Abstract
Objectives
To evaluate the effects of alprostadil (prostaglandin [PGE1] analog) and iloprost (prostacyclin [PGI2] analog) on renal, lung, and skeletal muscle tissues after ischemia reperfusion (I/R) injury in an experimental rat model.
Materials and methods
Wistar albino rats underwent 2 hours of ischemia via infrarenal aorta clamping with subsequent 2 hours of reperfusion. Alprostadil and iloprost were given starting simultaneously with the reperfusion period. Effects of agents on renal, lung, and skeletal muscle (gastrocnemius) tissue specimens were examined.
Results
Renal medullary congestion, cytoplasmic swelling, and mean tubular dilatation scores were significantly lower in the alprostadil-treated group than those found in the I/R-only group (P<0.0001, P=0.015, and P<0.01, respectively). Polymorphonuclear leukocyte infiltration, pulmonary partial destruction, consolidation, alveolar edema, and hemorrhage scores were significantly lower in alprostadil- and iloprost-treated groups (P=0.017 and P=0.001; P<0.01 and P<0.0001). Polymorphonuclear leukocyte infiltration scores in skeletal muscle tissue were significantly lower in the iloprost-treated group than the scores found in the nontreated I/R group (P<0.0001).
Conclusion
Alprostadil and iloprost significantly reduce lung tissue I/R injury. Alprostadil has more prominent protective effects against renal I/R injury, while iloprost is superior in terms of protecting the skeletal muscle tissue against I/R injury.
Introduction
Ischemia reperfusion (I/R) injury during abdominal aortic aneurysm reconstructions and aortoiliac disease operations is a consequence of clamping and subsequent declamping of the infrarenal aorta.Citation1 Clamping (ischemia period) and declamping (reperfusion period) the aorta result in both local tissue (muscle tissue, distal regions of clamped aorta) effects and distant organ injury which includes kidney, lung, brain, heart, and other organs.Citation2–Citation4 Renal failure incidence after aorta surgery is reported to be between 0.7 and 8.8, while mortality rates are reported to be between 50% and 90% after renal replacement therapy.Citation5,Citation6 Pulmonary failure after acute renal failure results in high morbidity and mortality.Citation7 I/R injury and systemic inflammatory response syndrome may lead to acute respiratory distress and/or noncardiogenic pulmonary edema.Citation8 Mortality rates is high as 80% of acute renal injury is complicated with acute respiratory failure.Citation9
Alprostadil is a prostaglandin-analog drug (PGE1) and a derivative of dihomo-g-linoleic acid; it has anti-inflammatory, potent vasodilatory, collagenase inhibitor activities with the inhibition of in vitro platelet aggregation, thromboxane secretion, and proliferation of vascular smooth muscle cells.Citation10 PGE1 exerts protective effects against ischemia of oxygen and glucose in ischemic cells.Citation11 Several studies demonstrated protective effects of alprostadil against I/R injury of different organs and tissues such as lung, kidney, heart, brain, and skeletal muscle.Citation12,Citation13
Iloprost is an analog of prostacyclin; PGI2 has important properties including inhibition of platelet aggregation, vasodilation, inhibition of leukocyte activation, and cytoprotection achieved by decreasing the inflammatory reaction following I/R injury.Citation14–Citation16 Also, iloprost inhibits the release of endothelin-1, tumor necrosis factor-α, and soluble adhesion molecules and also the production of growth factors such as transforming growth factor and connective tissue growth factor.Citation17,Citation18 Pulmonary arterial hypertension, ischemic episodes of Raynaud’s phenomenon, scleroderma and systemic sclerosis,Citation19 peripheral vascular disease, and diabetic foot are main areas of iloprost usage. Besides clinical usage, protective roles of iloprost on I/R injury of different tissues have been demonstrated.Citation20,Citation21
In this study, the effects of alprostadil and iloprost on kidney, lung, and skeletal muscle tissues after I/R injury secondary to infrarenal aorta clamping and declamping were investigated.
Materials and methods
Animals and experimental protocol
This study was conducted in the Physiology Laboratory of Kirikkale University with consent from the Experimental Animals Ethics Committee of Gazi University. All of the procedures were performed according to accepted standards of the International Guide for the Care and Use of Laboratory Animals.
