Abstract
Objective
Approximately 10% of pregnant women suffer from pregnancy-associated depression. Fluoxetine, as a selective serotonin reuptake inhibitor, is being employed as a therapy for depressive disorders. The present study aimed to determine the effects of fluoxetine on neonatal lung development.
Methods
Thirty pregnant Wistar rats (weighing 200–250 g) were treated daily with 7 mg/kg fluoxetine from gestation day 0 to gestation day 21, via gavage. The control group received a similar volume of distilled water only. Following delivery, the newborns and their lungs were immediately weighed in both of the groups. The right lung was fixed for histological assessments while the left lung was used for evaluation of the expression of SPC and HoxB5 by the real-time polymerase chain reaction method.
Results
Results have indicated that even though the body weight and the number of neonatal rats in both groups were the same, the lung weight of neonates exposed to fluoxetine was significantly different compared to the control group (P<0.05). Expression of both genes was increased, nonetheless, only elevation of HoxB5 was significant (P<0.05). Histological studies demonstrated that lung tissue in the fluoxetine treatment group morphologically appears to be similar to the pseudoglandular phase, whereas the control group lungs experienced more development.
Conclusion
According to the upregulated expression of HoxB5 concerning histological findings, results of the present study showed that fluoxetine can influence lung growth and may in turn lead to delay in lung development. So establishment of studies to identify the effects of antidepressant drugs during pregnancy is deserved.
Keywords:
Introduction
Approximately 350 million people are suffering from depression as a frequent disorder worldwide. This disorder affects several aspects of life, including an individual’s relationship, capacity to work, and financial status and can lead to self-harm and even suicide. The prevalence of depression in females is two-fold higher than in males. Women are at increased risk of depression during periods of hormone changes in various states such as during maturity, pregnancy, and menopause.Citation1
Evidences revealed that depression is a risk factor for both preterm birth and low birth weight, and hence it is important to cure gestational depression.Citation2 A number of studies have suggested that selective serotonin reuptake inhibitors (SSRIs) are the first-line treatment drugs for depression therapy.Citation3 Fluoxetine is an SSRI medication that is known as an important antidepressant drug for gestational depression which has been used during the last 2 decades.Citation4 Fluoxetine is able to cross the placenta and therefore induces some side effects on fetal development, including risk of persistent pulmonary hypertension of the newborn (PPHN). PPHN has been reported to occur in one to two per 1<000 live-born infants and is associated with some important diseases and mortalities in newborns. Epidemiological studies have reported positive statistical correlation between PPHN and fluoxetine consumption during pregnancy.Citation5
Several studies have demonstrated that fluoxetine affects both the level of pulmonary hypertensionCitation6,Citation7 and alters the thickness of the pulmonary arterial wall.Citation8,Citation9 Although many studies have examined the effects of fluoxetine on exposed neonates, there exists no studies to evaluate the evolutionary changes in bronchial tree and alveolar cells. Concerning the most recent studies, it is essential to evaluate the effect of fluoxetine on lung tissue and evolution of the bronchial tree. The expression of the HoxB5 gene is reported to commence at the earliest stages of lung development, peak at the end of the pseudoglandular stage, and finally diminish when branching morphogenesis is quite completed. After the birth, HoxB5 is expressed by type I, while SPC genes are expressed in type II alveolar epithelial cells.Citation10–Citation13 The SPC gene provides a direction for the building of one of the four surfactant proteins and mutations in the SPC gene that will lead to chronic interstitial lung diseases in both infants and adults.Citation14,Citation15 Therefore, the present study aimed to evaluate the effects of fluoxetine on the processes of lung development via measuring the expression of SPC and HoxB5 levels in neonatal rat lung.
