Abstract
Duchenne muscular dystrophy (DMD), an incurable and a progressive muscle wasting disease, is caused by the absence of dystrophin protein, leading to recurrent muscle fiber damage during contraction. The inflammatory response to fiber damage is a compelling candidate mechanism for disease exacerbation. The only established pharmacological treatment for DMD is corticosteroids to suppress muscle inflammation, however this treatment is limited by its insufficient therapeutic efficacy and considerable side effects. Recent reports show the therapeutic potential of inhibiting or enhancing pro- or anti-inflammatory factors released from DMD skeletal muscles, resulting in significant recovery from muscle atrophy and dysfunction. We discuss and review the recent findings of DMD inflammation and opportunities for drug development targeting specific releasing factors from skeletal muscles. It has been speculated that nonsteroidal anti-inflammatory drugs targeting specific inflammatory factors are more effective and have less side effects for DMD compared with steroidal drugs. For example, calcium channels, reactive oxygen species, and nuclear factor-κB signaling factors are the most promising targets as master regulators of inflammatory response in DMD skeletal muscles. If they are combined with an oligonucleotide-based exon skipping therapy to restore dystrophin expression, the anti-inflammatory drug therapies may address the present therapeutic limitation of low efficiency for DMD.
Introduction
Duchenne muscular dystrophy (DMD), the most common form of muscular dystrophy, involves progressive deterioration of muscle function, affecting up to one in 3,800–6,000 live male births.Citation1 DMD is caused mainly by a frameshift deletion, nonsense, or duplication mutations in the DMD gene on the X chromosome (Xp21.2), which encodes the protein dystrophin.Citation2 Dystrophin is a member of the spectrin superfamily of cytoskeletal proteins. Its full-length mRNA is mainly expressed in skeletal and cardiac muscles, as well as in small amounts in brain. In healthy muscle, dystrophin is located on the intracellular surface of the sarcolemma, along with the sarcomeres.Citation3 The structure is called the costamere, and it constitutes the structural cornerstone of muscular cells. At costameres, dystrophin assembles with the dystrophin-associated glycoprotein complex, which stabilizes the sarcolemma during muscular contractions. Dystrophin acts as a bridging and anchoring protein by binding to F-actin through its cytoplasmic N-terminal domain and to β-dystroglycan through its extracellular C-terminal domain.Citation4,Citation5 The loss of dystrophin disrupts the dystrophin-associated glycoprotein complex and causes membrane instability making it susceptible to damage and myofiber necrosis. Such circumstances provoke an abnormal persistence of inflammatory macrophages in the muscle, inducing chronic inflammation with impaired regeneration and ultimately fibrosis associated with the replacement of muscle by connective tissue, and consequently severe muscle dysfunction.Citation6–Citation8 Eventually, the loss of dystrophin results in severe muscle atrophy, respiratory and cardiac failure, and death before the age of 30 years.Citation9 Despite these findings, the molecular mechanisms initiating and perpetuating inflammation in DMD are poorly understood.
The most promising therapeutic candidate to overcome the deletion of dystrophin in DMD is exon-skipping therapy using antisense oligonucleotides (ASOs). ASOs can switch splicing patterns by targeting specific sequences of pre-mRNA elements involved in exon recognition and/or consensus splice sites in a sequence-specific manner.Citation10 Targeting splice sites or putative exon splicing enhancers with ASOs can induce the removal of exons from the mature DMD transcript so that a nonsense mutation is bypassed, or alternately removal of exons around a genomic deletion can restore the mRNA reading frame. ASOs have been extensively tested in disease models and are currently being evaluated in several clinical trials including ASO-based exon53 skipping therapy at our institute in Japan (http://www.ClinicalTrials.gov; NCT02081625).Citation11,Citation12
However, each ASO has a specific antisense sequence for single exon in the DMD gene and is considered a new drug; therefore, ASOs for separate exons have to undergo expensive and lengthy clinical trial stages. Furthermore, challenges involving exon skipping for duplications exist given the necessity to skip the only duplicated exon, in addition to the lack of an original exon for in-frame restoration and also the inability to recruit sufficient patients for clinical trials for exon skipping for rare mutations in DMD.Citation13 Thus, in addition to the dystrophin restoration, an efficient treatment should consider the possibility of inhibiting the muscle inflammation associated with dystrophin deletion that is common to all DMD patients.
Regarding skeletal muscle inflammation, the functions of skeletal muscle as a secretory organ should be considered. The cytokines released by skeletal muscle are called “myokines”; they are considered as autocrine and paracrine factors and are important mediators of communication between skeletal muscle and other organs in the endocrine system. They can exert profound effects on glucose and lipid metabolism and can be important mediators in inflammatory processes. As such, they are involved in energy homeostasis and the pathogenesis of obesity, diabetes, and other diseases.Citation14
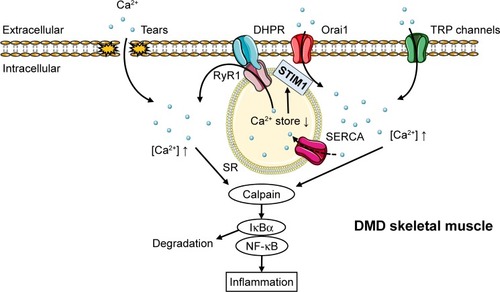
In this review, the recent findings on the inflammatory mechanisms in DMD are discussed, especially focusing on inflammatory factors released from skeletal muscle, which are regulated by calcium influx, reactive oxygen species (ROS), and nuclear factor-kappa B (NF-κB) (). This review emphasizes the future directions in DMD therapy targeting those master regulators of inflammation as well as each releasing factor regulated in DMD muscles.
Figure 1 The hypothetical mechanism of Ca2+ overflow-induced inflammation response in DMD muscles.
Abbreviations: DHPR, dihydropyridine receptor; DMD, Duchenne muscular dystrophy; EC, excitation-contraction; ER, endoplasmic reticulum; NF-κB, nuclear factor-kappa B; RyR, ryanodine receptor; SERCA, sarcoplasmic/endoplasmic-reticulum Ca2+-ATPase; SR, sarcoplasmic reticulum; STIM, stromal interaction molecule; TRP, transient receptor potential.
Broad anti-inflammatory drug – corticosteroids and nonsteroidal anti-inflammatory drugs targeting cyclooxygenases
The only currently accepted pharmacological therapy for DMD is corticosteroid-based anti-inflammatory treatment. In short-term clinical trials, corticosteroids have been shown to improve muscle strength and function without clinically severe adverse effects.Citation15,Citation16 In addition, nonrandomized trials have been shown significant beneficial effects on ambulation and cardiac function, delayed onset of both scoliosis and respiratory dysfunction, and a general amelioration of quality of life in treatments with prednisone or deflazacort for >2 years.Citation17
However, tolerance to chronic use and heterogeneous response to treatment are well-known drawbacks of corticosteroid therapy.Citation18 Glucocorticoids act through multiple mechanisms of action, making it unclear and controversial which molecular pathways provide the efficacy in DMD treatment and which pathways are responsible for detrimental effects. For example, glucocorticoids have a side effect of impaired growth in children with asthma,Citation19,Citation20 but the related limited muscle workload and delayed muscle maturation have been proposed to contribute to efficacy in DMD.Citation21
Like corticosteroids, treatment with commonly used nonsteroidal anti-inflammatory drugs (NSAIDs) broadly inhibiting cyclooxygenase (COX) enzymes had only partial therapeutic effects in an animal study. COXs generate prostaglandins and lipid autacoids from arachidonic acid, leading to pathogenic mechanisms, including the inflammatory response.Citation22 Daily treatment with NSAIDs (COX inhibitors) such as aspirin and ibuprofen was effective in ameliorating muscle morphology and reducing macrophage infiltration and necrosis but did not modify the percentage of regenerating myofibers. In addition, isometric tension did not differ in treated and untreated muscles; however, resistance to fatigue decreased by treatment with aspirin and not with ibuprofen.Citation23 In this study, parecoxib, a COX-2-selective inhibitor, was ineffective against DMD pathogenesis. COX-2 is known to be activated mainly in leukocytes, not muscles. In addition, steroids showed robust inhibition of COX-2 in leukocytes, which had only partial effects as mentioned earlier.Citation24 Therefore, it is possible that in order to treat DMD efficiently, the inflammatory process needs to be inhibited not only in leukocytes but in muscles as well.
