Abstract
Colorectal cancer (CRC) is one of the most prevalent cancers and has a high mortality rate. Insensitivity and the limited therapeutic efficacy of its standard chemotherapeutic drug, 5-fluorouracil (5-FU), represents an important challenge in CRC treatment. The robust antitumor properties of thymoquinone (TQ), the main bioactive constituent of Nigella sativa, have recently been demonstrated on different cancers. We investigated whether TQ could potentiate the chemopreventive effect of 5-FU to eradicate the early stages of CRC and elucidated its underlying mechanisms. An intermediate-term (15 weeks) model of colorectal tumorigenesis was induced in male Wistar rats by azoxymethane (AOM), and the animals were randomly and equally divided into five groups: control, AOM, AOM/5-FU, AOM/TQ, and AOM/5-FU/TQ. TQ (35 mg/kg/d; 3 d/wk) was given during the seventh and 15th weeks post-AOM injection, while 5-FU was given during the ninth and tenth weeks (12 mg/kg/d for 4 days; then 6 mg/kg every other day for another four doses). At week 15, the resected colons were subjected to macroscopic, histopathological, molecular, and immunohistochemical examinations. Interestingly, 5-FU/TQ combination therapy resulted in a more significant reduction on AOM-induced colorectal tumors and large aberrant crypts foci than treatment with the individual drugs. Mechanistically, 5-FU and TQ remarkably cooperated to repress the expression of procancerous Wnt, β-catenin, NF-κB, COX-2, iNOS, VEGF, and TBRAS and upregulate the expression of anti-tumorigenesis DKK-1, CDNK-1A, TGF-β1, TGF-βRII, Smad4, and GPx. Overall, our findings present the first report describing the in vivo enhancement effect of combined TQ and 5-FU against early stages of CRC; however, further studies are required to determine the value of this combination therapy in an advanced long-term model of CRC and also to realize its clinical potential.
Introduction
Colorectal cancer (CRC) is one of the most prevalent life-threatening malignant diseases across the world.Citation1,Citation2 According to the World Health Organization’s International Agency for Research on Cancer, CRC is recognized as the third most frequent cancer in men, the second most frequent inwomen, and the fourth leading cause of cancer-related deaths worldwide.Citation2 Despite the extensive efforts that have been made to improve its diagnostic and screening tools, the limited therapeutic efficacy of its current therapy represents the most important challenge in CRC management. In this aspect, 5-fluorouracil (5-FU)-based therapy, either alone or in combination with other cytotoxic agents (eg, irinotecan, leucovorin, or oxaliplatin), remains the standard chemotherapeutic approach.Citation3,Citation4 Overall, 5-FU still exhibits limited efficacy with a low tumor response rate ranging between 7% and 17% with monotherapy and 35% and 39% when given with other chemotherapeutic agents.Citation4,Citation5 More importantly, although the recent addition of new targeted agents, such as bevacizumab and cetuximab, to the standard chemotherapy provides hope for more effective therapy in advanced CRC, they have shown only modest benefit and they are also subject to both primary and secondary resistance like traditional chemotherapy, which ultimately leads to treatment failure.Citation4 Thus, development of potential alternative combinational therapeutic strategies is of paramount importance to improve the prognosis and the curing rate of this deadly cancer. To that end, thymoquinone (TQ) is being pursued as one of the attractive agents in this setting.Citation6,Citation7
Several natural products and nutraceuticals have shown increasing health benefits when used as adjuvants or alternative remedies to conventional drugs.Citation1,Citation6 For instance, Nigella sativa, or black seed, and its main bioactive constituent “TQ” have been shown to exert pleiotropic pharmacological actions and biological activities including antitumor, anti-inflammatory, immunomodulatory, antifibrotic, analgesic, antihypertensive, antidiabetic, antimicrobial, antioxidant, and tissue protective properties in a variety of medical disorders.Citation6–Citation9 As an anticancer agent, TQ’s potent antitumorigenic cellular and molecular effects on the activities of various precancerous and tumor suppressor genes/molecules that impact tumor cell proliferation, differentiation, angiogenesis, apoptosis, invasion, and other important aspects of tumorigenesis have recently attracted a great deal of attention for the treatment of different cancers.Citation9,Citation10 Its favorable chemopreventive and tumoricidal properties have been observed in a variety of in vitro and in vivo models of human cancers.Citation11–Citation13 More importantly, TQ was shown to chemosensitize and augment the cytotoxic effects of the conventional chemotherapeutic drugs on cancer cells.Citation11–Citation14 Furthermore, some clinical trials on different diseases have shown TQ’s promising safety profile, suggesting its potential to be developed as a novel drug in clinical aspect.Citation9,Citation10,Citation15
Taken together, TQ may be a potential adjuvant agent in therapy of CRC, especially in the chemoresistance subset.Citation16 Therefore, the present study aimed to investigate whether combined therapy with TQ would improve and potentiate the anti-CRC effect of 5-FU on colorectal neoplasia induced by an intermediate-term model of azoxymethane (AOM) in rats, and to elucidate the possible mechanisms that would mediate such beneficial synergistic interaction between the two drugs. AOM-induced colorectal tumors and carcinogenesis in rats and mice have been proven to be outstanding rodent models that closely mirror the phases and features of human CRC (aberrant crypts foci [ACF]–adenoma–adenocarcinoma and carcinoma sequence) in a time-dependent manner post AOM injection, and commonly used to assess new chemopreventive and therapeutic strategies and to provide new insights into the pathophysiologic mechanisms and risk factors of human CRC.Citation17,Citation18 Our findings show that though monotherapy with either TQ or 5-FU results in a significant chemopreventive effect on this model, their combination exhibits a more significant efficacy to repress the morphological, histopathological, and molecular changes that were observed in this model. Further studies are still essential to realize the potential clinical value of this combination in patients with early CRC disease, as well as to illustrate its possible usefulness in advanced stages of this dark cancer.