In this study, 24 Wistar Albino rats weighing between 200 and 250 g, raised under similar environmental conditions, were used. The rats were kept under 20°C–21°C at cycles of 12-hour daylight and 12-hour darkness and had free access to food until 2 hours before the anesthesia procedure. The animals were randomly separated into four groups, each containing six rats. Midline laparotomy was performed under ketamine anesthesia.
Control group
Midline laparotomy was done alone without any additional surgical intervention. Animals in the control group remained under surgery similar to animals in other study groups (4 hours); they were then sacrificed and renal, lung, and skeletal muscle tissue specimens were collected for histopathological investigation.
I/R group
Midline laparotomy was done in a similar manner. The infrarenal segment of the aorta was clamped for 2 hours. After removing the clamp, reperfusion was established for another 2 hours. Finally, all rats received ketamine 100 mg kg−1 intraperitoneally and sacrificed; renal, lung, and skeletal muscle tissue specimens were collected for histopathological investigation.
I/R group with alprostadil
Similar steps were followed, but in addition to the aforementioned procedure, alprostadil was given (20 mcg kg−1) intravenously (via tail vein) and simultaneously with the reperfusion period. Rats were sacrificed at the end of re perfusion period and subsequently renal, lung, and skeletal muscle tissue specimens were collected for histopathological investigation.
I/R group with iloprost
Similar steps were followed but in addition to the aforementioned procedure, iloprost was given (10 mcg kg−1) intravenously (via tail vein) and simultaneously with the reperfusion period. Rats were sacrificed at the end of the reperfusion period, and subsequently renal, lung, and skeletal muscle tissue specimens were collected for histopathological investigation.
Histopathological examination
Tissue samples (skeletal muscle [gastrocnemius], renal, and lung) were identified, sliced, and immersed in 10% buffered formalin solution, and then dehydrated and embedded in paraffin for 24 hours. Subsequently, tissue sections of 4 μm were mounted on slides for staining with hematoxylin and eosin.
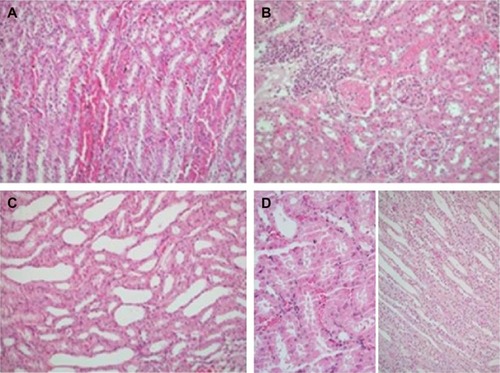
Acute renal injury was evaluated based on the presence or absence of interstitial capillary congestion, interstitial inflammation, tubular dilatation, swelling of tubular epithelium (ballooning of cytoplasm), and tubular necrosis. Medullary congestion was scored semiquantitatively as follows: (0) no congestion or negligible congestion (congestion identified in original magnification ×400), (1) mild (identified in original magnification ×200), (2) moderate (identified in original magnification ×100), and (3) severe (identified in original magnification ×40). Renal injury was scored semiquantitatively using the extent of affected (confirmed with the presence of tubular necrosis, swelling of tubular epithelium [ballooning of cytoplasm], tubular dilatation, and interstitial inflammation) parenchymal area as follows: (0) no injury, (1) mild <25%, (2) moderate between 25% and 50%, and (3) severe >50%.
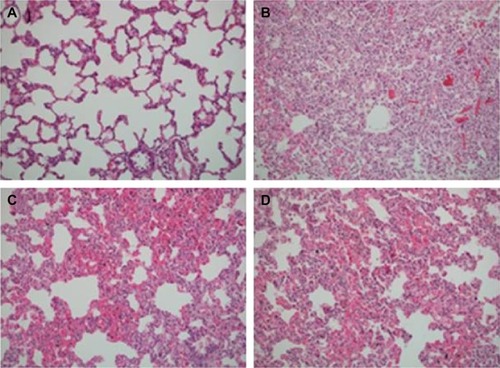
Following histological preparation of specimens as explained earlier, lung injury was scored semiquantitatively using the extent of affected (confirmed with the presence of several parameters including the enlargement of interalveolar septum and congestion, neutrophilic infiltration, perivascular alveolar edema, alveolar hemorrhage, and partial pulmonary destruction) parenchymal areas as follows: (0) no significant change, (1) mild pulmonary injury (mild to moderate interstitial congestion accompanied by mild neutrophilic infiltration), (2) moderate neutrophilic infiltration, perivas-cular edema, and partial destruction on lung parenchyma, (3) complete pulmonary destruction accompanied by severe neutrophilic infiltration.
