?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In situ administration of 5-fluorouracil (5FU) “thermosensitive” gel effectively reduced systemic side effects in treating colon rectal cancer; however, the penetration efficacy of the formulation was considerably low due to the poor lipid solubility of 5FU. The aim of this study was to develop thermosensitive gel-mediated 5FU water-in-oil microemulsion (TG-5FU-ME) for improving the infiltration of 5FU. An in vitro release test showed that TG-5FU-ME sustained the drug’s release up to 10 hours. TG-5FU-ME exhibited good stability, and the microemulsion entrapped did not show any change in morphology and 5FU content during the 4-month storage. Transportation test in the Caco-2 cell monolayer showed that TG-5FU-ME had a permeability 6.3 times higher than that of 5FU thermosensitive gel, and the intracellular uptake of 5FU increased by 5.4-fold compared to that of 5FU thermosensitive gel. In vivo tissue distribution analysis exhibited that the TG-5FU-ME group had drug levels in rectal tissue and mesenteric lymph nodes, which were significantly higher than those of 5FU thermosensitive gel group, with very low blood levels of 5FU in both groups. Furthermore, TG-5FU-ME was not associated with detectable morphological damage to the rectal tissue. Conclusively, TG-5FU-ME might be an efficient rectal delivery system to treat colorectal cancer.
Introduction
Colorectal cancer is the third most commonly diagnosed cancer in males and the second in females,Citation1 with more than 1.4 million new cases and 694,000 deaths in 2012.Citation2 It was estimated that colorectal cancer is one of the four most frequently diagnosed types of cancer and continued to be the most common causes of cancer death in 2014.Citation3 5-Fluorouracil (5FU) is one of the main chemotherapeutic agents used in the clinic for the treatment of colon rectal cancer.Citation4 Due to its low lipophilicity (logP, −0.83), 5FU shows poor oral bioavailability.Citation5,Citation6 For intravenous administration, a high dose of 5FU is required because of its rapid clearance from plasma (a mean half-life of ∼16 minutes).Citation7,Citation8 High dose of 5FU is often accompanied by severe toxic effects in gastrointestinal, hematological, neural, cardiac, or dermatological systems.Citation9,Citation10 Intravenous administration of 5FU also causes physical and psychic pain, as well as hypertrophy or atrophy at the subcutaneous injection site. Therefore, finding an alternative delivery form is desirable in hoping to improve the current chemothrapeutic regimen.Citation11
Delivering 5FU directly to the rectal site is considered a promising approach to reduce systemic side effects and provide effective tumor diffusion.Citation12,Citation13 Numerous studies have shown that the colon suppository administration of 5FU appeared to be efficient and showed pharmacokinetics similar to that of intravenous administration, but with decreased systemic side effects and increased rectal tissue drug concentration.Citation14–Citation19 Some animal studies demonstrated that administration of 5FU emulsion in the rectal mucous improved drug accumulation in regional lymph nodes, suggesting that 5FU emulsion therapy might be of potential in treating lymph node metastasis from rectal cancer.Citation20,Citation21 However, the solid form, ie, suppositories, was confined to limited drug contact area with the lesion and poor drug permeability crossing rectal mucous membrane. Other disadvantages of 5FU suppositories include anal pain, tenesmus, anal bleeding, reddened mucous, and histological changes coinciding with acute colitis.Citation15,Citation22 In addition, rectal administration of liquid forms often causes anal leakage, leading to inadequate dosing.Citation13
The aim of this study was to develop an effective rectal delivery system of 5FU by simultaneously employing nanotechnology and thermosensitive gel technique,Citation23–Citation25 in which the microemulsion might improve drug permeation, sustain drug release, and reduce drug mucosal irritation.Citation26–Citation29 We assume that loading the 5FU microemulsion in the thermosensitive gel might improve its stability, tissue deposition, and patient compliance.Citation30–Citation33 To the best of our knowledge, this is the first attempt to combine the two technologies for rectal drug delivery. Indeed, thermosensitive gel-mediated 5FU water-in-oil microemulsion (TG-5FU-ME) sustained the drug release, increased the drug permeation, improved the drug stability, elevated the drug deposition in disease tissue, and meliorated the use convenience. Animal experiments exhibited that TG-5FU-ME group had significantly high drug levels in the rectal tissue and mesenteric lymph nodes, while it had low drug levels in the blood system with no detectable morphological damage to rectal tissue. The results suggest that TG-5FU-ME is a promising rectal drug delivery system in treating rectal cancer.
