?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Nasal administration is a high-potential delivery system, particularly because it can provide a pathway from the nose to the brain. The objective of this research is to characterize puerarin transport across a Calu-3 cell monolayer used as a model of the nasal mucosa and to evaluate the influence of puerarin in combination with paeoniflorin and menthol to explore the enhanced mechanism of the permeability at the cell level. The apparent permeability coefficients (Papp) of puerarin bidirectional transport were both <1.5×10−6 cm/s, and the efflux ratio was <1.5, indicating that puerarin alone exhibited poor absorption and that its transport primarily occurred by passive diffusion through the cell monolayer. When puerarin was coad ministered with paeoniflorin, the Papp was not changed (P>0.05). However, the addition of menthol significantly (P<0.05) improved the Papp of puerarin in both directions. Moreover, based on immunofluorescence experiments and transepithelial electrical resistance measurements, the data indicated that the drug compatibility opened tight junctions and weakened the barrier capabilities of epithelial cells, thereby promoting the permeability of puerarin.
Introduction
Nasal delivery is a method of drug administration in which drugs are absorbed from the nasal mucosa to produce a local or systemic effect; this administration method has received extensive attention in recent years.Citation1 By nasal administration, the drug can obtain a rapid absorption, a high bioavailability, excellent effectiveness, and brain targeting.Citation2,Citation3 Particularly due to the pathway from the nose to the brain, an important physiological feature of the nose, significant efforts have been made over the past 10 years to develop nasal delivery systems that can potentiate the delivery of drugs to the central nervous system (CNS).Citation4,Citation5 Because of its functions of filtering and homeostatic maintenance, the nasal mucosa forms a nose–brain barrierCitation6 to limit the transmission and bioavailability of the drugs to the CNS, which poses a challenge to nasal administration. To accurately elucidate the mechanisms underlying drug absorption and to determine how to increase drug permeability after nasal administration, various methods have been employed both in vivo and in vitro. Of these methods, in vitro cell culture models that accurately simulate the physiological properties of transport and permeation through nasal epithelial cells at the cellular level have become widely recognized.Citation7,Citation8 These cell culture models are divided into two types: real nasal mucosa models based on primary cultures of nasal epithelial cells from various species and surrogate nasal mucosa models, which generally use Calu-3 cells, RPMI2650 cells, or other appropriate cell lines.Citation9–Citation14 In this research, we chose to use Calu-3 cells to stimulate the nasal mucosa barrier function in vitro because Calu-3 cells have been previously used to study nasal drug absorption.Citation15–Citation17
Tongqiaosanyu, a traditional Chinese medicine used to treat acute cerebral stroke, mainly consists of the kudzu root (Radix Puerariae Lobatae), white peony root (Radix Paeoniae Alba), and menthol (Herba Menthae). In traditional Chinese medicine, kudzu rootsCitation18–Citation21 are usually used to treat cardiovascular disorders and ischemic stroke; puerarin (), the major isoflavone glycoside isolated from kudzu root, exhibits a therapeutic effect when prescribed to treat cerebral disease.Citation22 White peony root is usually administered together with kudzu root to treat acute cerebral stroke in traditional Chinese medicine clinics.Citation23–Citation25 Pharmacodynamic screening has revealed that the effective constituent of white peony root is paeoniflorin (),Citation26 a monoterpene glucoside with many therapeutic functions, such as antithrombotic activity, anti-inflammatory activity, enhancement of glucose uptake, and neuroprotective effects.Citation27,Citation28 However, because puerarin and paeoniflorin display low absorption and bioavailability when administered orally, their applications are restricted in clinical settings. Menthol () is a major constituent of peppermint oil and has been confirmed to excite the CNS and enhance permeation.Citation29–Citation31 Our ultimate aim is to provide the full benefits of puerarin in Tongqiaosanyu for the treatment of certain brain diseases by developing a nasal drug delivery system that takes advantage of the compatibility of puerarin, paeoniflorin, and menthol.
Previous research examining this medication evaluated the effects of different administration routes and compatibility on its pharmacokinetic behavior in vivo. To study the pharmacokinetic behavior of puerarin in rats following different methods of administration, Tongqiaosanyu was administered to rats by caudal vein injection, nasal administration, and oral administration, and the concentration of the puerarin in mice plasma and brain was analyzed by reversed phase high-performance liquid chromatography (HPLC). The result showed that the area under the plasma concentration-time curve from zero to infinity (AUC0−∞) of caudal vein injection was 787.99±70.44 mg⋅min⋅L−1; AUC0–∞ of nasal administration was 376.56±93.93 mg⋅min⋅L−1; AUC0−∞ of oral administration (the dose was ten times higher than that of caudal vein injection and nasal administration) was 491.18±110.64 mg⋅min⋅L−1; the absolute bioavailability of puerarin was 47.78% by nasal administration; the brain targeting coefficient (Re) was 132.25% by intranasal administration, and the brain drug targeting index was 2.70, which were significantly higher than those by the injections and oral administration. These results indicated that the bioavailability of puerarin by intranasal administration is significantly higher than that by oral administration and the brain drug targeting of puerarin by intranasal administration is higher than that by injection and oral administration, which shows the advantage on the medicine absorption into the brain by the intranasal administration.Citation32–Citation34 However, for these compounds, the transport across the nasal mucosa and the influence of compatibility on nasal epithelial cells remain unclear. Thus, Calu-3 cell culture was used in this study to simulate the nasal mucosa in vitro to elucidate the permeability of puerarin and the contribution of mutual compatibility to enhancing permeability. Additionally, further analysis at the cell level was performed to determine the drug actions on the barrier functions and the associated protein structures of the mono-layer. These experiments will provide fundamental data for the future study of the complicated nose–brain pathway.