Considering how problematic and insufficient corticosteroids and NSAIDs appear to be as anti-inflammatory agents in the treatment of DMD, it is possible that more specific, safer, and more effective drugs need to be developed in this area.
Potential drugs targeting inflammatory mechanisms in DMD
Store-operated Ca2+ entry and transient receptor potential calcium channels
Based on the mechanism of inflammation in DMD, initial targets for anti-inflammatory drugs are calcium channels. Loss of dystrophin leads to a structurally weaker plasma membrane that is easily damaged during muscle contraction and allows both extracellular calcium influx and release of endogenous ligands. The upregulated cytoplasmic Ca2+ leads to calpain overactivation in DMD, which can activate the NF-κB inflammatory pathways mediated by degradation of IkBα (an NF-κB inhibitor)Citation25 and stimulate inflammatory cytokine release, which further impairs muscle regeneration (). This pathway induces abnormal persistence of inflammatory cells such as macrophages.Citation26,Citation27 In fact, elevated Ca2+ influx through calcium channels was sufficient to induce a dystrophic phenotype, including increased central nucleated myofibers, fibrosis, and infiltration of inflammatory cellsCitation28 (). However, the mechanism of calcium over-influx in DMD muscle is not clearly understood. One of the proposed models suggest that extracellular calcium leaks into dystrophin-deficient myofibers during muscle contraction, inducing local hypercontraction after repeated contraction, which magnifies membrane damage on adjacent lengthened regions in the same or adjacent muscle fibers.Citation29 In contrast, dystrophin deficiency increases calcium concentration mediated by 1) store-operated Ca2+ entry (SOCE) machinery as a result of by reduction of intracellular Ca2+ stores, 2) concentrated stretch-activated channels on plasma membrane such as the transient receptor potential (TRP) cation channel, and 3) the oxidized, overactivated “leaky” ryanodine receptor 1 (RyR1) described in “ROS and ryanodine receptor 1” section ().
The depletion of endoplasmic/sarcoplasmic reticulum (ER/SR) Ca2+ stores promotes the translocation of stromal interaction molecule 1 (STIM1), a calcium sensor in the ER/SR membrane, to regions close to the plasma membrane, where STIM1 activates Orai, a pore-forming unit that allows Ca2+ influx through the plasma membrane into the cytosol.Citation30 The expression of Orai1 was elevated in the dystrophic muscles, whereas STIM1 levels remained largely unchanged, together with increased SOCE activity in adult muscles of mdx mice. When Orai1 was inhibited with BTP-2, a specific SOCE inhibitor injected for 2 weeks in mdx mice, the cytosolic calpain1 activity in myofibers was significantly reduced, indicating that upregulation of Orai1-mediated SOCE pathway contributed to the disrupted Ca2+ homeostasis in mdx muscle.Citation30
TRP channels promote calcium overload from extracellular calcium as a result of their expression in plasma membrane. In skeletal muscle, several isoforms of the TRPC (canonical), TRPV (vanilloid), and TRPM (melastatin) subfamilies are expressed. In particular, TRPC1, C3, and C6; TRPV1, V2, and V4; and TRPM4 and M7 have been consistently found in cultured myoblasts or in adult muscles; however, only some of the TRPC and TRPV isoforms have been studied in skeletal muscle.Citation31
In the TRPC subfamily, expression of TRPC1, C3, and C6 was increased in dystrophic muscle.Citation30,Citation32 The abnormal activation of these channels in mdx fibers might be mediated by ROS production and Src kinase activation.Citation32 TRPC1 may contribute through binding with STIM1–Orai1 complex as a result of Ca2+ store depletion.Citation31 Additionally, Ca2+ influx through TRPC3 and C6 channels has been shown to be sufficient to induce muscle dystrophy.Citation28
In the TRPV subfamily, Iwata et alCitation33 reported that TRPV2 was abundantly expressed in the sarcolemma of dystrophic myocytes and that cyclic cellular stretch increased TRPV2 translocation to the membrane. It was found that dominant-negative inhibition of endogenous TRPV2 in mdx mice suppressed the calcium increase in muscle fibers and eased dystrophic pathology, that is, the increased number of central nucleus and fiber size variability/fibrosis/apoptosis, elevated serum creatine kinase levels, and reduced muscle performance.Citation33 Tranilast, a clinically used antiallergic drug, is also known to be a TRPV2 blocker experimentally,Citation34 and its administration was found to be efficacious in reducing serum creatine kinase levels in mdx miceCitation35 and fibrosis in mdx skeletal muscles, leading to improved resistance to muscle fatigue.Citation36
Thus far, the possible involvement of TRPV4 in DMD has not been investigated; however, it is notable that TRPV4 was activated by phospholipase A2,Citation37 an enzyme involved in SOCE in dystophic myofibers,Citation38 and that its expression is increased by 10- to 40-fold in dystrophinopathiesCitation39 ().
Table 1 Regulation of Ca2+ transport-related proteins in DMD muscles
ROS and ryanodine receptor 1
In addition to calcium influx, elevated ROS production in DMD is a potential target for anti-inflammatory therapy, as suggested by the reports showing the inhibitory effect of the antioxidant N-acetylcysteine on muscle damage in mdx mice accompanied with reduced NF-κB activityCitation40 and also the contribution of ROS to RyR1 or TRPV1-induced intracellular Ca2+ increase.Citation41,Citation42
The mechanical distension of the sarcolemma induced by contraction causes the formation of superoxide anion radicals by the action of nicotinamide adenine dinucleotide phosphate oxidases (NOX), including NOX type 2 and type 4 in skeletal muscle. This radical cannot be scavenged by nitric oxide to form peroxynitrite because of its low presence in DMD muscles, followed by the oxidization of RyRs and indirect activation of stretch-activated channelsCitation41 (). Notably, NOX inhibition with diapocynin, a dimer of the commonly used NOX inhibitor apocynin, was shown to inhibit the loss of strength of skeletal muscles induced by eccentric contractions in mdx mice, a model of DMD, to levels similar to those in wild-type mice.Citation43 In a potential therapy targeting downstream of ROS production, stabilization of the closed state of RyRs by Rycals, a RyR channel inhibitor, restored cardiac and skeletal muscle function by normalizing oxidized and overactivated RyRs.Citation44,Citation45
Figure 2 Overview of inflammatory mechanism in DMD muscles and related potential drugs.