Materials and methods
Drugs and chemicals
TQ (2-isopropyl-5-methyl-1,4-benzoquinone), AOM, and 5-FU were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). Unless otherwise stated, all other chemicals used were of the highest commercial grade.
Animals, induction of colorectal tumorigenesis, and treatment approach
All experimental protocols and procedures of the current study were approved by the Institutional Animal Care and Use Committee of the University of Umm Al-Qura, and were in accordance with The US Public Health Service Policy on Humane Care and Use of Laboratory Animals. Sixty adult male Wistar rats of 230±20 g body weight were housed in sterile polyvinyl cages (five rats per cage) at a controlled temperature of 22°C±3°C with a 12-hour light/dark cycle, and maintained on standard laboratory pellet diet and water ad libitum. The rats were allowed to acclimatize for 1 week before starting the experiments. For induction of colorectal tumorigenesis, AOM was dissolved in normal saline and injected subcutaneously at a dose of 15 mg/kg, once weekly for 2 weeks as previously described.Citation18 According to the subsequent treatment schedules, the rats were randomly categorized into the following five groups (12 rats/group): group 1 (normal controls), not injected with AOM and received only normal saline; group 2 (AOM group), AOM-injected rats and left without treatment; group 3 (AOM/5-FU group), AOM-injected rats and then treated with 5-FU; group 4 (AOM/TQ group), AOM-injected rats and then treated with TQ; and group 5 (AOM/5-FU/TQ group), AOM-injected rats and then treated with 5-FU plus TQ. In the designated groups, 5-FU was freshly prepared in normal saline and injected intraperitoneally during the ninth and tenth weeks post AOM injection in a dosage regimen similar to that used in the treatment of human CRC (12 mg/kg/d for four successive days, then 6 mg/kg every other day for four doses), while TQ was freshly prepared by dissolving it in 0.5% dimethylsulfoxide followed by dilution using olive oil, and then given orally by gastric gavage at the following dosage regimen: 35 mg/kg/d, 3 d/wk; starting from the seventh week post AOM injection and continued till the end of the study (ie, week 15). The dissolving process and dose of TQ were chosen on the basis of pilot experiments and of previously published reports.Citation10,Citation19
Blood sampling, whole colon resection, and macroscopic examination for grown tumors and large aberrant crypts foci
At the end of the experimental period, rats of the different groups were weighed, fasted overnight, and then euthanized under general anesthesia using diethyl (Thermo Fisher Scientific, Waltham, MA, USA). Following euthanasia, the whole blood sample was collected from vena cava into a plane tube and used to measure the serum levels of liver function enzymes (alkaline phosphatase, alanine aminotransferase, and aspartate aminotransferase) and kidney function tests (creatinine, urea, and blood urea nitrogen) by using a Cobas e411 analyzer (Hoffman-La Roche Ltd., Basel, Switzerland) according to the manufacturer’s instructions. Subsequently, the whole colon from rectum to cecum was gently resected, flushed with phosphate-buffered saline, and slit open longitudinally. The surface area of each isolated colon (length × width in cm2) was measured, followed by immersing the whole colon in 10% (v/v) neutralized formalin overnight between layers of filter papers with the mucosa on the upper side. The grown tumors on the mucosa of the isolated colons were blindly counted by naked eye by two observers. Next, the colon was cut into three portions: proximal, middle, and distal segments. Each segment was stained with 0.2% methylene blue solution for 1.5–2 minutes, placed on a microscope slide with the mucosal side upward, and examined under a dissecting microscope to count both small tumors that were not detected by the naked eye, and large ACF (containing four or more aberrant crypts) according to previously established criteria.Citation20 Thereafter, by using a microfeather scalpel blade and under the dissecting microscope, the colorectal specimens that had tumors and ACF were excised from the surrounding normal colorectal tissues and subsequently prepared for histopathological, molecular, enzyme-linked immunosorbent assay (ELISA), and immunohistochemical (IHC) examinations.
Histopathological examination
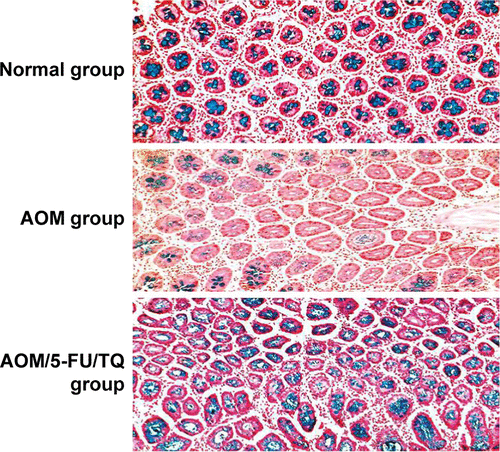
The tissue specimens were first immersed in 80% ethanol for removing the methylene blue and then embedded in paraffin, sectioned (4–5 μm tissue sections), deparaffinized, and stained with hematoxylin and eosin by using conventional methods. The stained colorectal tissue sections were microscopically examined for the presence of ACF, colonic adenomas, and adenocarcinomas. Based on the crypt architecture and nuclear features, ACF were microscopically classified into hyperplastic ACF (no dysplasia) or dysplastic ACF (elongated, crowded, and pseudo-stratified nuclei, increased nucleus-to-cytoplasm ratio, reduced number of goblet cells, back-to-back glands, and markedly decreased interglandular stroma) as previously described.Citation21 A colonic adenoma consists of proliferative and hyperplastic glands, while colonic adenocarcinoma is composed of dysplastic glands invading the submucosa and muscular layers.Citation17,Citation21 In addition, since the so-called mucin-depleted foci (MDFs) were recently recognized as a better precancerous predictor of colorectal carcinogenesis than ACF, colorectal tissue slides of the different groups were subsequently stained with Alcian blue to stain mucin and detect these MDFs as previously desribed.Citation17