Materials and methods
Materials
Puerarin, paeoniflorin, and menthol were obtained from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, People’s Republic of China). Calu-3 cells were purchased from China Infrastructure of Cell Line Resources (Beijing, People’s Republic of China). Polyethylene terephthalate (PET) cell culture inserts and 12-well plates (12 mm diameter, 0.4 μm pore size) were purchased from Corning Incorporated (Corning, NY, USA). A rabbit anti-occludin antibody (ab31721) was obtained from Abcam Shanghai Co., Ltd (Shanghai, People’s Republic of China). A mouse anti-claudin-1 antibody (2H10D10) was purchased from Thermo Fisher Scientific (Waltham, MA, USA). A tetramethylrhodamine isothiocyanate (red)-conjugated anti-rabbit immunoglobulin G (IgG) antibody was obtained from Beijing Zhongshan Golden Bridge Biotechnology Co. Ltd (Beijing, People’s Republic of China). A fluorescein isothiocyanate (green)-conjugated anti-mouse IgG antibody was purchased from Kangwei Century Biotechnology Co. Ltd (Beijing, People’s Republic of China). Acti-stain488 (green) fluorescent phalloidin was procured from Cytoskeleton Inc. (Denver, CO, USA). In this research, all experimental protocols were approved by the Review Committee for the Use of Human or Animal Subjects of Beijing University of Chinese Medicine.
Cell culture
Calu-3 cells were purchased from the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences (Beijing, People’s Republic of China) and were negative for the mycoplasma infection test. The cells were used at passage numbers 5–10 and were grown in minimum essential medium (Thermo Fisher Scientific) with 10% heat-inactivated fetal bovine serum (Thermo Fisher Scientific), 100 U/mL penicillin, and 100 μg/mL streptomycin in a humidified atmosphere with 5% CO2 at 37°C. Upon reaching 90% confluence, the cells were trypsinized using 0.25% trypsin and 0.1% ethylenediaminetetraacetic acid. Post-trypsinization, the Calu-3 cell suspension (0.5 mL) was inoculated at a density of 5×105 cells/mL on to 0.4 μm pore, collagen-coated clear PET membranes in 12 mm Transwell chambers, and medium (0.5 mL) was added to the basolateral chamber. On day 5, the medium on the apical surface was removed to produce air interface feeding conditions, and the transepithelial electrical resistance (TEER) values were measured by chopstick electrodes and an epithelial volt–ohm meter (EMD Millipore, Billerica, MA, USA) every other day when the medium in the basolateral chamber was replaced. After an additional 10 days, when the cells had reached maximum confluence on ~100% of the permeable support membranes and the TEER values had increased to >500 Ω cm2 under the conditions described earlier, the experiments were performed on polarized Calu-3 cell layers. For all the experiments, similar passage numbers were used.
Cytotoxicity assays
The levels of puerarin, paeoniflorin, and menthol that could interfere with the growth of Calu-3 cells were determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dye assay. The cells were seeded onto 96-well microtiter plates with flat-bottomed wells in a total volume of 100 μL of culture medium at a density of 1×105 cells/mL and incubated in a humidified atmosphere with 5% CO2 at 37°C. After 48 hours, the medium was removed and replaced with fresh medium containing different compounds at various concentrations. After culturing for an additional 48 hours, 15 μL of 5 mg/mL MTT in phosphate-buffered saline (PBS) was added to each well, and the mixtures were incubated at 37°C for 4 hours until purple deposits became visible. This assay measures the production of purple formazan, an MTT reaction product generated by mitochondrial dehydrogenase that indicates cell viability; in this reaction, formazan production is proportional to reductive activity. After the MTT solution was discarded, the colored reaction products were completely dissolved by the addition of 150 μL of dimethyl sulfoxide, and the absorbance was measured at 490 nm using a Multiskan GO microplate reader (Thermo Fisher Scientific). The mean absorbance of five measurements for each compound was expressed as the percentage of the absorbance of the untreated control and plotted against the concentration of the compound.