Abbreviations: ASO, antisense oligonucleotide; DAMPs, danger-associated molecular patterns; DMD, Duchenne muscular dystrophy; HGF, hepatocyte growth factor; HMGB, high-mobility group box protein; HSP, heat shock protein; IGF, insulin-like growth factor; IL, interleukin; MIF, macrophage migration inhibitory factor; NF-κB, nuclear factor-kappa B; NOX, nicotinamide adenine dinucleotide phosphate oxidase; ROS, reactive oxygen species; RyR, ryanodine receptor; SR, sarcoplasmic reticulum; TLR, Toll-like receptor; TNF, tumor necrosis factor; TNFR, TNF receptor; TRPV, transient receptor potential vanilloid.
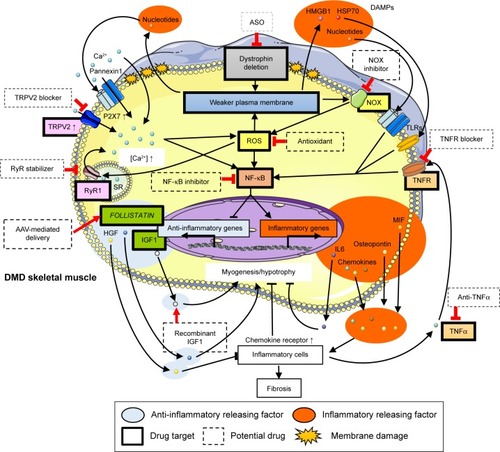
In contrast, Ca2+ overload in mitochondria directly increases ROS levels,Citation46 which further contributes to membrane permeability through sarcolemmal lipid peroxidation.Citation47 The antioxidant idebenone (Catena®; Santhera Pharmaceuticals, Liestal, Switzerland), a synthetic analog of ubiquinone (coenzyme Q10), is currently under clinical trials to test its efficacy in the prevention of skeletal muscle function loss and heart pump function reduction. Santhera Pharmaceuticals has confirmed the safety of idebenone in DMD patients (https://ClinicalTrials.gov/ct2/show/NCT00654784). There is an ongoing Phase II extension trial to test its safety and efficacy in the long-term. A Phase III trial was also carried out to assess the effect of idebenone on pulmonary function, motor function, muscle strength, and quality of life in patients not treated with corticosteroids. The results show that idebenone was well tolerated, and a slower decline in respiratory function was observed for treated patients compared to that observed for the placebo group (https://ClinicalTrials.gov/ct2/show/NCT01027884). These beneficial effects of idebenone can be explained by its ability to protect against mitochondrial respiratory chain dysfunction and reduce ROS production.Citation48
Santhera plans to file for marketing authorization in 2016. Furthermore, it is planning a placebo-controlled Phase III study in corticosteroids-treated patients. Additionally, preclinical studies with green tea extract and its active ingredient epigallocatechin gallate revealed histological and functional improvement in mdx mice.Citation49,Citation50 Furthermore, early treatment with green tea extract reduced dystrophic muscle pathology, potentially by regulating NF-κB activity in regenerating fibers.Citation51 Epigallocatechin gallate for DMD patients is now under Phase II/III trials at Charité University Hospital Berlin, Germany (https://www.ClinicalTrials.gov/ct2/show/NCT01183767). Flavocoxid is another antioxidant that has been tested in a Phase I trial in DMD patients to assess its safety (https://www.ClinicalTrials.gov/ct2/show/CAT-1004) (; ).
Table 2 The potential drugs for DMD anti-inflammatory therapy
NF-κB, tumor necrosis factor-α, and chemokines
NF-κB, one of the major transcription factor regulating inflammatory pathways, is also an important target for anti-inflammatory therapy. CAT-1004, an inhibitor of NF-κB has completed Phase I clinical trial (https://ClinicalTrials.gov/ct2/show/VBP15) ().
NF-κB is activated in dystrophic muscles, possibly following calcium influx and/or ROS production,Citation25,Citation40 and targets a wide range of genes, including inflammatory cytokines and chemokines.Citation52 Interestingly, NF-κB is activated among the earliest histological features of DMD neonates,Citation53–Citation55 years before symptoms appear. It suggests that very early treatment of DMD patients with NF-κB suppression therapy may prevent or delay the onset of muscle dysfunction as a result of improved muscle regeneration and reduction of fibrosis.
Ablation of one allele of the p65 subunit of NF-κB was sufficient to improve pathology in mdx mice. In addition, a study explored conditional deletion of IκB kinase (IKK) β in mdx mice, a component of the IKK complex with IKKα as catalytic subunits that induce NF-κB activation by IκB degradation. It was found that NF-κB in activated macrophages promotes inflammation and muscle necrosis in skeletal muscle fibers, resulting in limited regeneration through the inhibition of muscle progenitor cells. In addition, specific pharmacological inhibition of IKK resulted in improved pathology and muscle function in mdx mice.Citation26
Recently, Heier et al identified VBP15, a compound structurally related to glucocorticoids with similar anti-inflammatory properties but without steroidal side effects.Citation56 VBP15 inhibited NF-κB-induced tumor necrosis factor (TNF)-α release mediated by the glucocorticoid receptor, independently of the glucocorticoid response elements (eg, classical steroid receptor transactivation or hormonal properties), activation or upregulation that is implicated in a number of glucocorticoid side effects. It also had protective physicochemical effects on the cell membrane. The translation of these drug mechanisms into mdx mice improved muscle strength, live imaging, and pathology through both preventive and post-onset intervention regimens. VBP15 is now under a Phase I clinical trial ().
Furthermore, the inhibitors of TNF-α, a chemokine that induces NF-κB activation, have shown some potential in improving DMD pathology in animal studies. Remicade, a human anti-TNF-α antibody and etanercept, a blocker of soluble TNF-α receptor, are two candidates clinically used to treat inflammatory disorders (). Remicade delayed and reduced the inflammation and disruption of dystrophic muscle without adverse effects in mdx mice.Citation57 Etanercept inhibited exercise-induced force reduction in skeletal muscleCitation58 and protected against exercise-induced myofiber necrosis in mdx mice.Citation59 Furthermore, a murine-specific TNF-α antibody significantly reduced contractile dysfunction and myofiber necrosis.Citation60
Chemokines, whose expression is regulated by NF-κB, are major contributors to DMD pathogenesis promoting persistent inflammatory cells such as CD4+ and CD8+ T-cells, neutrophils, eosinophils, and inflammatory macrophages.Citation27 Besides immune cells, the main source of chemokines in skeletal muscle, muscle fibers can also chemoattract myeloid cells secreting chemokines. We recently found that NF-κB is activated in C2C12 myotubes upon muscle contraction and upregulate C-C motif chemokine ligand (CCL) 2 secretion, inducing THP-1 monocyte chemoattraction.Citation61 In addition, several CC chemokines including CCL2, which are the components of the largest family of chemokines, and chemoattractive for diverse inflammatory cells, showed increased expression in mdx muscle.Citation62 These findings suggest that increased CCL2 secretion from DMD muscles may also contribute to monocyte chemoattraction into skeletal muscles. Furthermore, CCL receptor (CCR) 2 is the only CCL2 receptor whose expression was upregulated in muscles of mdx mice.Citation63 CCR2 is expressed highly in Ly6Chigh inflammatory monocytes, which polarize CD11bhigh inflammatory macrophages. CCR2 deficiency in mdx mice preferentially reduced the CD11bhigh population of macrophages and promoted the recovery of normal macrophage polarization characteristics. Consequently, these mice improved characteristic histopathological features of the disease and increased the force-generating capacity of the diaphragm.Citation6
Also, C-X-C motif chemokine ligands (CXCL) such as CXCL1, CXCL2, CXCL3, CXCL8, and CXCL11 for neutrophils contributing to the inflammatory phase during regeneration and absent from normal muscle fibers were induced in DMD myofibers.Citation62 In addition, we found the secretion of macrophage migration inhibitory factor (MIF), an inflammatory cytokine and chemoattractant, was regulated by contraction in C2C12 myotubes.Citation64 MIF is known to be expressed mainly in leukocytes. However, in an immunohistochemical study, MIF was detected not only in immune cells but also in muscle fiber membrane areas of infiltration, necrosis, myophagocytosis, degeneration, and regeneration in muscular dystrophy samples.Citation65 MIF binds to the CXC chemokine receptors CXCR2 and CXCR4. CXCR2 is the receptor for CXCL1, 2, 3, and 8, which are the CXCLs upregulated in DMD muscles, and CXCR4 is the receptor for CXCL12, which is also upregulated in regenerating myofibers from DMD patientsCitation62 and thus can contribute to the inflammation. However, CXCL12 and CXCR4 may be difficult to use as candidates for anti-inflammation therapy because of their major role in muscle regeneration.Citation66 describes the potential of chemoattractants and these receptors as future therapeutic targets for DMD.