RNA extraction, cDNA synthesis, and gene expression analysis
To explore the underlying molecular mechanisms that could be involved in the induction or attenuation of this colorectal tumorigenesis model, the colorectal mRNA levels of Wnt, β-catenin, NF-κB, and COX-2 (as representatives of procancerous genes) and those of DKK-1, CDNK-1A, TGF-β1, and Smad4 (as representative of tumor suppressor/anti-cancerous genes) were assessed in all animal groups. First, the colorectal specimens were homogenized in RNAlater solution (Ambion, Waltham, MA, USA), and then the total RNA was isolated by using the Purelink RNA mini kit (Thermo Fisher Scientific), following the manufacturer’s instructions. The quality and the quantity of the extracted RNA were measured by the Nanodrop equipment (BioSpecnano; Shimadzu Corporation, Kyoto, Japan). Next, up to 200 ng of the extracted total RNA was reverse-transcripted to cDNA by using a high-capacity RNA-to-cDNA reverse transcription kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. Finally, quantitative real-time polymerase chain reaction (qRT-PCR) was conducted. All reactions were performed in triplicate by using the SYBR Green PCR Master Mix (Thermo Fisher Scientific), an ABI 7500 RT-PCR system (Thermo Fisher Scientific), and the 2−ΔΔCT method, as previously described.Citation22 Briefly, the reactions were performed in triplicate in a total volume of 20 μL each containing 10 μL SYBR Green PCR master mix, 7 μL DNase/RNase-free water, 1 μL (5 pmol) of each primer, and 1 μL (25 ng) cDNA, and the amplification was conducted under the following conditions: 40 cycles, 15 seconds at 95°C, and 1 minute at 65°C. The data were analyzed using a comparative threshold cycle (Ct) technique, normalized against the Ct values of β-actin, and expressed as fold-change compared with the normal control group. The nucleotide primer sequences of rat’s Wnt (NM_001105714.1), β-catenin (AF397179.1), NF-κB (NM_001008349.1), COX-2 (AF233596.1), DKK-1 (NM_001106350.1), CDNK-1A (NM_080782.3), TGF-β1 (NM_021578.2), and Smad4 (NM_019275.3), in addition to β-actin (NM_031144.3) as an internal reference (housekeeping) gene, are summarized in .
Table 1 Primer sequences used in the real-time quantitative PCR for detection of the transcription activities of Wnt, β-catenin, NF-κB, COX-2, DKK-1, CDNK-1A, TGF-β1, Smad4, and β-actin genes including the corresponding gene’s accession numbers
Enzyme-linked immunosorbent assay
ELISA was used to quantitatively measure the colorectal expression pattern of β-catenin, TGF-β1, and COX-2 at their protein level. Furthermore, the colorectal concentrations of vascular endothelial growth factor (VEGF, as an index of tumor angiogenesis), glutathione peroxidase (GPx, as an index of antioxidant status), and thiobarbituric acid reactive substances (TBARS, as an index of lipid peroxidation) were also measured by the ELISA technique. For the assays, the colorectal tissue specimens of the different studied groups were homogenized in RIPA lysis buffer containing protease inhibitors (Santa Cruz Biotechnology Inc., Dallas, TX, USA), centrifuged, and then their harvested supernatants were stored in −20°C till use. During the assays, the total protein concentration in each sample was adjusted, and the concentrations of the candidate molecules were measured by using commercial ELISA kits (Cusabio, Hubei, People’s Republic of China, for β-catenin, TGF-β1, COX-2, and VEGF; and Cayman Chemical, Ann Arbor, MI, USA for GPx and TBARS) following the manufacturers’ protocols.
Immunohistochemistry analysis
IHC staining for TGF-β1 and the related TGF-βRII and Smad4, β-catenin, and inducible nitric oxide synthase (iNOS) was performed on the colorectal paraffin tissue sections of the different animal groups and following the conventional protocol. The primary antibodies (1:100; Santa-Cruz Biotechnology Inc.) used were as follows: poly-clonal goat IgG antibodies against rat’s β-catenin (C-18), TGF-β1 (C-16), and Smad4 (C-20); and polyclonal rabbit IgG antibodies against rat’s TGF-βRII (L-21) and iNOS (N-20). Anti-goat or anti-rabbit avidin-biotinylated horseradish peroxidase (1:200; Santa-Cruz Biotechnology Inc.) were used as secondary antibodies. Staining was developed by 3,3′-diaminobenzidine chromogen substrate and counter-stained with Gill’s hematoxylin. The intensity of staining was assessed by using the H-score formula as follows: H-score = ƩPí(í+1), where í represents the intensity of positive stained cells (0= negative, 1= weak, 2= moderate, and 3= strong) and Pí is the percentage (0%–100%) of positive-stained cells.Citation23
Statistical analysis
Results are expressed as mean ± SD. The statistical significance of differences between the values of the different groups was determined using one-way analysis of variance, with post hoc comparisons using Dunnett’s multiple comparison test. The differences between data were considered to be statistically significant when P<0.05, and to be very significant when P<0.01.