Cell viability (percentage) from the MTT assay was calculated using the following equation:
Evaluation of transport across Calu-3 cells
To determine the permeability of puerarin and the effect of different drugs on its penetration, drug transport in cell monolayers was analyzed. First, Calu-3 cells were seeded at a density of 5×105 cells/mL on to the PET membranes in Transwell chambers and allowed to form monolayers (TEER >500 ΩΩcm2). Before each experiment, the cells were washed three times with Hank’s balanced salt solution (HBSS) and equilibrated for 30 minutes at 37°C. The drug solution (0.5 mL) was added to the apical (A) side, and HBSS (1.5 mL) was added to the basolateral (B) side to measure A→B transport. The cells were incubated at 37°C with shaking. Samples (600 μL) were collected from the B side at 30 minutes, 60 minutes, 90 minutes, 120 minutes, 150 minutes, and 180 minutes. The amount of puerarin transported was measured with HPLC using a Hibar C18 column (4×200 mm2, 5 μm), and the samples were analyzed via UV detection (λ=250 nm). The mobile phase consisted of methanol and 1% acetate solution (37:63, v/v) and was pumped at a flow rate of 1 mL/min, and the injected volume was 20 μL. Under these conditions, the retention time of puerarin was ~6 minutes. B→A transport was evaluated by adding 1.5 mL of drug solution to the B side and 0.5 mL of HBSS to the A side. Samples (200 μL) were collected from the A side at the same time and measured with the same HPLC method used to assess A→B transport.
The apparent permeability coefficients (Papp) for puerarin were calculated according to the following equation:
The efflux ratio (ER) was calculated according to the following equation:
Measurement of cell TEER changes after exposure to different compounds
In addition to determining the integrity of monolayers, the cell TEER values were measured to investigate changes in the intercellular compactness of the Calu-3 cells. After the cells had formed monolayers, the drug solution (0.5 mL) was added to the A side and HBSS (1.5 mL) was added to the B side to simulate A→B transport. B→A transport was evaluated by adding the drug solution (0.5 mL) to the B side and HBSS (1.5 mL) to the A side. The TEER values of the untreated cells and the cells treated with different drugs were determined at 30 minutes, 60 minutes, 90 minutes, 120 minutes, 150 minutes, and 180 minutes. The measured TEER before the experiment was set as 100%, and all the other values were calculated relative to this value. Then, the relative TEER at each time point was compared with the control group value and statistically analyzed.
Immunofluorescence microscopy
For the immunocytochemical assessment of tight junction (TJ) proteins, cells were seeded at a density of 5×105 cells/mL onto the PET membranes in Transwell chambers until they formed monolayers. Next, the cells were cultured with media containing different compounds for 3 hours. At the beginning of the experiment, the cells were fixed with cold 4% paraform-aldehyde for 30 minutes. After a rinse in PBS, the cells were incubated with goat serum as a blocking buffer for 1 hour, and the samples were subsequently incubated with a polyclonal anti-occludin (1:100) or monoclonal anti-claudin-1 (1:100) primary antibody at 4°C overnight. After being washed three times with PBS again, the cells were incubated with the secondary antibody, tetramethylrhodamine isothiocyanate (red)-conjugated anti-rabbit IgG or fluorescein isothiocyanate (green)-conjugated anti-mouse IgG. Some cells were stained only with Acti-stain 488 (green) fluorescent phalloidin (1:150) at room temperature for 30 minutes. DAPI (Solarbio, Beijing Solarbio Science and Technology Co., Ltd, Beijing, People’s Republic of China) was used to counterstain cell nuclei. The membranes were carefully excised from Transwell inserts, mounted on a glass slide with 80% glycerol, and covered with a 15 mm coated glass coverslip. The morphology and fluorescence of TJ proteins were visualized using an inverted fluorescence microscope equipped with an appropriate filter (Olympus Corporation, Tokyo, Japan) and a laser-scanning confocal microscope, and images were obtained using the accompanying analysis software. The settings for image collection were identical, and the average optical density (AOD) of the images was calculated using ImageJ software.
The immunofluorescence images were semiquantitatively determined using ImageJ software according to the following equation:
The AOD percentage corresponding to the TJ protein was calculated using the following equation:
Data analysis and statistics
Each set of results shown is representative of three separate experiments. The results are given as the mean ± SD. The data were analyzed with one-way analysis of variance followed by the Dunnett’s test to compare differences between multiple groups and the control group (using SPSS 17.0 statistical software; SPSS Inc., Chicago, IL, USA). Significance was set at P<0.05.
Results
Cytotoxicity of compounds in Calu-3 cells
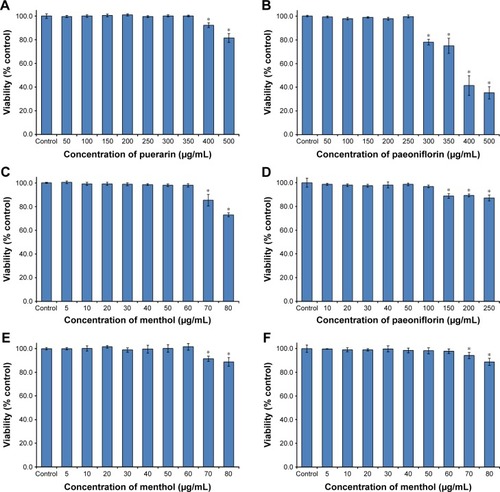
The cytotoxicity results are shown in . The puerarin, paeoniflorin, and menthol groups showed no cytotoxicity in the concentration ranges of 0–350 μg/mL, 0–250 μg/mL, and 0–60 μg/mL, respectively (). The puerarin plus paeoniflorin group (puerarin:paeoniflorin, 1:0.4, w/w) showed no cytotoxicity at concentrations of 0–100 μg/mL, which was graphed against paeoniflorin concentration (). The puerarin plus menthol group (puerarin:menthol, 1:0.5, w/w) showed no cytotoxicity at concentrations of 0–60 μg/mL, which was graphed in terms of the menthol concentration (). The puerarin plus paeoniflorin and menthol group (puerarin:paeoniflorin:menthol, 1:0.4:0.5, w/w/w) showed no cytotoxicity at concentrations of 0–60 μg/mL, which was graphed against the menthol concentration ().