Table 3 The upregulated inflammatory factors and their receptors in DMD muscles as the future targets for anti-inflammatory drugs
A second mechanism by which IKK/NF-κB signaling regulates the dystrophic process is inhibition of muscle regeneration. Muscle regeneration after injury represents a coordinated sequence of events from the activation of quiescent satellite cells into myoblasts to their subsequent fusion to newly formed myotubes.Citation67 NF-κB has been previously shown to inhibit the differentiation sequence by MyoD repressionCitation68 and by preventing myoblast fusion in vitro.Citation69 Additionally, an in vivo study showed that TNF-α, a potent inducer of NF-κB signaling, inhibited myogenesis through repression of MyoD.Citation68 Genetically or pharmacological blocking of NF-κB function promoted the formation of new myofibers in response to degeneration.Citation26 IKK deletion in muscles in response to acute injury could also lead to improved regenerationCitation70 ().
In addition to upregulating inflammatory cytokines, NF-κB suppresses hepatocyte growth factor (HGF), an anti-inflammatory cytokine. The mdx; NF-κB p65+/− muscle at 4 weeks of age showed significantly higher HGF expression, correlating with reduced leukocyte infiltration and increased muscle fiber formation. The phenotypic improvements of muscle in these mice were reversed by silencing HGF preferentially in myogenic cells, resulting in significant degeneration of the diaphragm.Citation71
Danger-associated molecular patterns
The disruption of plasma membrane or necrosis following calcium influx in DMD leads to the release of cytoplasmic molecules called danger-associated molecular patterns (DAMPs) that include nucleotides, high-mobility group box protein 1 (HMGB1), hyaluronic acid, biglycan, and heat shock proteins (HSPs). DAMPs can induce chronic inflammation as the ligands of Toll-like receptors (TLRs)Citation72 (). Recent proteomic studies of DMD subjects and mdx mice revealed an increased presence of several potential TLR2 ligands in the circulation, including serum amyloid A, HSP70, and fibrinogen.Citation73,Citation74 Other studies detected HMGB1, a shared TLR2/4 ligand, as a potential early inflammatory target in mdx mice,Citation7 in addition to increased HSP70 expression in DMD muscles,Citation75 and fibrinogen as a driver of fibrosis in dystrophic muscleCitation76 ().
These reports suggest that endogenous TLR ligands released from injured skeletal muscle may be important signals for stimulating chemokine expression in both muscle fibers and hematopoietic progenitor cells mediated by TLR-dependent inflammasome and/or NF-κB activation, thereby helping to attract monocytes/macrophages to the site of damaged skeletal muscle. These TLR2 ligands also affected the function of other inflammatory cell types in DMD muscles such as T lymphocytes, which play an important role in regulating macrophage function and are also implicated in the pathogenesis.Citation77,Citation78 In fact, TLR2 deletion in mdx mice led to reduced macrophage numbers within DMD muscle during the acute inflammatory phase of the disease. The reduced macrophage infiltration within diaphragms of mdx-TLR2−/− mice was associated with a significant amelioration of DMD pathogenesis, that is, reduced necrotic injury, larger regenerated myofibers, and decreased fibrosis, all of which led to a higher force-generating capacity of the muscle. In detail, TLR2 deletion in this context not only reduced macrophage infiltration but also significantly modified macrophage polarization into iNOS−CD206+, anti-inflammatory macrophages.Citation79 Furthermore, mdx mice lacking the TLR signaling adaptor protein MyD88 showed less myofiber necrosis in the diaphragm and improved limb muscle strength at 12 months of age.Citation80 Similarly, TLR4 ablation in mdx mice resulted in significant reduction of the amount of inflammatory macrophages within the diaphragm early in the pathogenesis (6–12 weeks of age), together with improved muscle histology and strength.Citation7
In DMD, ATP release, another major pattern in DAMPs, due to the fragility of myofibers can activate plasma membrane receptors for extracellular nucleotides termed “P2 receptors”. P2X7 was substantially upregulated in skeletal muscle from mdx mice and in myoblasts isolated from DMD patients.Citation81–Citation85 Additionally, exposure of mdx myoblasts to extracellular ATP induced a significant increase in P2X7/pannexin1 channel-dependent Ca2+ influx and release of interleukin (IL)-1β, suggesting that nucleotides released from dystrofic muscle can trigger inflammatory response in DMD through purinergic signaling.Citation86 It was recently reported that in vivo blocking of the extracellular ATP/P2X purinergic signaling pathway by periodate-oxidized ATP delayed the progression of DMD pathogenesis and ameliorated the local inflammatory response in mdx mice, including reduced leukocyte infiltration and IL-6 expressionCitation87 (; ).
IL-6
IL-6 is one of the most extensively investigated myokines, and its secretion is regulated by exercise. Additionally, it can regulate glucose and lipid homeostasis affecting liver, adipose tissue, and skeletal muscle itself.Citation88 IL-6 expression in skeletal muscle was found to be also upregulated in obesity and to contribute to increased white adipose tissue mass.Citation89
IL-6 is present at high levels in the circulation and muscles from DMD patients and from young mdx mice. It also follows the disease time-course in DMD patients.Citation90–Citation92 IL-6 contributes to satellite cell proliferationCitation93 and muscle growth,Citation94,Citation95 in addition to its major role of inducing the transition from acute neutrophil infiltration to chronic mononuclear cell infiltration.Citation96 Therefore, repeated cycles of degeneration/regeneration, induced by chronic IL-6 upregulation in DMD, might induce the exhaustion of satellite cells, leading to enhancement of the dystrophic phenotype. In fact, IL-6-overexpressing mdx mice showed an exacerbation of the dystrophic phenotype wherein increased circulating levels of IL-6 promoted muscle degeneration, inflammation, exhaustion of muscle stem cells, and accumulation of fibro/adipogenic progenitors.Citation92 Importantly, the neutralization of IL-6 activity in mdx mice using an anti-IL-6 receptor antibody resulted in increased robustness for the dystrophic muscle, impeded the chronic inflammatory response, reduced muscle necrosis, and promoted muscle differentiation and maturation. These events led to the reduction of exercise-induced fiber damage and mitigation of diminished muscle strength.Citation91 The Food and Drug Administration has approved a number of novel compounds blocking IL-6 signaling for the treatment of other inflammatory disorders in the last decade; the application of these drugs to DMD patients can be due to their anti-inflammatory properties (; ).