Specimens of rat skeletal muscle tissue were prepared using the same method as mentioned earlier in the first paragraph of the histopathological examination section, and tissue injury was scored semiquantitatively as follows: (0) no injury, (1) mild, (2) moderate, and (3) severe. Injury was evaluated with respect to the following parameters: disorganization of muscle fibers, degeneration, necrosis, inflammatory cell infiltration, and interstitial edema were used to evaluate tissue injury. All parameters were scored semiquantitatively using the extent of affected parenchymal areas as follows: (0) normal, (1) mild, (2) moderate, and (3) severe.
Statistical analysis
The statistical analyses were performed with SPSS 17.0 software program (SPSS Inc., Chicago, IL, USA), and P<0.05 was considered statistically significant. The findings were expressed as mean ± standard deviation. The data were evaluated with Kruskal–Wallis variance analysis. The variables with significance were evaluated with Bonferroni corrected Mann–Whitney U-test.
Results
Significant differences were found between groups in terms of renal medullary congestion, cytoplasmic swelling, and tubular dilatation (P=0.032, P=0.045, and P=0.010, respectively). Medullary congestion scores were significantly lower in Group C (control group) and Group IR-A (I/R group with alprostadil) than in Group IR (P=0.023, P<0.0001 respectively). Similarly, cytoplasmic swelling scores were higher for those in Group IR than for those in Group C and Group IR-A (P=0.015). Mean tubular dilatation scores were significantly lower in all groups than those in Group IR (, ).
Table 1 Scores obtained from light microscopy of kidney tissues (mean ± standard deviation)
Figure 1 Nephrectomy specimens of four groups.
Significant differences were found between groups in terms of polymorphonuclear leukocyte (PMNL) infiltration, alveolar edema, hemorrhage, pulmonary partial destruction, and consolidation (P=0.002, P=0.010, P<0.0001, respectively). PMNL infiltration scores obtained in Group C, Group IR-A, and Group IR-I were significantly lower than those in Group IR (P<0.0001, P=0.017, P=0.001, respectively). Similarly, pulmonary partial destruction and consolidation scores in Group C, Group IR-A, and Group IR-I (I/R group with iloprost) were significantly lower than those in Group IR (P<0.0001). Alveolar edema and hemorrhage were significantly higher in Group IR than those in other study groups (, ).
Table 2 Scores obtained from light microscopy of lung tissues (mean ± standard deviation)
Figure 2 Lung parenchyma of four groups.
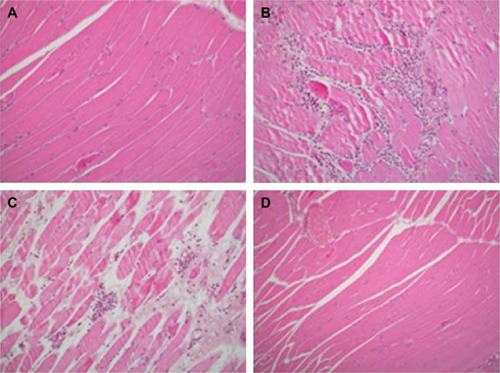
Significant differences were found between polymorphonuclear cell infiltration scores in skeletal muscle tissue specimens of groups (P=0.002). PMNL infiltration scores were significantly lower in Group C and Group IR-I than scores found in Group IR (P<0.0001) (, ).
Table 3 Scores obtained from light microscopy of skeletal muscle tissues (mean ± standard deviation)
Figure 3 Skeletal muscle biopsies of four groups.
Discussion
We investigated the effects of two prostaglandin analogs (alprostadil [PGE1] and iloprost [PGI2]) on three different tissues/organs after an experimental I/R injury model that included clamping and subsequent declamping of the infrarenal aorta. To the best of our knowledge, this is the first study investigating the effects of these agents on different tissues/organs in a single experimental design.