Figure 2 Cytotoxicity of puerarin, paeoniflorin, menthol, and their combinations as assessed by MTT test in Calu-3 cells for 24 hours.
Abbreviations: MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; SD, standard deviation.
Transport of puerarin
Puerarin transport across Calu-3 cells was studied at three concentrations: 30 μg/mL, 60 μg/mL, and 120 μg/mL. The results are shown in . The Papp (A→B) of puerarin was between 1.220×10−6 cm/s and 1.238×10−6 cm/s, whereas the Papp (B→A) of puerarin was between 1.174×10−6 cm/s and 1.266×10−6 cm/s in Calu-3 cells. The fluxes of puerarin from the A side to the B side showed no significant difference compared with the fluxes from the B side to the A side (P>0.05). The ER for each puerarin concentration was ~1.
Table 1 Transport of increasing puerarin concentrations across Calu-3 cell monolayers
The effects of different drug interactions on puerarin transport in Calu-3 cells are shown in . The effects of paeoniflorin were studied at 12 μg/mL, 24 μg/mL, and 48 μg/mL concentrations in the presence of 60 μg/mL puerarin. In combination with different concentrations of paeoniflorin, the Papp (A→B and B→A) values did not differ significantly from those of the control group (P>0.05), indicating that paeoniflorin could not alter the puerarin flux. The effects of menthol were studied at 15 μg/mL, 30 μg/mL, and 60 μg/mL concentrations in the presence of 60 μg/mL puerarin. Menthol significantly increased the puerarin flux in both directions in a concentration-dependent manner (30 μg/mL and 60 μg/mL in Calu-3 cells, P<0.05). The effects of both paeoniflorin and menthol were studied at the aforementioned concentrations in the presence of 60 μg/mL puerarin. Paeoniflorin and menthol together also significantly increased the puerarin flux in both directions in a concentration-dependent manner (24 μg/mL paeoniflorin and 30 μg/mL menthol or 48 μg/mL paeoniflorin and 60 μg/mL menthol, P<0.05).
Table 2 Effects of paeoniflorin (Pa) and menthol (Me) on puerarin (Pu) transport in Calu-3 cells
Changes in TEER after treatment with various compounds
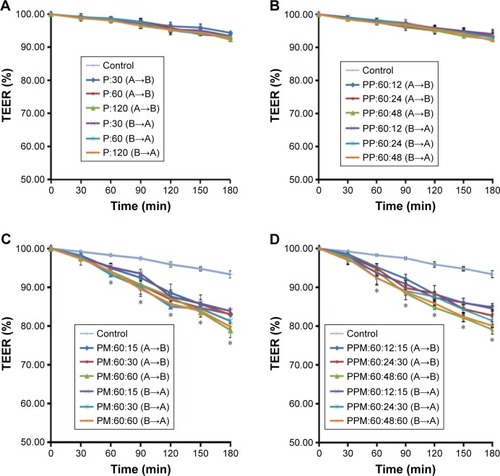
TEER was measured in barrier function assays to investigate changes to the TJs in Calu-3 cells after exposure to puerarin, paeoniflorin, and menthol. The results are shown in . In Calu-3 cells, the puerarin group (30 μg/mL, 60 μg/mL, and 120 μg/mL puerarin) () and the PP group (60 μg/mL puerarin combined with 12 μg/mL, 24 μg/mL, and 48 μg/mL paeoniflorin) () showed no difference from the control group regarding the trend of TEER change. In the PM group (60 μg/mL puerarin plus 15 μg/mL, 30 μg/mL, and 60 μg/mL menthol) () and the PPM group (60 μg/mL puerarin plus 12 μg/mL, 24 μg/mL, and 48 μg/mL paeoniflorin and 15 μg/mL, 30 μg/mL, and 60 μg/mL menthol) (), statistical analysis indicated that TEER began to decrease at 60 minutes (P<0.05) for both the A→B and B→A transport processes. The decreased TEER, which resulted from compatibility with menthol, showed concentration-dependent behavior in the studied range.
Figure 3 The trend of TEER change in A→B and B→A transport in Calu-3 cells after treatment with puerarin, either alone or in combination with paeoniflorin and menthol.