Osteopontin
Osteopontin (OPN) is a secreted and chemotactic phosphoprotein that plays important roles in tissue remodeling following injury.Citation97,Citation98 Recently, elevated serum OPN levels were found in the dystrophic dogs compared with those in the wild type just before and an hour after a cesarean section birth and at the age of 3 months. In addition, the serum OPN level was significantly correlated with the phenotypic severity of dystrophic dogs at the onset of muscle weakness. Immunohistologically, OPN was upregulated in infiltrated macrophages and developmental myosin heavy chain-positive regenerating muscle fibers in the dystrophic dogs.Citation99 It is reported that a promoter polymorphism on the OPN gene was associated with severity of DMD. The G allele in the locus (dominant model; 35% of subjects) inhibiting the binding of transcription factors, specificity protein-1 was associated with more rapid progression and less grip strength, suggesting OPN is a genetic modifier of DMD.Citation100 In addition, the promoter structure of the OPN gene has multiple predicted steroid hormone enhancers and a NF-κB promoter element.Citation101
Genetic ablation of OPN in mdx mice caused significant reduction in the amount of intramuscular neutrophils and natural killer T-like cells and an increased amount of regulatory T-cells. This anti-inflammatory state resulted in a net decrease in the amount of transforming growth factor-beta (TGF-β) in the later stages of DMD pathogenesis. Diminished TGF-β levels were correlated with a marked decrease in fibrosis of both diaphragmatic and cardiac muscles. These studies identified OPN as an immunomodulator and pro-fibrotic cytokine in DMD muscleCitation78 (; ).
Myostatin and follistatin
Myostatin, a member of TGF-β family, is a negative regulator of skeletal muscle growthCitation102,Citation103 and is produced pre dominantly in muscle and blood.Citation102,Citation104 Its expression was significantly higher in mdx mice than in wild-type mice.Citation105 The inhibitory effect of myostatin on postnatal growth is mediated by its negative regulation of satellite cell activation, proliferation, and self-renewalCitation106 as well as myoblast proliferation and differentiation.Citation103,Citation107
Blockade of endogenous myostatin using blocking antibodies for 3 months resulted in increased body weight, muscle mass, muscle size, and absolute muscle strength in mdx muscle, along with decreased muscle degeneration and concentrations of serum creatine kinase.Citation108 Myostatin propeptide also ameliorated the symptoms in mdx mice.Citation109
However, clinical studies targeting myostain have not shown sufficient therapeutic effects. MYO-029, a human myostatin antibody,Citation110 increased the muscle mass in immunodeficient mice by ~30% in 3 months.Citation108 In contrast, a double-blind randomized clinical trial in Becker muscular dystrophy indicated that MYO-029 was safe but not efficacious.Citation110 Another myostatin inhibitor, ACE-031, a soluble activin type IIB receptor was found promising in increasing muscle mass and whole body pulling tension in mdx mice.Citation111 However, a safety–tolerability study of ACE-031 in DMD patients was prematurely terminated because of minor nosebleeds and/or gum bleeding.
Follistatin, a secreted glycoprotein and functional antagonist of the TGF-β family, which includes myostatin, is likely to be more effective as a therapeutic target to promote muscle growth than inhibition of only myostatin, as indicated by a report that heterozygous loss of follistatin resulted in retention of reduced muscle mass in a myostatin-null background.Citation112 This implies that follistatin inhibits other TGF family members in addition to myostatin to regulate muscle size. Delivery of the FOLLISTATIN gene by adeno-associated virus (AAV1. CMV.FS344) in Becker muscular dystrophy patients in a Phase I/IIa clinical study showed safety and efficacy as determined by the distance walked in a 6-minute walk test, along with improved muscle histopathology.Citation113 Based on this positive result, the same strategy for DMD is currently under a Phase I/II clinical study (https://ClinicalTrials.gov/ct2/show/NCT02354781) (; ).
Insulin-like growth factor-1
Contrary to the inhibition of inflammatory response, there is also the strategy of enhancement of anti-inflammatory response and hypotrophic factor targeting by insulin-like growth factor-1 (IGF-1) in skeletal muscles. The transgenic mdx mice expressing muscle-restricted IGF-1 (mIGF-1) (mdx:mIgf+/+) exhibited increase in muscle mass by at least 40% leading to similar increases in force generation in extensor digitorum longus muscles compared with mdx mice. The diaphragms from transgenic mdx:mIgf+/+ mice exhibited significant hypertrophy and hyperplasia at all ages. In addition, the enhanced IGF-1 expression significantly diminished the amount of fibrosis normally observed in diaphragms of aged mdx mice. Decreased myonecrosis was also observed in diaphragms and quadriceps from the transgenic mice compared with age-matched mdx animals. Furthermore, signaling pathways associated with muscle regeneration and protection against apoptosis were significantly elevated in the transgenic mice.Citation114 Interestingly, mIGF-1 repressed the expression and activity of MIF, HMGB1, and NF-κB.Citation115
IGF-1 is currently approved for severe primary IGF deficiency by the Food and Drug Administration. A Phase II clinical trial of recombinant IGF-1 (INCRELEX™) has been initiated in glucocorticoid-treated DMD patients to test its ability to preserve muscle function over 6 months (https://ClinicalTrials.gov/ct2/show/NCT01207908) ().
Conclusion
From the point of view of inflammatory mechanisms in DMD skeletal muscles, calcium channel, ROS production, and NF-κB pathway are potential targets for treatment as the master regulators. In fact, RyR channel blockers, antioxidants, and NF-κB inhibitors are under clinical trial. Those potential drugs targeting specific molecules are promising as more effective and less toxic compared with the current corticosteroid therapy used as a broad anti-inflammatory treatment. Furthermore, those master regulators of DMD inflammation eventually induce the release of inflammatory factors such as DAMPs and inflammatory cytokines and suppress anti-inflammatory cytokines such as HGF and IGF-1 that mediate DMD pathogenesis (). Thus, we emphasize the need to understand the underlying mechanisms of DMD pathogenesis and the secretory functions of skeletal muscle as both contributor and healer of DMD pathogenesis in order to develop the next generation of DMD drugs.
Future perspectives
Dystrophin restoration by exon skipping in DMD is the most promising therapy, because it directly addresses the underlying pathogenic cause. Combined with this therapy, the anti-inflammatory treatments might show improved therapeutic potential. Interestingly, there are already trials using dual exon skipping of dystrophin and myostatin pre-mRNAs using ASOs.Citation116 Although the additional or synergistic effect of this combination of dystrophin restoration and muscle growth promotion is not yet clear, this ASO-using epochal idea can be applied to the treatments targeting other inflammatory factors discussed in this review with dystrophin restoration at the same time.