Results
Effects of TQ, 5-FU, and their combination therapy on the development of colorectal gross tumors and large ACF
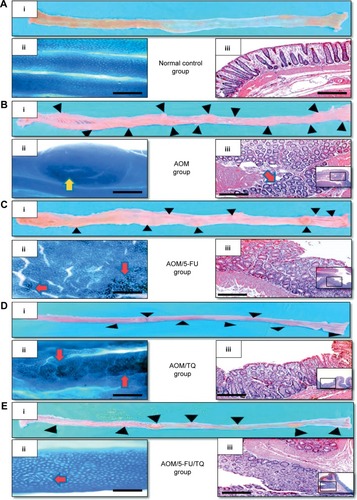
In the present study, the therapeutic efficacy of monotherapy and combination therapy with TQ and 5-FU on early colorectal tumorigenesis and tumor growth was evaluated in an intermediate-term model (15 weeks) of AOM-induced colorectal carcinogenesis in rats. In this model, rats that received AOM and were left without treatment developed a significant number of gross tumors and distortions on their colorectal mucosa (; ) compared to the colorectal tissues of normal controls (; ). However, treatment of these AOM-injected rats with either 5-FU (; ) or TQ (; ) led to a significantly decreased number of the grown tumors and topographic mucosal alterations. Importantly, a significant synergistic interaction was achieved via 5-FU/TQ combination therapy, as shown by the lowest number of grown colorectal tumors (; ). Similarly, examination of the resected colons by the dissecting microscope following methylene blue staining showed the presence of several microtumors and larger ACF in the colorectal tissues of AOM-injected rats and left without treatment (; ), and monotherapy with either 5-FU (; ) or TQ (; ) markedly reduced the total number of microtumors and larger ACF as compared to the AOM group (). More interestingly, the colorectal mucosal architecture was more preserved and the total number of microtumors and larger ACF was further and significantly reduced in the TQ/5-FU combined treatment group (; ) compared to 5-FU or TQ treatment alone. The histopathological findings () were also consistent with the macroscopic/microscopic observations and showed the presence of many large ACF (>4 crypts/focus) with hyperplastic and dysplastic features (), precancerous MDFs (), as well as multiple tubular adenomas in the colorectal tissues of rats injected with AOM and left without any treatment (AOM group) (). By contrast, treatment of these AOM-injected rats with 5-FU (), TQ (), or 5-FU plus TQ ( and ) attenuated the development of such pre-neoplastic large ACF and MDF, as well as tubular adenomas. And the highest attenuating effect was also observed with 5-FU/TQ dual therapy. Taken together, these results indicate that therapy with TQ not only attenuated CRC initiation but also had the capability to enhance the tumoricidal and chemopreventive efficacy of 5-FU in this disease modality.
Table 2 Effects of thymoquinone (TQ) and/or 5-fluorouracil (5-FU) therapy
Figure 1 Representative photos of macroscopic and microscopic appearance of colorectal mucosa.
Abbreviations: AOM, azoxymethane; 5-FU, 5-fluorouracil; TQ, thymoquinone; H&E, hematoxylin and eosin.
qRT-PCR findings of gene expression study
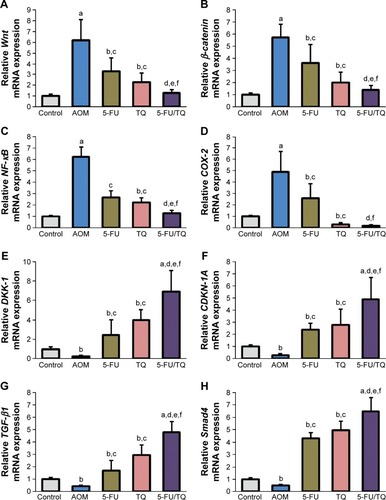
To understand the possible underlying mechanisms of the observed chemopreventive effects of TQ and 5-FU therapy on this rodent model of early stages of CRC, gene expression study was performed for four procancerous genes (Wnt, β-catenin, NF-κB, and COX-2) and for four anti-cancerous/tumor-suppressor genes (DKK-1, CDNK-1A, TGF-β1, and Smad4), which collectively play an important role in controlling CRC development and progression. As demonstrated in , qRT-PCR showed a remarkable elevation in the mRNA level of Wnt (), β-catenin (), NF-κB (), or COX-2 (), and a significant decrease in the mRNA level of DKK-1 (), CDNK-1A (), TGF-β1 (), or Smad4 () genes in the colorectal tissues of the AOM group compared with normal control group. On the contrary, co-therapy with TQ and 5-FU synergistically cooperated to modify the altered mRNA expression patterns of these pro-oncogenic and tumor suppressor genes in this AOM model of colorectal tumorigenesis ().
Figure 2 Findings of quantitative real-time polymerase chain reaction.
ELISA findings
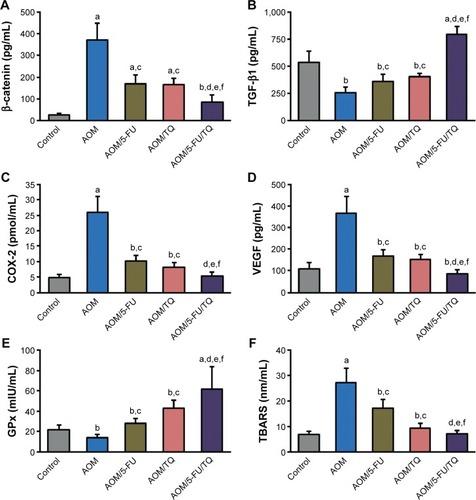
The colorectal levels of β-catenin, TGF-β1, COX-2, VEGF, GPx, and TBARS were determined by ELISA. shows the significant effects of AOM on the induction of β-catenin (), COX-2 (), VEGF (), and TBARS () in the colorectal tissues compared with the normal control group. However, the concentrations of these molecules were significantly reduced when the AOM-injected rats were subsequently treated with either TQ and/or 5-FU; and a more significant reduction was obtained with TQ and 5-FU combination therapy (). In contrast to these data, the colorectal levels of TGF-β1 () and GPx () were significantly decreased in the AOM group compared to the normal group. Interestingly, TQ and 5-FU acted synergistically not only to restore the expression levels of TGF-β1 and GPx but also to upregulate their levels than those of normal controls ().
Figure 3 Enzyme-linked immunosorbent assay findings.