Materials and methods
Materials
5FU was purchased from Xing Galaxy Chemical Company (Hubei, People’s Republic of China). Poloxamers were gifted from BASF (Ludwigshafen, Germany), and sodium alginate was supplied by Huzhou Zhanwang Pharmaceutical Co., Ltd (Zhejiang, People’s Republic of China). Tween 80 and Span 80 were supplied by Huamei Co., Ltd (Beijing, People’s Republic of China). Soybean oil was supplied by Sigma-Aldrich Co. (St Louis, MO, USA). All other reagents were at analytical grade and used without further purification.
Methods
Preparation and characterization of 5FU microemulsion
Preparation of 5FU microemulsion
As described in previous reports,Citation26,Citation27,Citation29 5FU water-in-oil microemulsion was prepared by gradually adding 33 mL of drug–water solution into 33 mL oil phase containing liquid soybean oil, Span 80, Tween 80, and propylene glycol (21:5:1:6) under continuous stirring at 2,000 rpm for 10 minutes at 40°C.
Characterization of 5FU microemulsion
Morphology test: A drop of drug-loaded sample was placed on a copper grid, and the excess was removed with filter paper. Phosphotungstic acid was applied for several minutes as a negative stain. The solution that overflowed was cleared and dried in the air. The grid was examined under a transmission electron microscope (Hitachi H-7650; Hitachi Ltd., Tokyo, Japan).
Particle size, polydispersity index, viscosity, and zeta potential test: The viscosity of each of the drug-loaded sample was tested using a DV-E Viscometer (Brookfield Engineering Laboratories, Inc., Middleboro, MA, USA), and the particle size, polydispersity index, and zeta potential of each of the drug-loaded sample were estimated using a Mastersizer (NICOMP™ 380 ZLS; Malvern, Santa Barbara, CA, USA) after dilution with filtered oil. The samples were diluted five times to keep the intensity between 300 kHz and 350 kHz, and the scattering angle was 173.
Entrapment efficiency test: Entrapment efficiency was determined by dialysis. Drug-loaded sample was added to a dialysis bag MD34 (MWCO 7,000; Viskase Co., Darien, IL, USA) and dialyzed for 24 hours at 4°C. The concentrations of the drugs in the dialysis fluid were analyzed using high-performance liquid chromatography (HPLC) with the mobile phase of acetonitrile–water (95:5, pH 3.5) and a flow rate of 1 mL min−1. The standard curve, A=59.957X+10.373 (r=0.9992), was plotted with a peak area of 5FU as ordinate against its concentration as abscissa. The linear range was 0.1–20 µg mL−1; the recovery rate was 99.2%; the relative standard deviation (RSD) detected at 0 hour, 4 hours, 12 hours, and 24 hours was 2.1%; and the RSD of six repetitions was 2.2% by detecting 5FU peak area. The entrapment efficiency was calculated as follows:
Preparation and physicochemical properties of TG-5FU-ME
Preparation of TG-5FU-ME
Appropriate quantities of P407, P188, and sodium alginate were added to the deionized water and mixed. The liquid was placed at 4°C until a clear solution was obtained. The microemulsion formulation was mixed with thermosensitive gel under mechanical stirring at 4°C for 30 minutes at 700 rpm followed by increasing the temperature slowly to ambient temperature.
Physicochemical properties of TG-5FU-ME
Measurement of gelation temperature: The measurement of gelation temperature was detected as described earlier.Citation28–Citation32 In brief, a 20 mL transparent beaker containing a magnetic bar and 10 g liquid gel was placed in a low-temperature thermostat water bath. The liquid gel was then heated at a constant rate while stirring. When the stirring magnet was arrested due to gelation, the temperature displayed on the thermistor was identified as the gelation temperature.
Measurement of gel strength: As described by Klimaszewsk, et al.,Citation34 CT3 Texture Analyzer (Brookfield Engineering Laboratories, Inc.) was used to test the gel strength. Samples were prepared in 25×150 mm culture tubes until the liquid almost reached the top of the tube and equilibrated at 25°C±1°C for 24 hours. The gel strength was tested with a 4 mm-diameter stainless steel cylinder probe, with the trigger force fixed at 4 g, the test distance depth at 2 mm, and the test speed at 1 mm s−1. The gel strength was represented as the maximum peak force. For each sample, three replicates from different tubes were measured.