Effects of alprostadil and iloprost on renal tissue after I/R injury
We found that alprostadil had significant protective effects after I/R injury which were proved by lower medullary congestion, cytoplasmic swelling, and tubular dilatation scores found in the Alprostadil-treated group (IR-A) when compared with Group IR (P<0.0001, P=0.015, and P=0.01, respectively). Alprostadil is an exogenous form of PGE1, and several studies have shown positive effects of alprostadil on renal hemodynamics and perfusion.Citation1,Citation22,Citation23 Renal vasodilation and improved renal artery perfusion are possible mechanisms underlying the protective effects of alprostadil on the renal tissue.Citation1,Citation23 Xu et alCitation22 found protective effects of alprostadil when combined with hydration in patients with contrast-induced nephropathy, and they concluded that this finding is a consequence of vasodilatory and perfusion-improving effects of alprostadil. Soares et alCitation24 conducted a study with a similar design as that of our study except for shorter ischemia and reperfusion periods (30 minutes/1 hour vs 2 hours/2 hours). They found lower expressions of Intracellular Adhesion Molecule 1 (ICAM-1) and tissue architecture disarrangement in the alprostadil-treated group. Additionally, they found less leukocyte tubular adhesion and tissue necrosis in the alprostadil-treated group. They concluded that the protective effect of alprostadil could be implemented through anti-inflammatory routes such as inhibiting the expression of ICAM-1 and other inflammatory parameters. Although the expression or levels of inflammatory parameters have not been investigated, histological findings of our study are correlated with the study conducted by Soares et al.Citation24
Iloprost produced less promising findings regarding renal tissue protection that presented with only lower tubular dilatation scores when compared with the I/R group. Contrary to our results, Sahsivar et alCitation14 found protective effects of iloprost documented with all histological parameters including focal glomerular necrosis, dilatation of Bowman capsule, degeneration of tubular epithelium, necrosis in tubular epithelium, and tubular dilatation. In a study conducted by Sahsivar et al,Citation14 iloprost was given as continuous intravenous infusion of 20 ng kg/min during 60 minutes of ischemia and 120 minutes of reperfusion via tail vein, whereas in our study, iloprost was given at 10 mcg kg−1 dose through the tail vein for 30 minutes starting simultaneously with the reperfusion period. So we suggest that differences in the iloprost dose, infusion time, and starting of drug therapy may affect the results of studies.
Effects of alprostadil and iloprost on lung tissue after I/R injury
We found that alprostadil and iloprost had significant protective effects against lung I/R injury when compared with the I/R-alone group. PMNL infiltration, alveolar edema, hemorrhage, pulmonary partial destruction, and consolidation were significantly lower than those in the I/R-alone group (P=0.002, P=0.010, P<0.0001, respectively). Our findings are correlated with Oztay et alCitation25 and other different studies that showed protective effects of alprostadil on lung tissue after I/R injury.Citation12,Citation13,Citation25 Oztay et alCitation25 reported only a weak I/R injury effect on the lung structure that presented with edema, partly dilated alveoli, and slight leukocyte infiltration. Authors suggested that this weak effect of Renal Ischemia Reperfusion on the lung structure could be related to the duration of I/R (1 hour of ischemia and 1 hour of reperfusion). However, pulmonary edema induction after I/R injury is the most common evidence obtained,Citation26 and significant alveolar edema was accepted as sufficient finding for lung injury. In our study, the duration of I/R was longer (2 hours/2 hours), and so more significant lung injury findings in our study may be a consequence of longer duration of I/R periods.
Significant protective effects of iloprost were found on the lung tissue after I/R injury. Several studies showed decreased pulmonary injury after ischemia with iloprost treatment, and this result was attributed to important properties of iloprost such as preservation of the scavenging system, inhibition of platelet aggregation, vasodilatation, and cytoprotection.Citation27,Citation28 Erer et alCitation8 showed immunohistochemically and biochemically (measurement of total oxidant and antioxidant status) protective effects of iloprost using similarly designed experiments, and we think that results of histological evaluation in our study supports previous findings.