Immunohistochemistry findings
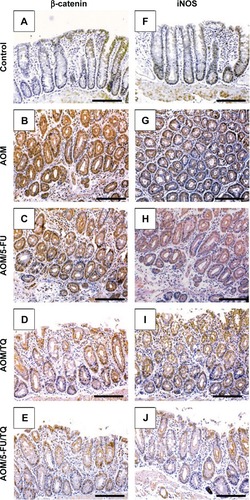
Next, IHC staining was performed to give further mechanistic insights. As shown in and , a remarkably low level of immunostaining for the TGF-β1 system (TGF-β1, TGF-βRII, and Smad4) was detected in the glandular epithelium of the colorectal tissues of the AOM group in comparison with normal control group (; ). Though monotherapy with either 5-FU (; ) or TQ (; ) significantly counteracted the repression effect induced by AOM on these three elements of TGF-β system, their single therapy failed to upregulate these repressed three TGF-β candidate molecules to reach their levels in the normal control group (). On the other hand, combined therapy with TQ/5-FU interestingly cooperated to upregulate the expression pattern of TGF-β1 (; ), TGF-βRII (; ), or Smad4 (; ) to reach a higher level compared to the control group (). In contrast to these data, the colorectal tissues of the AOM group showed marked elevations in the levels of β-catenin (; ) and iNOS (; ) compared to their normal expression pattern in the colorectal tissues of control group (; ); and therapy with TQ and 5-FU succeeded in repressing β-catenin and iNOS expression in a synergistic manner (; ).
Table 3 Immunohistochemistry scores for the effects of thymoquinone (TQ) and/or 5-fluorouracil (5-FU)
Figure 4 Representative photos of immunohistochemical findings.
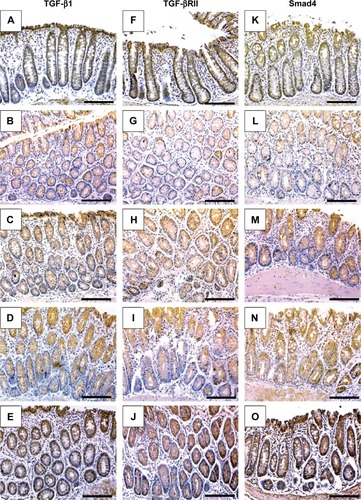
Figure 5 Representative photos of immunohistochemical findings.
Abbreviation: iNOS, inducible nitric oxide synthase.
Biochemical findings
As shown in , the biochemical analysis of the collected sera samples did not show any significant differences in the serum levels of liver function enzymes and renal function parameters between the different animal groups, reflecting the safety profile, at least at the hepatorenal level, of the applied TQ/5-FU combination therapy.
Discussion
5-FU-based therapy is still the standard protocol used in the treatment of CRC. Nevertheless, its limited treatment response, dose-limiting toxicity, and resistance devolvement remain major challenges.Citation3,Citation4 Therefore, identifying a promising combination strategy to improve 5-FU’s efficacy in CRC management is of paramount importance. TQ is a natural biological compound derived from N. sativa with well-known, robust, and pluripotent antitumor activity.Citation6–Citation10 We therefore investigated the in vivo ability of TQ to enhance the chemopreventive efficacy of 5-FU on an intermediate-term (15 weeks) model of colorectal tumorigenesis induced in rats by AOM, which is a commonly used in vivo experimental model because its different stages greatly mimic human CRC in several aspects.Citation17,Citation18 Interestingly, compared with the single therapy with either TQ or 5-FU, the two drugs remarkably cooperated to reduce the numbers of grown colorectal tumors and to inhibit the formation of microtumors and colonic preneoplastic lesions such as large ACF and MDF, which collectively represent the main macroscopic and microscopic features during this phase of colorectal tumorigenesis induced by AOM in rats.Citation17,Citation18 In support of our findings, earlier and recent in vitro studies have shown that TQ induces cellular damage on human colon cancer cells comparable to cells exposed to 5-FU.Citation24 Furthermore, TQ has been found to promote the chemotherapeutic effectiveness of 5-FU on human nasopharyngeal carcinoma and gastric cancer, suggesting that TQ may be a potential adjuvant agent with 5-FU in cancer therapy.Citation25,Citation26 Of note, much effort has been spent on recognizing and identifying the common preneoplastic colorectal mucosal lesions during the early steps of colorectal carcinogenesis. In this regard, ACF identified in both humans and this AOM-animal model of CRC disease have been widely used as intermediate biomarkers of colorectal neoplasia and carcinogenesis and also to assess the preventive potential of several investigational chemopreventive agents.Citation17,Citation18 Furthermore, efforts to identify better precancerous lesions that strongly predict colorectal carcinogenesis than ACF have led to the identification of MDF characterized by the absence or scant production of mucin mucus.Citation17 In the present study, it was noticed that the combined therapy with 5-FU and TQ had markedly interfered with development of both ACF and MDF in the colorectal mucosa of rats injected with AOM, suggesting their potential chemopreventive interaction during the early stages of CRC disease.