Materials and methods
Animals
All animal protocols were carried out in accordance with the guidelines for the care and use of laboratory animals at the Rafsanjan University of Medical Sciences and rules and regulations of the European Communities Council Directive, November 24, 1986 (86/609/EEC). Thirty female Wistar rats weighing 200–250 g were kept and used under controlled conditions at 22°C ±2°C and a constant 12 hours light/12 hours dark cycle. Rats had free access to food and water. Every three female rats were placed in contact with an adult male rat for mating. After 24 hours, vaginal smears were evaluated in female rats. The day of sperm detection in the vaginal smear was considered day 0 of pregnancy (gestation day 0).Citation16 Then, the female rats were randomly separated into treatment and control groups. The treatment group was initially treated by gastric gavage with fluoxetine at 10 mg/kg once per dayCitation17 from days 0 to 21 of gestation. Because rats could not tolerate this dose, the dose was reduced and the maximum dose for this study was 7 mg/kg. The control rats received a similar volume of distilled water. Immediately after delivery, the weights of pups were recorded and lungs were removed surgically. Then, lung weights were recorded, next, the left lung was fixed in TRizol reagent for real-time polymerase chain reaction (PCR) and the right lung was fixed in formalin for histological analysis.
Real-time PCR
Total RNA was extracted from the lung tissue using TRizol reagent according to the manufacturer’s protocol. Extracted RNA was purified by isolation kits and used as a template for reverse transcription in cDNA synthesis. The cDNA synthesis kit was used for cDNA synthesis using both oligo (dT) and random hexamer primers. Real-time PCR was performed on all lung samples. This reaction was undertaken for HoxB5, SPC, and β-actin (housekeeping gene) genes in triplicate. Designed primers for each gene were controlled thermodynamically, then, they were evaluated in the BLAST database to verify the absence of nonspecific binding to other regions of the genome. The sequences of primers used are described in .
Table 1 The sequence of primers used for real-time polymerase chain reaction in the study
Real-time PCR was performed using 10 µL of SYBR green master mix, 3 µL of cDNA, 5 µL of distilled water, 2 µL of each primer, either forward or reverse primer. The following program was programmed on a Bio-Rad CFX96 system (Bio-Rad Laboratories Inc., Hercules, CA, USA) for PCR amplification. There was one cycle of 95°C for 10 minutes to activate the hot start Taq polymerase enzyme and DNA denaturation, 40 cycles of 95°C for 15 seconds, and 40 cycles of 58°C for 1 minute. Drawing of separation curve was performed using the following cycle: 95°C for 15 seconds, 60°C for 30 seconds, and 95°C for 15 seconds, and the temperature was reduced from 95°C to 60°C at 0.03°C per second in this step. The relative quantification of PCR products was determined using the 2−ΔCt formula.Citation18 The melting curves, quantitative analyses, and dissociation stages of the data were performed using the CFX manager software.
Histological and morphological analyses
The right lung samples which had already been fixed in formalin were embedded in paraffin, then the tissues were sectioned at 8 µm and were stained with hematoxylin and eosin. The samples were studied under a light microscope.
Results
Body and lung weight
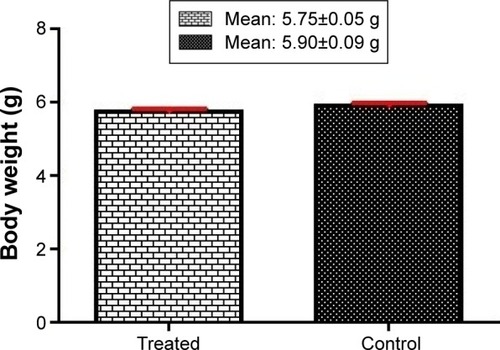
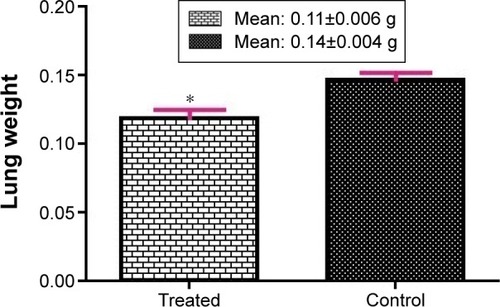
Our results showed that the mean body weight of newborns of the fluoxetine-exposed group (5.75±0.05 g) was not significantly reduced compared to the control group (5.90±0.09 g) (). We observed that fluoxetine-exposed newborns’ lung weight was significantly reduced compared to control newborns’ lung weight (0.11±0.006 g and 0.14±0.004 g, respectively) (P=0.001) ().