Measurement of coverage area: The coverage area was measured using the method described in our previous report.Citation13 In brief, a section of tissue was taken from the rabbit rectum and secured with the mucosal side facing out on a glass plate and storing at 36.5°C. Liquid gel (1 mL) was administered to the surface of the rabbit rectum, spreading freely until a gel film formed, and the area of the gel coverage was calculated.
Measurement of bioadhesive force: The bioadhesive force was determined using the method described by Koffi et alCitation35 with little modification. Briefly, the ample gel was placed in contact with a fragment of rabbit rectal mucosa of certain surface area under thermostated conditions, and then adhesive joint between the two surfaces was formed. The gel strength was recorded as the maximal detachment force. A mucosal fragment (the surface area was determined by the measurement of coverage described earlier) was placed at the surface of a metallic plot with a rubber band, and this metallic plot was fastened to the mobile traverse of CT3 Texture Analyzer. A hollow plastic support (10 mm in depth) was filled with a preset amount of 3 g of the sample and was immobilized in a beaker filled with 300 mL of water (37°C), which was immobilized in the texture analyzer apparatus. The whole system was placed in such a position that a perfect contact could be created between the surface of the gel and the mucous membrane. After being contacted for 10 minutes under an initial contact strength (0.5 N), the two surfaces were separated at a constant rate of displacement. The strength was recorded as the maximal detachment force, Fmax.
Formulation stability test
Three batches of 5FU microemulsion and TG-5FU-ME were stored in sealed glass containers under the temperature of 20°C±1°C for 4 months. The stability was detected periodically by visual appearance, microscopy, and 5FU content.
The appearances of 5FU microemulsion and TG-5FU-ME were examined for layering, creaming, coalescence, phase separation, or precipitation. The 5FU microemulsion samples were examined at 0 hour, 24 hours, 72 hours, and 1 week, and TG-5FU-ME samples were tested in fresh as well as at 1 month, 2 months, 3 months, and 4 months after preparation. Each visual examination was accompanied with microscopic test for droplet structure change, merging, or rupturing using the OLYMPUS-B201/IX2-sp (1,000×; Olympus, Tokyo, Japan). The drug content of 5FU microemulsion or TG-5FU-ME was extracted by 2 mol L−1 hydrochloride and analyzed by HPLC (as discussed in the “Entrapment efficiency test” section).
In vitro drug release
The in vitro release of 5FU was determined by dynamic dialysis. A certain amount of 5FU microemulsion or TG-5FU-ME was transferred to prepare dialysis channels, which were placed in a conical flask filled with 100 mL of phosphate buffer solution (pH 8.0) and magnetically stirred at 37°C at 150 rpm. Two milliliter sample was taken at 0.5 hours, l hour, 1.5 hours, 2 hours, 3 hours, 4 hours, 6 hours, 8 hours, 10 hours, 12 hours, and 14 hours, and the same volume of phosphate buffer solution was added into the flask simultaneously. The concentration of dialyzed drug was determined using HPLC, as mentioned earlier, to calculate the cumulative dissolution. The control samples for the experiment were the 5FU in water solution as well as the 5FU thermosensitive gel. The blank microemulsion and gel presented in the experimental system had no effect on the 5FU content measurement.
Trans Caco-2 cell test
As described earlier,Citation34,Citation35 Caco-2 cell lines obtained from the Institute of Biochemistry and Cell Biology (Shanghai, People’s Republic of China) were grown at 37°C in an atmosphere of 5% CO2 and 90% relative humidity in 25 cm2 culture dish with standard growth media of Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal bovine serum, 1% nonessential amino acids, 4.5 g/L glucose, 2 mmol/L l-glutamine, 100 U/mL penicillin, and 100 µg/mL streptomycin. Cells were seeded at a density of 6.25×104 cells/cm2 onto 12-well transwell culture plates coated with polycarbonate filters with a pore size of 0.4 µm and an area of 1.13 cm2 (Corning Incorporated, Corning, NY, USA). Growth media were added to the apical (AP) side and basolateral (BL) side. The medium was changed every other day. Cells were grown 21–28 days prior to use. The tightness of the cell monolayer was evaluated through the determination of trans-epithelial electrical resistance (TEER), which was measured using a Millicell-ERS Resistance Instrument (EMD Millipore, Billerica, MA, USA), and the permeation ratio of 14C mannitol was determined using a liquid scintillation counter (Wallac MicroBeta TriLux; Wallac, Turku, Finland) during the transport experiment. The cell monolayer was applied for transport experiments once the TEER was >600 Ω/cm2.