At the molecular level, CRC is a multifaceted and multigenic malignant disease. Aberrant activation of the Wnt/β-catenin signaling pathway and its downstream components has been suggested to be crucially implicated in the induction and dissemination of ~80% of all human CRCs.Citation27,Citation28 Mechanistically, this canonical tumorigenic pathway is initiated by binding of Wnt proteins with their target plasma membrane receptors/co-receptors, followed by the translocation of β-catenin into the nuclei of cells derived from the intestinal crypts wherein it stimulates the transcription of several pro-oncogenes.Citation28 On the opposite side, DKK-1, a well-known colon cancer suppression agent, has been described as an important inhibitor of the Wnt/β-catenin pathway by blocking Wnt signaling receptor complexes.Citation29–Citation31 Moreover, DKK-1 has also been suggested to have additional Wnt/β-catenin-independent tumor-suppressor, anti-angiogenesis, and anti-metastasis activities in CRC.Citation30,Citation32 In that respect, several colonic biopsies from CRC patients with chemoresistance and poor clinical outcome have shown a remarkable decrease in their colorectal levels of DKK-1.Citation28–Citation31 Likewise, a remarkable downregulation or even loss of activity of CDNK-1A, a tumor suppressor gene that encodes a potent cell cycle inhibitory factor known as p21, has been detected in most human CRC cases.Citation33,Citation34 Based on these facts, it is conceivable that blocking or repression of the Wnt/β−catenin pathway and/or stimulation of DKK-1 and CDNK-1A activity may hold tremendous therapeutic potential in CRC therapy.Citation28–Citation33 Interestingly, our findings are in harmony with this hypothesis, in that repression of Wnt and β-catenin and upregulation of activity of DKK-1 and CDNK-1A were significantly observed with TQ and 5-FU therapy and might be behind their observed augmented chemopreventive effect in this CRC model. In support, it has been reported that TQ attenuated colorectal tumor growth in APCMin mice by blocking the Wnt/β-catenin pathway,Citation35 and induced cell cycle arrest and DAN fragmentation in human CRC and other tumor cells via overexpression of p21.Citation14,Citation36 Similarly, blockade of the Wnt/β-catenin pathway has been associated with the inhibition of multidrug resistance system and cancer cell resistance to 5-FU and other chemotherapeutics.Citation37,Citation38
CRC is a life-threatening complication of inflammatory bowel diseases. In this regard, the aberrant activation of NF-κB and overproduction of pro-oncogenic inflammatory mediators such as COX-2 and iNOS may provide a crucial mechanistic link between inflammation and carcinogenesis.Citation39–Citation42 Aberrant activation of NF-κB can induce many tumorigenesis genes that influence tumor growth, angiogenesis, invasion, resistance to chemotherapy, and other potentially carcinogenic processes.Citation43 As well, overexpression of COX-2 and iNOS and the induction of lipid peroxidation and oxidative stress have been shown to play vital roles between colorectal inflammation and carcinogenicity.Citation40–Citation45 Like other cancers, angiogenesis mediated by VEGF and other pivotal angiogenic and tumor vascularization facilitators has a central role in the progression and metastasis of CRC.Citation46 Taken together, suppression of NF-κB, iNOS, COX-2, VEGF, and/or lipid peroxidation could represent a potential strategy in CRC therapy. Interestingly, our data are in harmony and showed that TQ/5-FU combination therapy significantly suppressed AOM-induced lipid peroxidation and the expression of NF-κB, iNOS, COX-2, and VEGF in the colorectal tissues. In agreement, results from previous studies have indicated that the anticancer and anti-inflammatory activities of TQ might be mediated in part through the suppression of NF-κB, iNOS, and COX-2 activation pathways and tumor angiogenesis, in addition to its potent antioxidant and tissue protective property.Citation9,Citation10,Citation47 Furthermore, 5-FU has been shown to inhibit the NF-κB pathway and NF-κB-mediated NO production in cancer cells,Citation48,Citation49 and selective inhibition of NF-κB and COX-2 has shown to enhance the in vitro cytotoxic effects of 5-FU on CRC cells and downregulate VEGF production and vascular density in tumor tissues.Citation40,Citation50,Citation51
TGF-β1 is a pleiotropic cytokine engaged in several cellular functions and processes. Importantly, dysfunction of the TGF-β1 signaling pathway is crucially involved in a variety of diseases including cancer, and its dual paradoxical roles have been well established in variable cancers, though the precise underlying mechanisms of this paradox have remained elusive.Citation52–Citation55 Of note, the activation of this pathway and its downstream components during the early stages of oncogenesis is generally associated with oncosuppression and the inhibition of epithelial cell transformation and hyperproliferation.Citation53,Citation54 The pleiotropic activities of TGF-β1 are mediated through Smad-dependent and independent mechanisms depending upon the cellular subset in which this cytokine exerts its actions.Citation55,Citation56 In the Smad-dependent pathway, signaling begins with the binding of TGF-β1 to its receptor (TGF-βRII) with downstream activation of complex pathways involving co-Smads, particularly Smad4, to transduce the signal into the nucleus and influence the transcription of several target genes.Citation53–Citation56 Considerably, earlier studies have shown that downregulation or loss of TGF-β1 and its receptors leads to increased susceptibility to gastrointestinal carcinogenesis, particularly colon cancer.Citation54 Moreover, downregulation or even loss of the Smad4 protein has been clinically detected in CRC and other neoplastic diseases, and would be used as a valuable prognostic factor.Citation56 On the other hand, the upregulation of this pathway has been previously explored as a part of the anti-tumor mechanisms of TQ therapy on human pancreatic and breast cancers,Citation57,Citation58 and 5-FU-exhibited activation of TGF-β pathway has been observed in human CRC cells both in vitro and in a xenograft model.Citation59 Interestingly, findings of the present study are in parallel, and demonstrate that combination therapy with TQ and 5-FU not only recovers AOM-induced suppression of TGF-β1, TGF-βRII, and Smad4 expression in the colorectal tissues but also to upregulate their levels more than those of normal controls, suggesting that the enhancement of TGF-β1–Smad4-dependent pathway could be a part of the underlying mechanisms by which TQ and 5-FU displayed their observed augmenting antitumor effect on this preclinical model of CRC disease.
Conclusion
The present study demonstrates the positive chemopreventive interaction between TQ and 5-FU against the early stages of colorectal tumors induced by AOM in rats by modulating the expression pattern of a number of pro and anticancerous pathways and molecules. Further studies are warranted to illustrate the reality of its clinical value and also to explore its possible usefulness in advanced stages of this dark cancer.
Authors contributions
OAK and AGE conceived and conceptualized the study and its design. All authors participated in the study methodologies and laboratory processes and analysis and interpretation of the results. All authors contributed toward drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
This project was funded by the National Science, Technology and Innovation Plan of (MARRIFAH)-King Abdul Aziz City for Science and Technology (KACST), the Kingdom of Saudi Arabia, Award Number (12-MED2965-10). OAK, AGE, BR, and AMM are the recipients of the grant.