Abbreviations: TEER, transepithelial electrical resistance; min, minutes; SD, standard deviation; P, puerarin; PP, puerarin and paeoniflorin; PM, puerarin and menthol; PPM; puerarin, paeoniflorin and menthol.
Effect of the compounds on TJ proteins
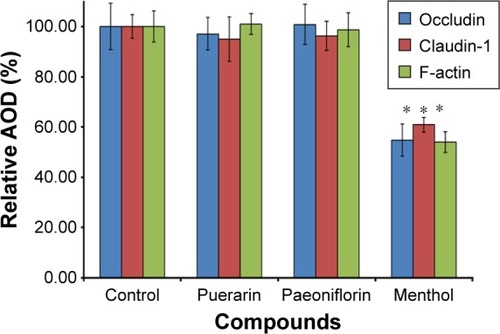
To investigate the effect of the compounds on TJ proteins in Calu-3 cells, immunohistochemistry was performed to detect occludin, claudin-1, and F-actin, and the AOD of the images was measured. The images are shown in , and the results calculated from the AOD values are shown in . Three TJ proteins were clearly observed in Calu-3 cells. Occludin was stained red, claudin-1 and F-actin were stained green, and the nucleus was stained blue. After treatment with puerarin and paeoniflorin, the immunostaining results for the three types of TJ proteins were similar to the control group. However, when the cells were incubated with menthol, the fluorescence intensity was weaker than that of the control group. Moreover, as calculated by ImageJ software and analyzed with SPSS 17.0, the control, puerarin, and paeoniflorin groups exhibited similar AOD values, whereas the difference was statistically significant when the menthol group was compared with the control group (P<0.05).
Figure 4 Effects of puerarin, paeoniflorin, and menthol on TJ proteins in Calu-3 cells.
Abbreviation: TJ, tight junction.
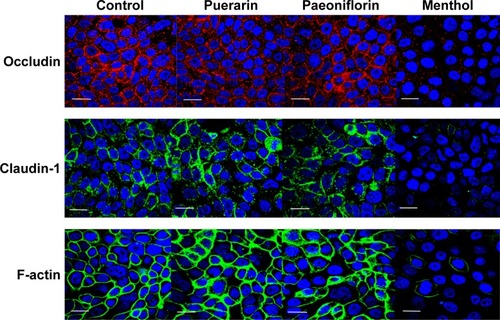
Figure 5 Changes to the relative AOD of occludin, claudin-1, and F-actin in Calu-3 cells after treatment with different compounds.
Abbreviations: AOD, average optical density; TJ, tight junction; SD, standard deviation.
Discussion
In this study, we were able to show the characteristics of puerarin transport and the influence of paeoniflorin and menthol in a cell model. We also demonstrated that menthol altered the physiological activity of the monolayer, resulting in improved puerarin transport across Calu-3 cells. Calu-3 cells, which are derived from a human lung adenocarcinoma cell line, can form ion channels, and express the cystic fibrosis transmembrane conductance regulator protein,Citation14,Citation17 are regularly used to mimic the nasal mucosal tissue to investigate puerarin uptake and the effects of mutual drug compatibility. In terms of cytotoxicity, living cells can reduce MTT to violet formazan dye, whereas dead cells cannot. Thus, the viability of cells after exposure to different compounds may be evaluated by measuring MTT metabolism. The experimental results indicated that menthol was the most toxic, whereas puerarin was safer and less irritating than paeoniflorin. In addition, the toxicities of the different compounds to the cells were concentration dependent; the maximum doses of the various compounds in the cells were determined from the results, which guided the other experiments in this study.
The transport of puerarin in Calu-3 cell monolayers at different concentrations showed that the Papp values (both A→B and B→A) exhibited no significant difference, and the puerarin transport speed increased with an increase in concentration, suggesting that the dominant transport mechanism of puerarin across the Calu-3 cell monolayer is the passive paracellular transport pathway. The ER values obtained for different puerarin concentrations were all ~1, and the transport of puerarin in both directions did not significantly differ; thus, the results in this study did not directly indicate that the transport of puerarin was related to the mucosal direction. It has been reported in the literature that the transport of puerarin in cell monolayers is a comprehensive process that mainly involves passive diffusion but may also involve active transportation mediated by P-glycoprotein (P-gp).Citation35,Citation36 P-gp is an important efflux pump involved in transport across the cell membrane that can transport compounds from inside the cell back into the extracellular fluid. It has also been noted that when the compounds are administered at a lower concentration, the effects of P-gp are more significant, whereas at higher concentrations, the simple diffusion rates of many P-gp substrates are much greater than the efflux. In the dosage range tested in this study, the results regarding transport indicated only the passive diffusion of puerarin. The assays evaluating the influence of drug compatibility on puerarin transport showed that paeoniflorin did not improve the permeability of puerarin. Some studies have reported that paeoniflorin is a P-gp substrate.Citation37,Citation38 In theory, when combined with paeoniflorin, the transport of puerarin should be improved due to competitive binding of the P-gp receptor by paeoniflorin. However, in our research, the Papp values obtained for puerarin in combination with different concentrations of paeoniflorin did not increase significantly. This may be because the transport of puerarin in Calu-3 cells mainly occurs via passive diffusion. Therefore, in this complex prescription, paeoniflorin is used mainly as a medicinal component to enhance the therapeutic effect. In addition, menthol significantly facilitated puerarin transport, and the Papp values of puerarin in the Calu-3 cell monolayers increased significantly with an increasing concentration of menthol. Many researchers have reported the use of menthol as a penetration enhancer.Citation39,Citation40 In previous in vivo studies, we have found that the brain targeting coefficient of puerarin combined with menthol and that the brain drug targeting index were significantly higher than