Effects of alprostadil and iloprost on skeletal muscle tissue after I/R injury
There was no difference between the alprostadil-treated group and the I/R-only group in terms of inflammatory changes in the skeletal muscle. Antonio et alCitation29 reported that alprostadil did not reduce the release of muscular enzymes, the consumption of tissue glycogen, or the effects of ischemia and reperfusion on the cell membrane, characterized by lipid peroxidation at the end of experimental study, which was designed in the same way as our study. However, infrarenal aorta ischemia period was longer (5 hours) and reperfusion period was shorter (1 hour) than the period used in this study. In contrast, Rowlands et alCitation30 reported improved skeletal muscle (gastrocnemius muscle of rats) blood flow and decreased muscle edema with alprostadil and iloprost treatment following 5.5 hours of ischemia and 4 hours during reperfusion periods. Luther et alCitation31 demonstrated significant reduction of free oxygen radical reactions and histological findings of protective alprostadil effects on tibialis anterior muscle following 3.5 hours of ischemia and then 4 hours of reperfusion. In another study conducted in pigs, supporting evidence for the possible protective role of alprostadil was found with regard to the improvement during initial reperfusion in regional blood flow (at 60 and 90 minutes of reperfusion), glucose consumption (at 30 minutes of reperfusion), and serum potassium regulation (at 30 and 90 minutes of reperfusion).Citation32
Contrary to findings in the alprostadil group, significantly lower PMNL infiltration was found in the iloprost-treated group when compared with Group IR only (P<0.0001). This finding is correlated with previous findings in studies investigating the effects of iloprost on skeletal muscle I/R injury.Citation2,Citation30 It is well known that iloprost decreases vascular resistance and increases blood flow. Additionally, iloprost has strong cytoprotective and antiaggregant properties via inhibiting thrombocyte aggregation and decreasing the synthesis of adhesion molecules. Iloprost also improves microcirculation and attenuates microvascular dysfunction. Tiryakioglu et alCitation2 found protective effects of iloprost in I/R injury of muscle tissue (gastrocnemius muscle) evidenced by a significant decrease in all injury parameters including hemorrhage, necrosis, loss of nuclei, PMNL, monocyte, and macrophage infiltration. Although significant differences were found only in PMNL infiltration, iloprost may have protective effects against I/R injury in the skeletal muscle tissue.
Limitations
First, we evaluated only histological/pathological injury findings without any biochemical and/or immunohistochemical evaluation. This may limit the effectivity of the study results. Second, limitation is related to the sample size of the study. The sample size is small to achieve a high statistical power. However, we think that future studies investigating the effects of different prostaglandin analogs on I/R injury of different tissues may benefit from the findings of this study.
Conclusion
We can conclude that both alprostadil and iloprost effectively decrease lung tissue injury following aortic cross-clamping-induced I/R injury, while alprostadil exerts more prominent protective effects against renal tissue injury. On the other hand, more significant results were achieved with iloprost treatment against skeletal muscle tissue injury. Future studies investigating the effects of alprostadil and iloprost on tissue levels and biochemical analysis after I/R injury will help to interpret these results in a better way.
Disclosure
The authors declare no conflicts of interest.
References
- DolegowskaBPikułaESafranowKMetabolism of eicosanoids and their action on renal function during ischaemia and reperfusion: the effect of alprostadilProstaglandins Leukot Essent Fatty Acids200675640341117011760
- TiryakiogluOErkocKTunerirBThe effect of iloprost and N-acetylcysteine on skeletal muscle injury in an acute aortic ischemia-reperfusion model: an experimental studyBiomed Res Int2015201545374825834818
- FaubelSAcute kidney injury and multiple organ dysfunction syndromeMinerva Urol Nefrol200961317118819773721
- LiXHassounHTSantoraRRabbHOrgan crosstalk: the role of the kidneyCurr Opin Crit Care200915648148719851101
- VemuriCWainessRMDimickJBEffect of increasing patient age on complication rates following intact abdominal aortic aneurysm repair in the United StatesJ Surg Res20041181263115093713
- PowellRJRoddySPMeierGHGusbergRJConteMSSumpioBEEffect of renal insufficiency on outcome following infrarenal aortic surgeryAm J Surg199717421261309293827