Number of newborns
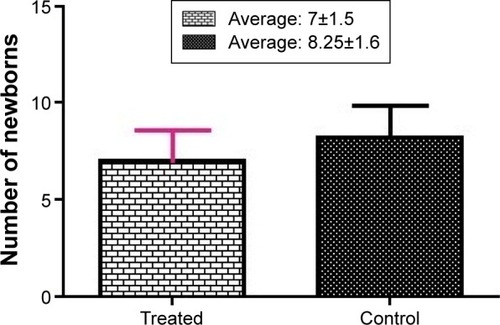
There were no statistically significant differences between the average number of live-born infants in the fluoxetine treatment group and the control group (average: 7±1.5 and 8.25±1.6, respectively) ().
HoxB5 and SPC gene expression
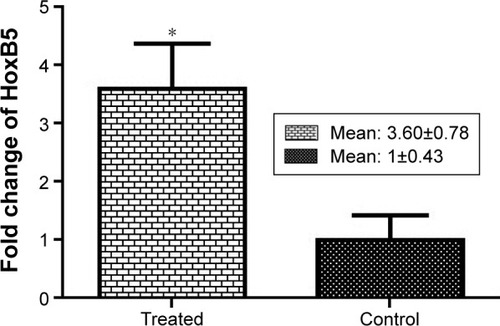
The mRNA of HoxB5 was expressed in rat lungs. When a comparison was made between the fluoxetine treatment group and the control group, a significant difference was observed. The expression of the HoxB5 gene in the fluoxetine treatment group was significantly increased compared with the control group (P=0.0276) ().
Figure 4 Fold changes of HoxB5 expression.
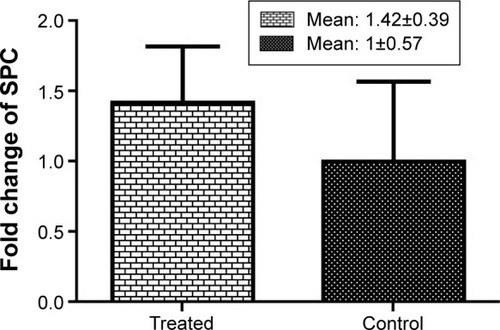
The mRNA of the SPC gene was expressed in type II alveolar cells. Although SPC was increased in the fluoxetine treatment group compared to the control group, this was nonsignificant (P=0.1226) ().
Results of histological analyses
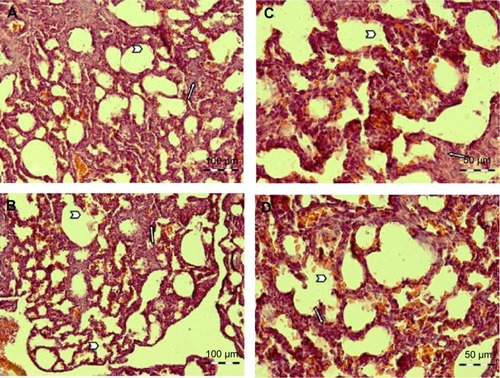
Histology survey showed that the amount of mesenchymal tissue in the fluoxetine treatment group was more than in the control group which was elevated relative to the developmental time. The alveolar cells were formed in the control group and the walls between the alveolar cells were thinner than the fluoxetine treatment group ().
Figure 6 A representative picture of hematoxylin and eosin staining of rat lungs.