those obtained with injections.Citation34 Through studying the influence of menthol on the mucosal permeability of puerarin in the abdominal skin of Rana catesbeiana as an in vitro model, we also found that when the mass concentration of menthol was 5 mg/mL after compatibility with puerarin was assessed, the Papp value was significantly higher than that obtained in the puerarin alone group.Citation41 All these in vitro and in vivo results prove that menthol can enhance the permeation of puerarin in nasal administration and is beneficial for the transport of puerarin from the nose to the brain. Interestingly, when we assessed the ER values of puerarin transport with menthol, we observed that although puerarin in combination with menthol effectively permeated into the tissues, it also easily flowed out of the tissue. Nevertheless, the research in vivo still confirmed that puerarin reached the brain with sufficient availability.Citation34 Therefore, we speculated that menthol promotes puerarin transport across the nasal mucosa and increases the chances of puerarin transport into the brain, but after penetrating the nasal mucosal epithelial cells, the mechanism of further transport could not be verified in that study and was necessary to study further.
There is very little information in the literature on the mechanism by which menthol enhances the permeability of the mucosal epithelial cells. Using a cell model of the nasal mucosa and the addition of menthol, we have described the reduction of TEER and the suppression of TJ proteins. Drug nasal absorption often involves paracellular transport.Citation42,Citation43 The regulation of paracellular transport across a monolayer involves multiple factors, in which the critical components are the degree of compactness and the physiological function between cells. TEER measurements were performed to evaluate the restrictiveness and to characterize the paracellular resistance of epithelial monolayers in vitro because the TEER value is affected by cell–substrate contact. If the distance between a cell and a substrate is small, the TEER value must be high. In this paper, the TEER value gradually decreased when menthol was added to the cells, whereas the values of the other groups were relatively stable during the test period. Thus, it was confirmed that menthol loosened the monolayer and weakened the nasal mucosa barrier to enhance puerarin paracellular transport. The mechanism by which menthol decreases the TEER values probably involves calcium influx and variations in the activity of intrinsic membrane proteins.Citation44 The TJs surrounding epithelial cells also play a vital role in drug transport by tightly connecting neighboring cells and establishing a defined intercellular space. The TJs separate the apical domain from the basolateral cell surface domain, generating cell polarity and performing barrier and fence functions that restrict the paracellular transport of drugs. TJs consist of a series of integral membrane proteins, including claudins, occludin, zonula occludens, and actin.Citation45,Citation46 In this study, representative TJ proteins were selected to study the influence of each compound on the TJ structure. Occludin, which is an ~60 kDa transmembrane protein with a short intracellular curve, two extracellular annuli, and N- and C-termini in the cytoplasm, is regarded as the primary module in TJ strands that maintains epithelial polarity.Citation47–Citation49 Claudin-1, an 18–27 kDa transmembrane protein with two extracellular annuli, a short N-terminus, and a C-terminus in the cytoplasmic domain, is also considered an important component of TJs and has even been referred to as the TJ backbone.Citation50,Citation51 Many recent studies have shown that claudin-1 is a critical component of TJs with cell adhesion activity that can directly affect the regulation of paracellular permeability and selectivity for solute size by interacting with itself to generate TJ strands. F-actin, one of the peripheral TJ membrane proteins, is involved in organelle movement, protoplasmic streaming, and intercellular junction regulation.Citation45,Citation52,Citation53 Based on the experimental results of immunofluorescence testing and the semiquantitative analysis of the calculated AOD of these chosen TJ proteins, it was speculated that menthol had a disruptive effect on the configuration and integrity of TJs. These results were consistent with the TEER measurements and revealed that menthol suppressed the function of TJ proteins to improve the puerarin permeability across the nasal mucosa. The mechanism underlying the effects of menthol may be based on the phosphorylation of TJ components, activation of protein kinases, or depletion of calcium.Citation54,Citation55
Most drugs used in traditional Chinese medicine are extracted from well-known plants, and as such, their effects have been studied over their long history of use. In many cases, the safety and efficacy of these drugs have been established, but traditional dosages are unable to accommodate modern needs. By doing this research, we hope to provide information regarding the transport of puerarin and the influences of paeoniflorin and menthol on puerarin permeability at the cell level, thus confirming the rationality of using nasal drug delivery systems and the validity of the drug compatibility. However, transport from the nose to the brain is a complicated and comprehensive process. Therefore, this study is not a complete description of this type of transport, and further research is required.