- FeltesCMVan EykJRabbHDistant-organ changes after acute kidney injuryNephron Physiol20081094p80p8418802379
- ErerDDursunADOktarGLThe effects of iloprost on lung injury induced by skeletal muscle ischemia-reperfusionBratisl Lek Listy2014115740541025077362
- ChertowGMChristiansenCLClearyPDMunroCLazarusJMPrognostic stratification in critically ill patients with acute renal failure requiring dialysisArch Intern Med199515514150515117605152
- FanYYChapkinRSImportance of dietary gamma-linolenic acid in human health and nutritionJ Nutr19981289141114149732298
- HotterGClosaDPiFArachidonate metabolism in ischemia-reperfusion associated with pancreas transplantationJ Lipid Mediat Cell Signal1994921351438012760
- de PerrotMFischerSLiuMProstaglandin E1 protects lung transplants from ischemia-reperfusion injury: a shift from pro- to anti-inflammatory cytokinesTransplantation20017291505151211707737
- AoeMTrachiotisGDOkabayashiKAdministration of prostaglandin E1 after lung transplantation improves early graft functionAnn Thorac Surg19945836556617944685
- SahsivarMONarinCKiyiciAToyHEgeESarigülAThe effect of iloprost on renal dysfunction after renal I/R using cystatin C and beta2-microglobulin monitoringShock200932549850219295492
- GrantSMGoaKLIloprost. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in peripheral vascular disease, myocardial ischaemia and extracorporeal circulation proceduresDrugs19924368899241379160
- KirisITekinIYilmazNSutcuRKarahanNOcalAIloprost down-regulates expression of adhesion molecules and reduces renal injury induced by abdominal aortic ischemia-reperfusionAnn Vasc Surg200923221222318774689
- Balbir-GurmanABraun-MoscoviciYLivshitzVAntioxidant status after iloprost treatment in patients with Raynaud’s phenomenon secondary to systemic sclerosisClin Rheumatol20072691517152117401513
- MittagMBeckheinrichPHausteinUFSystemic sclerosis-related Raynaud’s phenomenon: effects of iloprost infusion therapy on serum cytokine, growth factor and soluble adhesion molecule levelsActa Derm Venereol200181429429711720181
- WigleyFMWiseRASeiboldJRIntravenous iloprost infusion in patients with Raynaud phenomenon secondary to systemic sclerosis. A multicenter, placebo-controlled, double-blind studyAnn Intern Med199412031992067506013
- YakutNYasaHBahriye LafciBThe influence of levosimendan and iloprost on renal ischemia-reperfusion: an experimental studyInteract Cardiovasc Thorac Surg20087223523918056154
- KokselOOzdulgerAAytacoqluBThe influence of iloprost on acute lung injury induced by hind limb ischemia-reperfusion in ratsPulm Pharmacol Ther200518423524115777606
- XuRHMaGZCaiZXChenPZhuZDWangWLCombined use of hydration and alprostadil for preventing contrast-induced nephropathy following percutaneous coronary intervention in elderly patientsExp Ther Med20136486386724137279
- SketchMHJrWheltonASchollmayerEPrevention of contrast media-induced renal dysfunction with prostaglandin E1: a randomized, double-blind, placebo-controlled studyAm J Ther20018315516211344383
- SoaresBLde FreitasMAMonteroEFPittaGBJúniorFMAlprostadil attenuates inflammatory aspects and leucocytes adhesion on renal ischemia and reperfusion injury in ratsActa Cir Bras201429Suppl 2556025229516
- OztayFKara-KislaBOrhanNYanardaqRBolkentSThe protective effects of prostaglandin E1 on lung injury following renal ischemia-reperfusion in ratsToxicol Ind Health Epub2015416
- DengJHuXYuenPSStarRAAlpha-melanocyte-stimulating hormone inhibits lung injury after renal ischemia/reperfusionAm J Respir Crit Care Med2004169674975614711793
- BaltalarliAOzcanVBirFAscorbic acid (vitamin C) and iloprost attenuate the lung injury caused by ischemia/reperfusion of the lower extremities of ratsAnn Vasc Surg2006201495516378148
- FantoneJCMarascoWAElgasLJWardPAStimulus specificity of prostaglandin inhibition of rabbit polymorphonuclear leukocyte lysosomal enzyme release and superoxide anion productionAm J Pathol198411519166324595
- AntonioLGEvoraPRPiccinatoCEUse of alprostadil, a stable prostaglandin E1 analogue, for the attenuation of rat skeletal muscle ischemia and reperfusion injuryMinerva Chir200964655956420029353
- RowlandsTEGoughMJHomer-VanniasinkamSDo prostaglandins have a salutary role in skeletal muscle ischaemia-reperfusion injury?Eur J Vasc Endovasc Surg199918543944410610833
- LutherBLehmannCGruneTControlled reperfusion of ischemic extremity musculature to prevent free radical induced lesions)Zentralbl Chir19991244336343 German10355090
- Abdel-RahmanURisteskiPKlaefflingCThe influence of controlled limb reperfusion with PGE1 on reperfusion injury after prolonged ischemiaJ Surg Res2009155229330019524255