Discussion
Therapeutic drugs is a path to overcome depression during pregnancy. Approximately 2%–3% of pregnant women with depression receive SSRI drugs (such as fluoxetine).Citation19 Fluoxetine increases the serotonin level, and an acute increase in serotonin in turn reduces uterine blood flow and leads to preterm birth and reduced fetal growth.Citation3 The time of exposure to fluoxetine is important as Morrison et al demonstrated that first-trimester exposure to fluoxetine caused preterm birth, fetal growth restriction, and increased rates of miscarriage,Citation3 while a fetus exposed to fluoxetine during the third trimester will require aspiratory support and hospitalization after birth.Citation20
In the present study, the impact of maternal fluoxetine treatment was evaluated on the development of fetal rat lungs and the results showed the effect of fluoxetine on the fetal rat. In this study the number of live-born infants in the fluoxetine treatment group was lower than the control group, however it was nonsignificant, but the lung weights of fluoxetine-exposed newborns compared to the control newborns’ lung weights were significantly reduced (P<0.05). These weight changes and mortality were reported in other studies. Vorhees et al demonstrated that exposure to fluoxetine led to reduction of size in pregnant rats and increased mortality, although its mechanism is yet to be cleared.Citation21 Morrison et al reported that the cause of this reduction in weight and the number of the newborns are due to the reduction of uterine blood flow after exposure to fluoxetine.Citation22
The most important and novel result of this study is the effect of fluoxetine on expression of HoxB5 and SPC genes.
Analysis of the real-time PCR showed the expression of both genes was increased and this increase was significant for the HoxB5 gene (P=0.0276). HoxB5 is a homeobox-containing gene that plays an important role in the patterning of airway branches.Citation11,Citation23,Citation24 Strong expression of this gene is found in lung mesenchyme during branching morphogenesis, and its expression falls in the saccular stageCitation11 and after birth it is expressed only in alveolar type I cells. Regarding, increased or decreased expression of this gene can serve as an indicator to show a problem in lung development. Overexpression of this gene could be indicative of a delay in lung development; however, a reduction in expression of this gene can imply a reduction in the number of alveolar type I cells. According to the histological analysis results of this study, it was found that lung development is delayed in the fluoxetine treatment group compared with the control group. With regard to the expected evolutionary time in this study, the mesenchymal tissue in the lungs of the fluoxetine treatment group is more than the control group and the lung tissue morphology is similar to pseudoglandular phase whereas, mesenchymal tissue has decreased in the control group and more alveolar cells have been established.
We observed in this study that the expression of SPC was increased, however this increase was not significant. Thus, according to the alveolar type II cells, cells differentiate into type I, and relative overexpression of the SPC gene could be due to the lack of differentiation of alveolar type II cells toward type I and delay in development.
In 2007, Wang et al investigated the effect of alcohol on murine fetal lung and reported that alcohol delays the lung development and explained that the cause of overexpression of HoxB5 is delay in lung development.Citation25
In Xu et al’s study which evaluated the expression of HoxB5 and its role in neonatal rats with chronic lung disease, the cause of overexpression of SPC is related to the lack of differentiation of alveolar type II cells toward type I.Citation13
A number of studies also indicated the effects of fluoxetine on tissues and cells.
Swerts et al reported the effects of fluoxetine on nerve cells’ development. They stated that the effect of fluoxetine on the elevated levels of serotonin led to the reduction in uterine blood flow which in turn can have an impact on the development of nerve cells.Citation26
More recently in 2013, Kiryanova et al investigated the effects of fluoxetine on heart muscle cells and reported that exposure to fluoxetine during the neonatal period will lead to abnormality in cardiac growth and ventricular septal defects.Citation27
Therefore, in agreement with aforementioned studies, our findings may also confirm fluoxetine’s effects on development throughout fetal life. Some studies exist that suggest that SSRIs have an impact on pulmonary hypertension,Citation5,Citation28 and they have also reported that the highest concentration of fluoxetine accumulates within lungs after injection of fluoxetine in animalsCitation29,Citation30 which can lead to PPHN.Citation31 Even though the main cause of PPHN is not clear, one of the concerns could possibly be the increase in serotonin levels which results in increased pulmonary and smooth muscle cells’ vasoconstriction.Citation32
Conclusion
In the present study, we demonstrated that the exposure to fluoxetine during pregnancy caused increased expression of HoxB5 and SPC in neonatal rats. The expression of the HoxB5 gene began at the earliest stages of lung development, peaked at the end of the pseudoglandular stage, and then its expression was moderated in the saccular stage.Citation33 Regarding histological analysis, it appears that the overexpression of this gene in the fluoxetine treatment group is probably due to the delay in lung development.