Additionally, clustered regularly interspaced short palindromic repeat/Cas9-mediated genome editing was recently applied to correct the DMD gene mutation itself in the germ line of mdx mice, suggesting the potential to restore DMD protein levels.Citation117 Future advances of this technique may enable genome editing of postnatal somatic cells and correction of DMD gene mutations in the muscle tissue. The combination of this strategy with anti-inflammation drugs may cooperate to attenuate DMD pathogenesis in the future.
Acknowledgments
This work was supported by the Japan Society for the Promotion of Science Grant-in-Aid for Research Activity Start-up (grant to YA) (number 15H06883) and the Japan Agency for Medical Research and Development (AMED) (16ek0109154h0002 and 16am0301021h0002). We are grateful to Dr Urs T Ruegg and Dr Takashi Saito for their insightful comments and suggestions.
Disclosure
The authors report no conflicts of interest in this work.
References
- MendellJRShillingCLeslieNDEvidence-based path to newborn screening for Duchenne muscular dystrophyAnn Neurol201271330431322451200
- HoffmanEPBrownRHJrKunkelLMDystrophin: the protein product of the Duchenne muscular dystrophy locusCell19875169199283319190
- LjubicicVBurtMJasminBJThe therapeutic potential of skeletal muscle plasticity in Duchenne muscular dystrophy: phenotypic modifiers as pharmacologic targetsFASEB J201428254856824249639
- OguraYTajrishiMMSatoSHindiSMKumarATherapeutic potential of matrix metalloproteinases in Duchenne muscular dystrophyFront Cell Dev Biol201421125364719
- CirakSArechavala-GomezaVGuglieriMExon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation studyLancet2011378979159560521784508
- MojumdarKLiangFGiordanoCInflammatory monocytes promote progression of Duchenne muscular dystrophy and can be therapeutically targeted via CCR2EMBO Mol Med20146111476149225312642
- GiordanoCMojumdarKLiangFToll-like receptor 4 ablation in mdx mice reveals innate immunity as a therapeutic target in Duchenne muscular dystrophyHum Mol Genet20152482147216225552658
- VillaltaSANguyenHXDengBGotohTTidballJGShifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophyHum Mol Genet200918348249618996917
- FindlayARWeinNKaminohYClinical phenotypes as predictors of the outcome of skipping around DMD exon 45Ann Neurol201577466867425612243
- KoleRKrainerARAltmanSRNA therapeutics: beyond RNA interference and antisense oligonucleotidesNat Rev2012112125140
- WoodMJGaitMJYinHRNA-targeted splice-correction therapy for neuromuscular diseaseBrain2010133Pt 495797220150322
- KooTWoodMJClinical trials using antisense oligonucleotides in Duchenne muscular dystrophyHum Gene Ther201324547948823521559
- Aartsma-RusAFokkemaIVerschuurenJTheoretic applicability of antisense-mediated exon skipping for Duchenne muscular dystrophy mutationsHum Mutat200930329329919156838
- AhimaRSParkHKConnecting myokines and metabolismEndocrinol Metab2015303235245
- BonifatiMDRuzzaGBonomettoPA multicenter, double-blind, randomized trial of deflazacort versus prednisone in Duchenne muscular dystrophyMuscle Nerve20002391344134710951436
- EscolarDMHacheLPClemensPRRandomized, blinded trial of weekend vs daily prednisone in Duchenne muscular dystrophyNeurology201177544445221753160
- FalzaranoMSScottonCPassarelliCFerliniADuchenne muscular dystrophy: from diagnosis to therapyMolecules20152010181681818426457695
- HuizengaNAKoperJWDe LangePA polymorphism in the glucocorticoid receptor gene may be associated with and increased sensitivity to glucocorticoids in vivoJ Clin Endocrinol Metab19988311441519435432
- WolthersODPedersenSShort term linear growth in asthmatic children during treatment with prednisoloneBMJ199030167441451482202451
- AvioliLVGlucocorticoid effects on statural growthBr J Rheumatol199332Suppl 227308495277
- GroundsMDShavlakadzeTGrowing muscle has different sarcolemmal properties from adult muscle: a proposal with scientific and clinical implications: reasons to reassess skeletal muscle molecular dynamics, cellular responses and suitability of experimental models of muscle disordersBioEssays201133645846821500235
- RicciottiEFitzGeraldGAProstaglandins and inflammationArterioscler ThrombVasc Biol20113159861000
- SerraFQuartaMCanatoMInflammation in muscular dystrophy and the beneficial effects of non-steroidal anti-inflammatory drugsMuscle Nerve201246577378422847332
- MartinezLErmolovaNVIshikawaTOStoutDBHerschmanHRSpencerMJA reporter mouse for optical imaging of inflammation in mdx musclesSkelet Muscle201551525949789
- SchaecherKGoustJMBanikNLThe effects of calpain inhibition on IkB alpha degradation after activation of PBMCs: identification of the calpain cleavage sitesNeurochem Res20042971443145115202778
- AcharyyaSVillaltaSABakkarNInterplay of IKK/NF-kappaB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophyJ Clin Invest2007117488990117380205
- VillaltaSARosenbergASBluestoneJAThe immune system in Duchenne muscular dystrophy: friend or foeRare Dis201531e101096626481612
- MillayDPGoonasekeraSASargentMAMailletMAronowBJMolkentinJDCalcium influx is sufficient to induce muscular dystrophy through a TRPC-dependent mechanismProc Natl Acad Sci U S A200910645190231902819864620
- CooperSTMcNeilPLMembrane repair: mechanisms and pathophysiologyPhysiol Rev20159541205124026336031
- ZhaoXMoloughneyJGZhangSKomazakiSWeislederNOrai1 mediates exacerbated Ca(2+) entry in dystrophic skeletal musclePloS One2012711e4986223185465
- GaillyPTRP channels in normal and dystrophic skeletal muscleCurr Opin Pharmacol201212332633422349418
- GervasioOLWhiteheadNPYeungEWPhillipsWDAllenDGTRPC1 binds to caveolin-3 and is regulated by Src kinase – role in Duchenne muscular dystrophyJ Cell Sci2008121Pt 132246225518544631
- IwataYKatanosakaYAraiYShigekawaMWakabayashiSDominant-negative inhibition of Ca2+ influx via TRPV2 ameliorates muscular dystrophy in animal modelsHum Mol Genet200918582483419050039
- AoyagiKOhara-ImaizumiMNishiwakiCNakamichiYNagamatsuSInsulin/phosphoinositide 3-kinase pathway accelerates the glucose-induced first-phase insulin secretion through TrpV2 recruitment in pancreatic beta-cellsBiochem J2010432237538620854263
- IwataYKatanosakaYShijunZProtective effects of Ca2+ handling drugs against abnormal Ca2+ homeostasis and cell damage in myopathic skeletal muscle cellsBiochem Pharmacol200570574075116009351
- SwiderskiKTodorovMGehrigSMTranilast administration reduces fibrosis and improves fatigue resistance in muscles of mdx dystrophic miceFibrogenesis Tissue Repair201471124476069
- VriensJAppendinoGNiliusBPharmacology of vanilloid transient receptor potential cation channelsMol Pharmacol20097561262127919297520