Conclusion
We analyzed the cytotoxicity of compounds in Calu-3 cells, the bidirectional transport of puerarin across Calu-3 cell monolayers, and the influence of drug compatibility on mucosal permeation in vitro. The results confirmed that the transport of puerarin mainly occurred via passive diffusion; this transport was increased by menthol but not by paeoniflorin. Moreover, because menthol disrupted the TJ protein structure and weakened the barrier function of the epithelial cells, the use of menthol enhanced the mucosal permeation of puerarin. The experiments performed herein were conducted to better understand the fundamental pharmaceutical properties of puerarin in combination with paeoniflorin and menthol and to lay the foundation for deeper investigations into nasal administration and nose–brain pathways in vitro.
Acknowledgments
The authors thank Jing Han of the Beijing University of Chinese Medicine for her suggestions concerning immunofluorescence experiments and Zhen-Zhen Chen of Capital Medical University for his helpful advice for cell culture experiments. This project was supported by the National Natural Science Foundation of China (No 81473363) and the Self-Topic Fund of Beijing University of Chinese Medicine (No 2015-JYB-XS053).
Disclosure
The authors report no conflicts of interest in this work.
References
- HafnerAŠkrinjarDFilipovic-GrcicJNasal administration of medicinesFarmaceutski Glas2014705303321
- MistryAStolnikSIllumLNanoparticles for direct nose-to-brain delivery of drugsInt J Pharm2009379114615719555750
- DuchiSOvadiaHTouitouENasal administration of drugs as a new non-invasive strategy for efficient treatment of multiple sclerosisJ Neuroimmunol20132581–2324023517929
- StockhorstUPietrowskyROlfactory perception, communication, and the nose-to-brain pathwayPhysiol Behav200483131115501485
- SarkarADrug metabolism in the nasal mucosaPharm Res199291191589391
- OkuyamaSThe first attempt at radioisotopic evaluation of the integrity of the nose-brain barrierLife Sci19976021188118849154998
- CasettariLIllumLChitosan in nasal delivery systems for therapeutic drugsJ Control Release201419018920024818769
- KürtiLGáspárRMárkiÁIn vitro and in vivo characterization of meloxicam nanoparticles designed for nasal administrationEur J Pharm Sci2013501869223542493
- AguRUJorissenMWillemsTAugustijnsPKingetRVerbekeNIn-vitro nasal drug delivery studies: comparison of derivatised, fibrillar and polymerised collagen matrix-based human nasal primary culture systems for nasal drug delivery studiesJ Pharm Pharmacol200153111447145611732747
- HussainxAAHiraiSBawarshiRNasal absorption of natural contraceptive steroids in rats – progesterone absorptionJ Pharm Sci19817044664677229970
- SteeleVEArnoldJTIsolation and long-term culture of rat, rabbit, and human nasal turbinate epithelial cellsIn Vitro Cell Dev Biol198521126816874077807
- MerkleHPDitzingerGLangSRPeterHSchmidtMCIn vitro cell models to study nasal mucosal permeability and metabolismAdv Drug Deliv Rev1998291–2517910837580
- WengstAReichlSRPMI 2650 epithelial model and three-dimensional reconstructed human nasal mucosa as in vitro models for nasal permeation studiesEur J Pharm Biopharm201074229029719733661
- VllasaliuDFowlerRGarnettMEatonMStolnikSBarrier characteristics of epithelial cultures modelling the airway and intestinal mucosa: a comparisonBiochem Biophys Res Commun2011415457958522079636
- TayebatiSKNwankwoIEAmentaFIntranasal drug delivery to the central nervous system: present status and future outlookCurr Pharml Des2013193510526
- JereyJLRobertGTIntranasal Drug Delivery to the Brain. Drug Delivery to the BrainNew York, NYSpringer2014401431
- QinTYinYWangXWhole inactivated avian Influenza H9N2 viruses induce nasal submucosal dendritic cells to sample luminal viruses via transepithelial dendrites and trigger subsequent DC maturationVaccine201533111382139225613720
- MiyazawaMSakanoKNakamuraSKosakaHAntimutagenic activity of isoflavone from Pueraria lobataJ Agric Food Chem200149133634111170596
- WongKHLiGQLiKMRazmovski-NaumovskiVChanKKudzu root: traditional uses and potential medicinal benefits in diabetes and cardiovascular diseasesJ Ethnopharmacol2011134358460721315814
- ZhangHZhangLZhangQPuerarin: a novel antagonist to inward rectifier potassium channel (I K1)Mol Cell Biochem20113521–211712321327545
- LiuYXueQLiXAmelioration of stroke-induced neurological deficiency by lyophilized powder of Catapol and PuerarinInt J Biol Sci201410444845624719562
- YiguoZMeiXZhinnegYAnalysis of puerarin and chemical compositions changes in kudzu root during growth periodJ Chem2014201416
- ZengxiangQPharmacological action and application of GegentangChinese Traditional Patent Material19961844344 Chinese