According to the other studies,Citation5,Citation28 one of the reasons for the delay in lung development can be the increase of serotonin levels in this tissue. On the other hand, pulmonary vascular and the bronchial tree development are equivalent. Therefore, maybe the effect of fluoxetine on vessels developmentCitation9,Citation34,Citation35 can affect the development of the bronchial tree, although it’s not clear.
Considering the results of the present study and other studies, it is important to be more scrutinizing before taking fluoxetine during pregnancy, so, it is essential to study the effects of antidepressant drugs during pregnancy.
Acknowledgments
The authors of this article would like to thank Rafsanjan University of Medical Sciences as well as Dr Hasanshahi for editing the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- Vivian-TaylorJHickeyMMenopause and depression: is there a link?Maturitas201479214214624951102
- Kendall-TackettKHaleWTThe use of antidepressants in pregnant and breastfeeding women: a review of recent studiesJ Hum Lact201026218719519652194
- MorrisonJLRiggsKWRurakDWFluoxetine during pregnancy: impact on fetal developmentReprod Fertil Dev200517664165016263070
- StarkPFullerRWWongDTThe pharmacologic profile of fluoxetineJ Clin Psychiatry1985463 Pt 27133871767
- KielerHArtamaMEngelandASelective serotonin reuptake inhibitors during pregnancy and risk of persistent pulmonary hypertension in the newborn: population based cohort study from the five Nordic countriesBMJ2012344d801222240235
- PorzionatoAZaramellaPMacchiVFluoxetine may worsen hyperoxia-induced lung damage in neonatal ratsHistol Histopathol201227121599161023059890
- ZhaiFGZhangXHWangHLFluoxetine protects against monocrotaline-induced pulmonary arterial hypertension: potential roles of induction of apoptosis and upregulation of Kv1.5 channels in ratsClin Exp Pharmacol Physiol200936885085619298536
- LiXQWangHMYangCGZhangXHHanDDWangHLFluoxetine inhibited extracellular matrix of pulmonary artery and inflammation of lungs in monocrotaline-treated ratsActa Pharmacol Sin201132221722221217769
- DelaneyCGienJGroverTRRoeGAbmanSHPulmonary vascular effects of serotonin and selective serotonin reuptake inhibitors in the late-gestation ovine fetusAm J Physiol Lung Cell Mol Physiol20113016L937L94421908589
- GolponHAGeraciMWMooreMDHOX genes in human lung: altered expression in primary pulmonary hypertension and emphysemaAm J Pathol2001158395596611238043
- VolpeMVMartinAVosatkaRJMazzoniCLNielsenHCHoxb-5 expression in the developing mouse lung suggests a role in branching morphogenesis and epithelial cell fateHistochem Cell Biol199710864955049450632
- RottierRTibboelDFetal lung and diaphragm development in congenital diaphragmatic herniaSemin Perinatol2005292869316050526
- XuWYangNPanLFuJXueXThe expression of HoxB5 and its role in neonatal rats with chronic lung diseaseFetal Pediatr Pathol2012311112022233504
- BrownNJDohmMTBernardino de la SernaJBarronAEBiomimetic N-terminal alkylation of peptoid analogues of surfactant protein CBiophys J201110151076108521889444
- MulugetaSMaguireJANewittJLRussoSJKotorashviliABeersMFMisfolded BRICHOS SP-C mutant proteins induce apoptosis via caspase-4- and cytochrome c-related mechanismsAm J Physiol Lung Cell Mol Physiol20072933L720L72917586700