Confluent cultures of Caco-2 cell monolayers (cultured for ∼21 days) in Hank’s buffered salt solution (HBSS) were precultured at 37°C for 20 minutes in an incubator. After removing the buffer solution, the cell monolayers were washed three times with HBSS. The experiments were performed at 37°C for 150 minutes from the AP to BL (A→B) or the BL to AP (B→A) directions. The donor chambers were filled with 1.5 mL of a sample solution containing 5FU (200 µM), and the receiver compartments were filled with 2.6 mL of blank HBSS, respectively. Samples were taken at receiver side (100 µL) at different time points (30 minutes, 60 minutes, 90 minutes, 120 minutes, and 150 minutes), and the same volume of fresh HBSS was added immediately. At the end of the transport study, cells were recovered and washed three times with 2 mL of ice-cold HBSS; 800 µL of NaOH (0.2 N) was then added to lyse the cells, and the sample was back neutralized by adding 200 µL of HCl (0.8 N). The amount of drug accumulated in the cell was evaluated. Each treatment was accomplished with 3–5 wells, and the experiments were conducted in duplicate. The 5FU content was determined as described earlier. The apparent permeability coefficient (Papp, cm/s) for 5FU was calculated according to the following equation:
Measurement of rectal retention time
The method described by Choi et alCitation30 was adopted for this study with slight modification. Male BALB/c mice (∼20 g) were fasted and allowed free access to water for 24 hours prior to the experiments. 5FU microemulsion and TG5-FU-ME with 0.1% Cy7NHS ester (Seebio Biotech Co., Ltd., Shanghai, People’s Republic of China) in the water phase were administered (20 mg kg−1of 5FU) into the rectum 0.5 cm above the anus using a stomach probe needle. The formulations retained in the rectum were detected after administration using small animal imaging technology.
5FU distribution in vivo
As described in other reports,Citation17,Citation23,Citation24 male Sprague Dawley rats (∼300 g) were used for this study. A total of 58 rats were randomly divided into three groups (20, 18, and 20), fasted, and allowed free access to water for 24 hours prior to drug administration. 5FU microemulsion, TG-5FU-ME, and 5FU thermosensitive gel (30 mg kg−1of 5FU) were administered into the rectum using a stomach sonde needle fit into a plastic syringe. The 5FU dose used in the test was approximately three times higher than that of the human dose (10 mg kg−1).
The method of Galandiuk et alCitation16 was used in this investigation. At 0.5 hours, 1 hour, 3 hours, 6 hours, 9 hours, and 12 hours after drug administration, three animals from each group were anesthetized. Cardiac puncture was performed to collect systemic blood, and the rats were sacrificed with exsanguinations. Rectal tissues and regional lymph nodes were harvested to determine the 5FU tissue concentrations. Serum and tissue samples were stored at −70°C until analysis. All the surgeries were performed under sodium pentobarbital anesthesia, and all efforts were made to minimize the suffering.
The tissue and plasma samples were treated as described in our previous work.Citation13 Briefly, the tissue sample was mashed and ground into a homogenate by adding 2 mL of distilled water. After standing for 30 minutes, the precipitate was removed by centrifugation, washed, and filtered with 0.4 mL purified water three times. Subsequently, 1 mL of 0.5 mol L−1 ammonium sulfate was added to precipitate proteins. 5FU in the supernatant was extracted with addition of ethyl acetate for three times with 0.5 mL of ethyl acetate each. The solution was vortexed for 0.5 minutes each time after ethyl acetate was added. The ethyl acetate layer was collected and centrifuged at 4,000 rpm for 5 minutes. The plasma samples were treated with protein precipitation and drug extraction. An accurately measured 1 mL sample of supernatant was removed and added into an Eppendorf tube, followed by dryness over nitrogen gas in a water bath at 40°C. Then, the residue was dissolved in 100 µL of the mobile phase, 20 µL of which was measured accurately for 5FU concentration by HPLC (as discussed in the “Entrapment efficiency test” section).
Rectal irritation
Immediately after the last sampling, two animals from the TG-5FU-ME or 5FU thermosensitive gel group were collected, respectively. The rectum was isolated, washed with saline solution, fixed in 10% neutral carbonate-buffered formaldehyde, embedded in paraffin, and cut into slices. The tissues were stained with hematoxylin–eosin and examined under light microscopy.