Supplementary material
Figure S1 Corresponding histopathological features of colorectal tissues stained with Alcian blue.
Abbreviations: AOM, azoxymethane; TQ, thymoquinone; 5-FU, 5-fluorouracil.
Disclosure
The authors report no conflicts of interest in this work.
References
- RouhollahiEMoghadamtousiSZAl-HenhenaNThe chemo-preventive potential of Curcuma purpurascens rhizome in reducing azoxymethane-induced aberrant crypt foci in ratsDrug Des Devel Ther2015939113922
- FerlayJSoerjomataramIDikshitRCancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012Int J Cancer20151365E359E38625220842
- AhnJYLeeJSMinHYLeeHYAcquired resistance to 5-fluorouracil via HSP90/Src-mediated increase in thymidylate synthase expression in colon cancerOncotarget2015632326223263326416450
- HammondWASwaikaAModyKPharmacologic resistance in colorectal cancer: a reviewTher Addv Med Oncol2016815784
- StringerAMGibsonRJBowenJMKeefeDMChemotherapy-induced modifications to gastrointestinal microflora: evidence and implications of changeCurr Drug Metab2009101798319149515
- AgbariaRGabarinADahanABen-ShabatSAnticancer activity of Nigella sativa (black seed) and its relationship with the thermal processing and quinone composition of the seedDrug Des Devel Ther2015931193124
- DarakhshanSBidmeshki PourAHosseinzadeh ColagarASisakhtnezhadSThymoquinone and its therapeutic potentialsPharmacol Re201595–96138158
- BarakatEMEl WakeelLMHagagRSEffects of Nigella sativa on outcome of hepatitis C in EgyptWorld J Gastroenterol201319162529253623674855
- RahmaniAHAlzohairyMAKhanMAAlySMTherapeutic implications of black seed and its constituent thymoquinone in the prevention of cancer through inactivation and activation of molecular pathwaysEvid Based Complement Alternat Med2014201472465824959190
- AbukhaderMMThymoquinone in the clinical treatment of cancer: fact or fiction?Pharmacogn Rev201371411712024347919
- LiFRajendranPSethiGThymoquinone inhibits proliferation, induces apoptosis and chemosensitizes human multiple myeloma cells through suppression of signal transducer and activator of transcription 3 activation pathwayBr J Pharmacol2010161354155420880395
- AttoubSSperandioORazaHThymoquinone as an anticancer agent: evidence from inhibition of cancer cells viability and invasion in vitro and tumor growth in vivoFundam Clin Pharmacol201327555756922788741
- KunduJChunKSAruomaOIKunduJKMechanistic perspectives on cancer chemoprevention/chemotherapeutic effects of thymoquinoneMutat Res2014768223425847385
- KhalifeRHodrojMHFakhouryRRizkSThymoquinone from Nigella sativa seeds promotes the antitumor activity of noncytotoxic doses of topotecan in human colorectal cancer cells in vitroPlanta Med201682431232126848703
- AkhondianJKianifarHRaoofziaeeMMoayedpourAToosiMBKhajedalueeMThe effect of thymoquinone on intractable pediatric seizures (pilot study)Epilepsy Res2011931394321112742
- ChenMCLeeNHHsuHHThymoquinone induces caspase-independent, autophagic cell death in CPT-11-resistant lovo colon cancer via mitochondrial dysfunction and activation of JNK and p38J Agric Food Chem20156351540154625611974
- WashingtonMKPowellAESullivanRPathology of rodent models of intestinal cancer: progress report and recommendationsGastroenterology2013144470571723415801
- AlmagramiAAAlshawshMASaif-AliRShwterASalemSDAbdullaMAEvaluation of chemopreventive effects of Acanthus ilicifolius against azoxymethane-induced aberrant crypt foci in the rat colonPLoS One20149e9600424819728
- KhazdairMRThe protective effects of Nigella sativa and its constituents on induced neurotoxicityJ Toxicol2015201584182326604923
- GuanFLiGLiuABδ- and γ-tocopherols, but not α-tocopherol, inhibit colon carcinogenesis in azoxymethane-treated F344 ratsCancer Prev Res (Phila)20125464465422366914
- XiaoHHaoXSimiBGreen tea polyphenols inhibit colorectal aberrant crypt foci (ACF) formation and prevent oncogenic changes in dysplastic ACF in azoxymethane-treated F344 ratsCarcinogenesis200829111311917893236
- LivakKJSchmittgenTDAnalysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) methodMethods200125440240811846609
- PrakashATuckermanELairdSOlaBLiTCLedgerWLA preliminary study comparing the endometrial expression of inhibin, activin and follistatin in women with a history of implantation failure after IVF treatment and a control groupBJOG20081154532536 discussion 536–53718271891
- NorwoodAATanMMayMTucciMBenghuzziHComparison of potential chemotherapeutic agents, 5-fluoruracil, green tea, and thymoquinone on colon cancer cellsBiomed Sci Instrum20064235035616817633
- WilliamsSTucciMABenghuzziHAThe effect of combination treatments of epigallocatechin-3-gallate, thymoquinone, and 5-fluorouracil on fadu nasopharyngeal carcinoma cellsBiomed Sci Instrum20145036136625405445
- LeiXLvXLiuMThymoquinone inhibits growth and augments 5-fluorouracil-induced apoptosis in gastric cancer cells both in vitro and in vivoBiochem Biophys Res Commun2012417286486822206670
- SongLLiZYLiuWPZhaoMRCrosstalk between Wnt/β-catenin and Hedgehog/Gli signaling pathways in colon cancer and implications for therapyCancer Biol Ther20151611725692617
- FonsecaBFPredesDCerqueiraDMDerricin and derricidin inhibit Wnt/β-catenin signaling and suppress colon cancer cell growth in vitroPLoS One2015103e012091925775405