- MullerJCBoaretoACLourencoELIn utero and lactation exposure to fluoxetine in Wistar rats: pregnancy outcomes and sexual developmentBasic Clin Pharmacol Toxicol2013113213214023527813
- LiuXSmithBJChenCUse of a physiologically based pharmacokinetic model to study the time to reach brain equilibrium: an experimental analysis of the role of blood–brain barrier permeability, plasma protein binding, and brain tissue bindingJ Pharmacol Exp Ther200531331254126215743928
- SchmittgenTDLivakKJAnalyzing real-time PCR data by the comparative C(T) methodNat Protoc2008361101110818546601
- ACOG Committee on Obstetric PracticeCommittee Opinion No. 354: Treatment with selective serotonin reuptake inhibitors during pregnancyObstet Gynecol200610861601160317138801
- drugs.com [homepage on the Internet]Fluoxetine Available from: https://www.drugs.com/pro/Fluoxetine.htmlAccessed July 13, 2016
- VorheesCVAcuff-SmithKDSchillingMAFisherJEMoranMSBuelke-SamJA developmental neurotoxicity evaluation of the effects of prenatal exposure to fluoxetine in ratsFundam Appl Toxicol19942321942057982528
- MorrisonJLChienCRiggsKWGruberNRurakDEffect of maternal fluoxetine administration on uterine blood flow, fetal blood gas status, and growthPediatr Res200251443344211919327
- ChinoyMRVolpeMVCilleyREGrowth factors and dexamethasone regulate Hoxb5 protein in cultured murine fetal lungsAm J Physiol19982744 Pt 1L610L6209575880
- VolpeMVArchavachotikulKBhanILessinMSNielsenHCAssociation of bronchopulmonary sequestration with expression of the homeobox protein Hoxb-5J Pediatr Surg200035121817181911101743
- WangXGomutputraPWolgemuthDJBaxiLEffects of acute alcohol intoxication in the second trimester of pregnancy on development of the murine fetal lungAm J Obstet Gynecol20071973269.e1e417826415
- SwertsCACostaAMEstevesABoratoCESwertsMSEffects of fluoxetine and imipramine in rat fetuses treated during a critical gestational period: a macro and microscopic studyRev Bras Psiquiatr201032215215820027488
- KiryanovaVMcAllisterBBDyckRHLong-term outcomes of developmental exposure to fluoxetine: a review of the animal literatureDev Neurosci201335643744924247012
- ReeveHLNelsonDPArcherSLWeirEKEffects of fluoxetine, phentermine, and venlafaxine on pulmonary arterial pressure and electrophysiologyAm J Physiology19992762 Pt 1L213L219
- PohlandRCByrdTKHamiltonMKoonsJRPlacental transfer and fetal distribution of fluoxetine in the ratToxicol Appl Pharmacol19899821982052785300
- ShiueCYShiueGGCornishKGO’RourkeMFPET study of the distribution of [11C]fluoxetine in a monkey brainNucl Med Biol19952256136167581171
- ChambersCDHernandez-DiazSVan MarterLJSelective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newbornN Eng J Med20063546579587
- EddahibiSGuignabertCBarlier-MurAMCross talk between endothelial and smooth muscle cells in pulmonary hypertension: critical role for serotonin-induced smooth muscle hyperplasiaCirculation2006113151857186416606791
- VolpeMVRamaduraiSMMujahidSRegulatory interactions between androgens, Hoxb5, and TGF β signaling in murine lung developmentBiomed Res Int2013201332024924078914
- OrnoyAKorenGSelective serotonin reuptake inhibitors in human pregnancy: on the way to resolving the controversySemin Fetal Neonatal Med201419318819424321501
- FornaroELiDPanJBelikJPrenatal exposure to fluoxetine induces fetal pulmonary hypertension in the ratAm J Respir Crit Care Med2007176101035104017702969