- BoittinFXPetermannOHirnCCa2+-independent phospholipase A2 enhances store-operated Ca2+ entry in dystrophic skeletal muscle fibersJ Cell Sci2006119Pt 183733374216926189
- LindahlMBackmanEHenrikssonKGGorospeJRHoffmanEPPhospholipase A2 activity in dystrophinopathiesNeuromuscul Disord1995531931997633184
- WhiteheadNPPhamCGervasioOLAllenDGN-Acetylcysteine ameliorates skeletal muscle pathophysiology in mdx miceJ Physiol200858672003201418258657
- RueggUTPharmacological prospects in the treatment of Duchenne muscular dystrophyCurr Opin Neurol201326557758423995279
- ItoNRueggUTKudoAMiyagoe-SuzukiYTakedaSActivation of calcium signaling through Trpv1 by nNOS and peroxynitrite as a key trigger of skeletal muscle hypertrophyNat Med201319110110623202294
- IsmailHMScapozzaLRueggUTDorchiesOMDiapocynin, a dimer of the NADPH oxidase inhibitor apocynin, reduces ROS production and prevents force loss in eccentrically contracting dystrophic musclePloS One2014910e11070825329652
- MarxSOMarksARDysfunctional ryanodine receptors in the heart: new insights into complex cardiovascular diseasesJ Mol Cell Cardiol20135822523123507255
- AnderssonDCMeliACReikenSLeaky ryanodine receptors in beta-sarcoglycan deficient mice: a potential common defect in muscular dystrophySkelet Muscle201221922640601
- NetheryDCallahanLAStofanDMatteraRDiMarcoASupinskiGPLA(2) dependence of diaphragm mitochondrial formation of reactive oxygen speciesJ Appl Physiol2000891728010904037
- HauserEHogerHBittnerRWidhalmKHerknerKLubecGOxyradical damage and mitochondrial enzyme activities in the mdx mouseNeuropediatrics19952652602628552217
- BuyseGMVan der MierenGErbMLong-term blinded placebo-controlled study of SNT-MC17/idebenone in the dystrophin deficient mdx mouse: cardiac protection and improved exercise performanceEur Heart J200930111612418784063
- NakaeYHirasakaKGotoJSubcutaneous injection, from birth, of epigallocatechin-3-gallate, a component of green tea, limits the onset of muscular dystrophy in mdx mice: a quantitative histological, immunohistochemical and electrophysiological studyHistochem Cell Biol2008129448950118264714
- DorchiesOMWagnerSVuadensOGreen tea extract and its major polyphenol (-)-epigallocatechin gallate improve muscle function in a mouse model for Duchenne muscular dystrophyAm J Physiol20062902C616C625
- EvansNPCallJABassaganya-RieraJRobertsonJLGrangeRWGreen tea extract decreases muscle pathology and NF-kappaB immunostaining in regenerating muscle fibers of mdx miceClin Nutr201029339139819897286
- HaydenMSGhoshSSignaling to NF-kappaBGenes Dev200418182195222415371334
- ChenYWNagarajuKBakayMEarly onset of inflammation and later involvement of TGFbeta in Duchenne muscular dystrophyNeurology200565682683416093456
- PorterJDKhannaSKaminskiHJA chronic inflammatory response dominates the skeletal muscle molecular signature in dystrophin-deficient mdx miceHum Mol Genet200211326327211823445
- PorterJDMerriamAPLeahyPGongBKhannaSDissection of temporal gene expression signatures of affected and spared muscle groups in dystrophin-deficient (mdx) miceHum Mol Genet200312151813182112874102
- HeierCRDamskerJMYuQVBP15, a novel anti-inflammatory and membrane-stabilizer, improves muscular dystrophy without side effectsEMBO Mol Med20135101569158524014378
- GroundsMDTorrisiJAnti-TNFalpha (Remicade) therapy protects dystrophic skeletal muscle from necrosisFASEB J200418667668215054089
- PiernoSNicoBBurdiRRole of tumour necrosis factor alpha, but not of cyclo-oxygenase-2-derived eicosanoids, on functional and morphological indices of dystrophic progression in mdx mice: a pharmacological approachNeuropathol Appl Neurobiol200733334435917493014
- HodgettsSRadleyHDaviesMGroundsMDReduced necrosis of dystrophic muscle by depletion of host neutrophils, or blocking TNFalpha function with Etanercept in mdx miceNeuromuscul Disord2006169–1059160216935507
- PiersATLavinTRadley-CrabbHGBakkerAJGroundsMDPinnigerGJBlockade of TNF in vivo using cV1q antibody reduces contractile dysfunction of skeletal muscle in response to eccentric exercise in dystrophic mdx and normal miceNeuromuscul Disord201121213214121055937
- MiyatakeSBilanPJPillonNJKlipAContracting C2C12 myotubes release CCL2 in an NF-kappaB-dependent manner to induce monocyte chemoattractionAm J Physiol20163102E160E170
- De PaepeBCreusKKMartinJJDe BleeckerJLUpregulation of chemokines and their receptors in Duchenne muscular dystrophy: potential for attenuation of myofiber necrosisMuscle Nerve201246691792523225384
- PorterJDGuoWMerriamAPPersistent over-expression of specific CC class chemokines correlates with macrophage and T-cell recruitment in mdx skeletal muscleNeuromuscul Disord200313322323512609504
- MiyatakeSManabeYInagakiAMacrophage migration inhibitory factor diminishes muscle glucose transport induced by insulin and AICAR in a muscle type-dependent mannerBiochem Biophys Res Commun2014444449650124472542
- ReimannJSchnellSSchwartzSKappes-HornKDodelRBacherMMacrophage migration inhibitory factor in normal human skeletal muscle and inflammatory myopathiesJ Neuropathol Exp Neurol201069665466220467327
- BrzoskaEKowalskiKMarkowska-ZagrajekASdf-1 (CXCL12) induces CD9 expression in stem cells engaged in muscle regenerationStem Cell Res Ther201564625890097
- ChargeSBRudnickiMACellular and molecular regulation of muscle regenerationPhysiol Rev200484120923814715915
- GuttridgeDCMayoMWMadridLVWangCYBaldwinASJrNF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexiaScience200028954882363236611009425
- ThaloorDMillerKJGephartJMitchellPOPavlathGKSystemic administration of the NF-kappaB inhibitor curcumin stimulates muscle regeneration after traumatic injuryAm J Physiol19992772 Pt 1C320C32910444409
- MourkiotiFKratsiosPLueddeTTargeted ablation of IKK2 improves skeletal muscle strength, maintains mass, and promotes regenerationJ Clin Invest2006116112945295417080195
- ProtoJDTangYLuANF-kappaB inhibition reveals a novel role for HGF during skeletal muscle repairCell Death Dis20156e173025906153
- ChenGYNunezGSterile inflammation: sensing and reacting to damageNat Rev20101012826837
- HathoutYMarathiRLRayavarapuSDiscovery of serum protein biomarkers in the mdx mouse model and cross-species comparison to Duchenne muscular dystrophy patientsHum Mol Genet201423246458646925027324
- HathoutYBrodyEClemensPRLarge-scale serum protein biomarker discovery in Duchenne muscular dystrophyProc Natl Acad Sci U S A2015112237153715826039989
- PaepeBDCreusKKWeisJBleeckerJLHeat shock protein families 70 and 90 in Duchenne muscular dystrophy and inflammatory myopathy: balancing muscle protection and destructionNeuromuscul Disord2012221263321855341
