028320