- NiehrsCFunction and biological roles of the Dickkopf family of Wnt modulatorsOncogene200625577469748117143291
- QiLSunBLiuZLiHGaoJLengXDickkopf-1 inhibits epithelial-mesenchymal transition of colon cancer cells and contributes to colon cancer suppressionCancer Sci2012103482883522321022
- AguileraÓGonzález-SanchoJMZazoSNuclear DICKKOPF-1 as a biomarker of chemoresistance and poor clinical outcome in colorectal cancerOncotarget2015685903591725788273
- LiuZSunBQiLDickkopf-1 expression is down-regulated during the colorectal adenoma-carcinoma sequence and correlates with reduced microvessel density and VEGF expressionHistopathology201567215816624916146
- OginoSNoshoKShimaKp21 expression in colon cancer and modifying effects of patient age and body mass index on prognosisCancer Epidemiol Biomarkers Prev20091892513252119723919
- BishehsariFGachJSAkagiNWebberMKBauerJJungBHAnti-p21 autoantibodies detected in colorectal cancer patients: a proof of concept studyOncoimmunology201438e95220225960931
- LangMBorgmannMOberhuberGThymoquinone attenuates tumor growth in ApcMin mice by interference with Wnt-signalingMol Cancer20131214123668310
- ParamasivamARaghunandhakumarSPriyadharsiniJVJayaramanGIn vitro anti-neuroblastoma activity of thymoquinone against neuro-2a cells via cell-cycle arrestAsian Pac J Cancer Prev201516188313831926745078
- NodaTNaganoHTakemasaIActivation of Wnt/beta-catenin signalling pathway induces chemoresistance to interferon-alpha/5-fluorouracil combination therapy for hepatocellular carcinomaBr J Cancer2009100101647165819401692
- ShenDYZhangWZengXLiuCQInhibition of Wnt/β-catenin signaling downregulates P-glycoprotein and reverses multi-drug resistance of cholangiocarcinomaCancer Sci2013104101303130823822562
- DiDonatoJAMercurioFKarinMNF-kappaB and the link between inflammation and cancerImmunol Rev2012246137940022435567
- RangerGSCurrent concepts in colorectal cancer prevention with cyclooxygenase inhibitorsAnticancer Res201434116277628225368225
- JanakiramNBRaoCVThe role of inflammation in colon cancerAdv Exp Med Biol2014816255224818718
- CampanholoVMSilvaRMSilvaTDOral concentrated grape juice suppresses expression of NF-kappa B, TNF-α and iNOS in experimentally induced colorectal carcinogenesis in Wistar ratsAsian Pac J Cancer Prev201516394795225735387
- LiFZhangJArfusoFNF-kappaB in cancer therapyArch Toxicol201589571173125690730
- WalyMIAl-RawahiASAl RiyamiMAmelioration of azoxymethane induced-carcinogenesis by reducing oxidative stress in rat colon by natural extractsBMC Complement Altern Med2014146024533833
- AfrinSGiampieriFGasparriniMChemopreventive and therapeutic effects of edible berries: a focus on colon cancer prevention and treatmentMolecules201621216926840292
- ZhouLHHuQSuiHTanshinone II-a inhibits angiogenesis through down regulation of COX-2 in human colorectal cancerAsian Pac J Cancer Prev20121394453445823167360
- HarzallahHJGrayaaRKharoubiWMaaloulAHammamiMMahjoubTThymoquinone, the Nigella sativa bioactive compound, prevents circulatory oxidative stress caused by 1,2-dimethylhydrazine in erythrocyte during colon postinitiation carcinogenesisOxid Med Cell Longev2012201285406522570743
- JungIDYangSYParkCG5-Fluorouracil inhibits nitric oxide production through the inactivation of IkappaB kinase in stomach cancer cellsBiochem Pharmacol200264101439144512417257
- AotaKAzumaMYamashitaT5-Fluorouracil induces apoptosis through the suppression of NF-kappaB activity in human salivary gland cancer cellsBiochem Biophys Res Commun200027331168117410891390
- IrieTTsujiiMTsujiSSynergistic antitumor effects of celecoxib with 5-fluorouracil depend on IFN-gammaInt J Cancer2007121487888317450522
- VoborilRHochwaldSNLiJInhibition of NF-kappa B augments sensitivity to 5-fluorouracil/folinic acid in colon cancerJ Surg Res2004120217818815234211
- SmithALRobinTPFordHLMolecular pathways: targeting the TGF-β pathway for cancer therapyClin Cancer Res201218174514452122711703
- PrincipeDRDollJABauerJTGF-β: duality of function between tumor prevention and carcinogenesisJ Natl Cancer Inst20141062djt36924511106
- HahmKBLeeKMKimYBConditional loss of TGF-beta signalling leads to increased susceptibility to gastrointestinal carcinogenesis in miceAliment Pharmacol Ther200216suppl 211512711966532
- ZhangQYuNLeeCMysteries of TGF-β paradox in benign and malignant cellsFront Oncol201449424860782
- WitkowskaMSmolewskiPSMAD family proteins: the current knowledge on their expression and potential role in neoplastic diseasesPostepy Hig Med Dosw (Online)20146830130924662798
- TorresMPPonnusamyMPChakrabortySEffects of thymoquinone in the expression of mucin 4 in pancreatic cancer cells: implications for the development of novel cancer therapiesMol Cancer Ther2010951419143120423995
- RajputSKumarBNDeyKKPalIParekhAMandalMMolecular targeting of Akt by thymoquinone promotes G(1) arrest through translation inhibition of cyclin D1 and induces apoptosis in breast cancer cellsLife Sci2013932178379024044882
- RomanoGSantiLBiancoMRThe TGF-β pathway is activated by 5-fluorouracil treatment in drug resistant colorectal carcinoma cellsOncotarget Epub201633