- JianZMinZGegentang treated 36 post-stroke depression patientsJ Jilin Univ Med Ed200939569 Chinese
- JiaiYYueDShupangAPharmacological and clinical researches of GegentangArch Tradit Chin Med200725612751278
- MikageMOnoNHerbological study of red peony and white peony used in Chinese medicineKampo Med2009604419428
- ZhangYLiHHuangMPaeoniflorin, a monoterpene glycoside, protects the brain from cerebral ischemic injury via inhibition of apoptosisAm J Chin Med201543354355725967667
- XiaoLWangYZLiuJLuoXTYeYZhuXZEffects of paeoniflorin on the cerebral infarction, behavioral and cognitive impairments at the chronic stage of transient middle cerebral artery occlusion in ratsLife Sci200578441342016137717
- GelalAJacobPYuLBenowitzNLDisposition kinetics and effects of mentholClin Pharmacol Ther199966212813510460066
- PlevkovaJKollarikMPoliacekIThe role of trigeminal nasal TRPM8-expressing afferent neurons in the antitussive effects of mentholJ Appl Physiol2013115226827423640596
- AmatoALiottaRMulèFEffects of menthol on circular smooth muscle of human colon: analysis of the mechanism of actionEur J Pharmacol201474029530125046841
- ChenXDuSLuYStudy on pharmacokinetics of puerarin in rats following different methods of administration of Tongqiaosanyu prescriptionZhongguo Zhong Yao ZaZhi2011361723472349 Chinese
- XiaolanCShouyingDYangLStudy on pharmacokinetics of puerarin in rats by following different methods of administration of puerariae extractChin J Tradit Chin Med Pharmacy2011271024082411 Chinese
- XiaolanCYangLShouyingDStudy on the comparison the plasma pharmacokinetics with the brain pharmacokinetics based on the different administration routes of TongqiaoSanyu formulaChina J Tradit Chin Med Pharmacy201229826682672
- LiangXLZhaoLJLiaoZGTransport properties of puerarin and effect of radix Angelicae dahuricae extract on the transport of puerarin in Caco-2 cell modelJ Ethnopharmacol2012144367768223085309
- LiangXLZhangJZhaoGWMechanisms of improvement of intestinal transport of baicalin and puerarin by extracts of radix Angelicae dahuricaePhytother Res201529222022725312586
- LiuLZhaoXZhuDChengYQuHSimultaneous LC-MS/MS determination of danshensu and paeoniflorin for permeability studies in Caco-2 intestinal absorption modelChem Res Chin Univ2008244420426
- LiangXLZhuMLZhaoGWEffect of furan coumarins from Angelica dahuricae radix on intestinal transport absorption of puerarin, paeoniflorin, and vincristineTradit Herbal Drug201546710071011 Chinese
- XuXYuNBaiZEffect of menthol on ocular drug deliveryGraefes Arch Clin Exp Ophthalmol2011249101503151021597947
- HuangLBaiJYangHLiuJCuiHCombined use of borneol or menthol with labrasol promotes penetration of baicalin through rabbit cornea in vitroPak J Pharm Sci20152811725553687
- PengyueLYangLShouyingDXiaolanCStudy on the effect of menthol on mucosal permeability of Puerarin in vitroChin Pharmacy2012234744254427 Chinese
- GenterMBKrishanMAugustineLMCherringtonNJDrug transporter expression and localization in rat nasal respiratory and olfactory mucosa and olfactory bulbDrug Metab Dispos201038101644164720660103
- HuangYDonovanMDLarge molecule and particulate uptake in the nasal cavity: the effect of size on nasal absorptionAdv Drug Deliv Rev1998291–214715510837585
- TurnerJRBuschmannMMRomero-CalvoISailerAShenLThe role of molecular remodeling in differential regulation of tight junction permeabilitySemin Cell Dev Biol20143620421225263012
- TsukitaSFuruseMItohMMultifunctional strands in tight junctionsNat Rev Mol Cell Biol20012428529311283726
- SawadaNMurataMKikuchiKTight junctions and human diseasesMed Electron Microsc200336314715614505058
- BaldaMSWhitneyJAFloresCGonzálezSCereijidoMMatterKFunctional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane proteinJ Cell Biol19961344103110498769425
- FuruseMHiraseTItohMOccludin: a novel integral membrane protein localizing at tight junctionsJ Cell Biol19931236 pt 2177717888276896
- TsukamotoTNigamSKRole of tyrosine phosphorylation in the reassembly of occludin and other tight junction proteinsAm J Physiol19992765 pt 2F737F75010330056
- FuruseMFujitaKHiiragiTFujimotoKTsukitaSClaudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludinJ Cell Biol19981417153915509647647
- FuruseMSasakiHFujimotoKTsukitaSA single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblastsJ Cell Biol199814323914019786950
- SchneebergerEELynchRDThe tight junction: a multifunctional complexAm J Physiol Cell Physiol20042866C1213C122815151915
- HallARho GTpases and the actin cytoskeletonScience199827953505095149438836
- VelardeGAit-AissaSGilletCUse of transepithelial electrical resistance in the study of pentachlorophenol toxicityToxicol In Vitro1999134–572372720654541
- HirschMNoskeWThe tight junction: structure and functionMicron1993243325352