- VidalBSerranoALTjwaMFibrinogen drives dystrophic muscle fibrosis via a TGFbeta/alternative macrophage activation pathwayGenes Dev200822131747175218593877
- VillaltaSARosenthalWMartinezLRegulatory T cells suppress muscle inflammation and injury in muscular dystrophySci transl Med20146258258ra142
- VetroneSAMontecino-RodriguezEKudryashovaEOsteopontin promotes fibrosis in dystrophic mouse muscle by modulating immune cell subsets and intramuscular TGF-betaJ Clin Invest200911961583159419451692
- MojumdarKGiordanoCLemaireCDivergent impact of Toll-like receptor 2 deficiency on repair mechanisms in healthy muscle versus Duchenne muscular dystrophyJ Pathol20162391102226800321
- Henriques-PonsAYuQRayavarapuSRole of Toll-like receptors in the pathogenesis of dystrophin-deficient skeletal and heart muscleHum Mol Genet201423102604261724368419
- YoungCNBrutkowskiWLienCFP2X7 purinoceptor alterations in dystrophic mdx mouse muscles: relationship to pathology and potential target for treatmentJ Cell Mol Med20121651026103721794079
- JiangTYeungDLienCFGoreckiDCLocalized expression of specific P2X receptors in dystrophin-deficient DMD and mdx muscleNeuromuscul Disord200515322523615725584
- BuvinicSAlmarzaGBustamanteMATP released by electrical stimuli elicits calcium transients and gene expression in skeletal muscleJ Biol Chem200928450344903450519822518
- YeungDZablockiKLienCFIncreased susceptibility to ATP via alteration of P2X receptor function in dystrophic mdx mouse muscle cellsFASEB J200620661062016581969
- YoungCNSinadinosALefebvreAA novel mechanism of autophagic cell death in dystrophic muscle regulated by P2RX7 receptor large-pore formation and HSP90Autophagy201511111313025700737
- RawatRCohenTVAmpongBInflammasome up-regulation and activation in dysferlin-deficient skeletal muscleAm J Pathol201017662891290020413686
- GazzerroEBaldassariSAsseretoSEnhancement of muscle T regulatory cells and improvement of muscular dystrophic process in mdx mice by blockade of extracellular ATP/P2X axisAm J Pathol2015185123349336026465071
- PedersenBKFebbraioMAMuscles, exercise and obesity: skeletal muscle as a secretory organNat Rev201288457465
- KnudsenJGBertholdtLJoensenELassenSBHidalgoJPilegaardHSkeletal muscle interleukin-6 regulates metabolic factors in iWAT during HFD and exercise trainingObesity20152381616162426109166
- RufoADel FattoreACapulliMMechanisms inducing low bone density in Duchenne muscular dystrophy in mice and humansJ Bone Miner Res20112681891190321509823
- PelosiLBerardinelliMGDe PasqualeLFunctional and morphological improvement of dystrophic muscle by interleukin 6 receptor blockadeEBioMedicine20152428529326137572
- PelosiLBerardinelliMGForcinaLIncreased levels of interleukin-6 exacerbate the dystrophic phenotype in mdx miceHum Mol Genet201524216041605326251044
- KurosakaMMachidaSInterleukin-6-induced satellite cell proliferation is regulated by induction of the JAK2/STAT3 signalling pathway through cyclin D1 targetingCell Proliferat2013464365373
- KurekJBNouriSKannourakisGMurphyMAustinLLeukemia inhibitory factor and interleukin-6 are produced by diseased and regenerating skeletal muscleMuscle Nerve19961910129113018808655
- SerranoALBaeza-RajaBPerdigueroEJardiMMunoz-CanovesPInterleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophyCell Metabol2008713344
- GabayCInterleukin-6 and chronic inflammationArthritis Res Ther20068Suppl 2S316899107
- PagelCNWasgewatte WijesingheDKTaghavi EsfandouniNMackieEJOsteopontin, inflammation and myogenesis: influencing regeneration, fibrosis and size of skeletal muscleJ Cell Commun Signal2014829510324318932
- UaesoontrachoonKWasgewatte WijesingheDKMackieEJPagelCNOsteopontin deficiency delays inflammatory infiltration and the onset of muscle regeneration in a mouse model of muscle injuryDis Model Mech20136119720522917925
- KuraokaMKimuraENagataTSerum osteopontin as a novel biomarker for muscle regeneration in Duchenne muscular dystrophyAm J Pathol201618651302131226963343
- PegoraroEHoffmanEPPivaLSPP1 genotype is a determinant of disease severity in Duchenne muscular dystrophyNeurology201176321922621178099
- BarfieldWLUaesoontrachoonKWuCSEccentric muscle challenge shows osteopontin polymorphism modulation of muscle damageHum Mol Genet201423154043405024626632
- McPherronACLawlerAMLeeSJRegulation of skeletal muscle mass in mice by a new TGF-beta superfamily memberNature1997387662883909139826
- ThomasMLangleyBBerryCMyostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferationJ Biol Chem200027551402354024310976104
- ZimmersTADaviesMVKoniarisLGInduction of cachexia in mice by systemically administered myostatinScience200229655721486148812029139
- AbeSSoejimaMIwanumaOExpression of myostatin and follistatin in Mdx mice, an animal model for muscular dystrophyZoolog Sci200926531532019715499
- McCroskerySThomasMMaxwellLSharmaMKambadurRMyostatin negatively regulates satellite cell activation and self-renewalJ Cell Biol200316261135114712963705
- LangleyBThomasMBishopASharmaMGilmourSKambadurRMyostatin inhibits myoblast differentiation by down-regulating MyoD expressionJ Biol Chem200227751498314984012244043
- BogdanovichSKragTOBartonERFunctional improvement of dystrophic muscle by myostatin blockadeNature2002420691441842112459784
- BogdanovichSPerkinsKJKragTOWhittemoreLAKhuranaTSMyostatin propeptide-mediated amelioration of dystrophic pathophysiologyFASEB J200519654354915791004
- WagnerKRFleckensteinJLAmatoAAA phase I/IItrial of MYO-029 in adult subjects with muscular dystrophyAnn Neurol200863556157118335515
- CadenaSMTomkinsonKNMonnellTEAdministration of a soluble activin type IIB receptor promotes skeletal muscle growth independent of fiber typeJ Appl Physiol2010109363564220466801
- LeeSJLeeYSZimmersTARegulation of muscle mass by follistatin and activinsMol Endocrinol201024101998200820810712
- MendellJRSahenkZMalikVA phase 1/2a follistatin gene therapy trial for Becker muscular dystrophyMol Ther201523119220125322757
- BartonERMorrisLMusaroARosenthalNSweeneyHLMuscle-specific expression of insulin-like growth factor I counters muscle decline in mdx miceJ Cell Biol2002157113714811927606
- PelosiLGiacintiCNardisCLocal expression of IGF-1 accelerates muscle regeneration by rapidly modulating inflammatory cytokines and chemokinesFASEB J20072171393140217264161
- Lu-NguyenNBJarminSASalehAFPopplewellLGaitMJDicksonGCombination antisense treatment for destructive exon skipping of myostatin and open reading frame rescue of dystrophin in neonatal mdx miceMol Ther20152381341134825959011
- LongCMcAnallyJRSheltonJMMireaultAABassel-DubyROlsonENPrevention of muscular dystrophy in mice by CRISPR/Cas9-mediated editing of germline DNAScience201434562011184118825123483