All animal studies were carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee of the Ethics of Animal Experiments of the Chinese Academy of Medical Sciences and Peking Union Medical College.
Statistics
The data were analyzed using the SPSS (11.5) software (SPSS Inc., Chicago, IL, USA). After validation of the test for homogeneity of variance, differences between or among study groups were examined by Student’s t-test or by one-way analysis of variance followed by the Newman–Keuls test for multiple comparisons. The P-value <0.05 was considered statistically significant.
Results and discussion
Evaluation of 5FU microemulsion
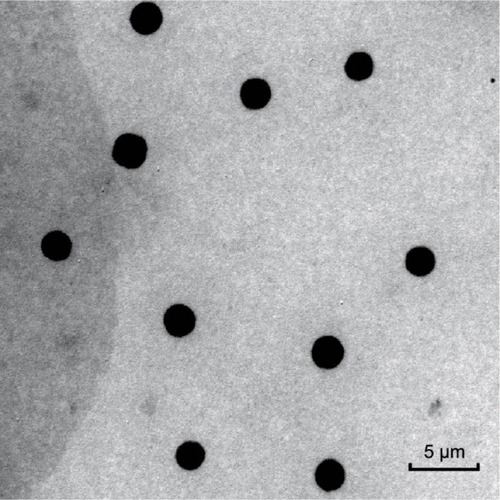
All the 5FU microemulsion vesicles were in round shape and free of aggregation, as assessed by the transmission electron microscope (). Sample characteristics are given in . The average size of 5FU microemulsion was 2.43±0.49 µm, with a narrow size distribution. Zeta potential value was +1.06±0.11. Encapsulation efficiency was 80.11%±0.58%.
Table 1 Characteristics of 5FU microemulsion
Physicochemical properties of TG-5FU-ME
The physicochemical properties of drug-free thermosensitive gel and TG-5FU-ME, such as gel temperature, gel strength, coverage area, and bioadhesive force, are shown in . Addition of 5FU microemulsion into the thermosensitive gel decreased the gelling temperature from 54.5°C to 19.2°C, and reduced the coverage area from 1.42 cm2/mL to 0.81 cm2/mL. However, the bioadhesive force and gel strength of thermosensitive gel were dramatically increased from 1.47 N to 4.18 N and 11.21 g to 30.14 g, respectively. The TG-5FU-ME was designed for gelling at ambient temperature, turning into liquid form within 10 minutes below 10°C and returning to gel form at body temperature. Most likely, after applying as liquid forms to the rectum, a layer of gel containing 5FU microemulsion forms and spreads broadly on rectal mucous membrane, with reasonable gel strength and bioadhesive force, which might benefit the tissue deposition of drug and improve the patient compliance.
Table 2 Physicochemical properties of the drug-free thermosensitive gel and TG-5FU-ME
Formulation stability study
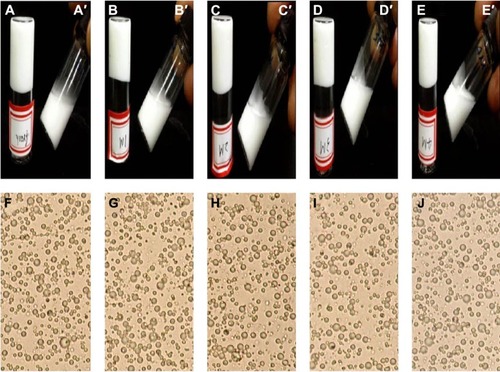
The results of naked eye evaluation, microscopic examination, and 5FU content test of 5FU microemulsion and TG-5FU-ME are shown in and , as well as in and . The 5FU microemulsion was unstable under the temperature of 20°C±1°C, with layer occurring in 24 hours after preparation, and the oil precipitation took place in 1 week (). Microscopically, the droplet of microemulsion began to merge in 24 hours and became large bulbs in 1 week () with the disappearance of the microemulsion droplet structure. In contrast, TG-5FU-ME was remarkably stable for the period of study (4 months) at ambient temperature. This system gelled at room temperature () and turned into liquid form (′–E′) within 10 minutes when the temperature was below 10°C. The sign of instability, such as layering, creaming, coalescence, phase separation, and precipitation, was not detected; significant morphological changes were detected negative as well (). The 5FU content did not change during the storage time in both the 5FU microemulsion and TG5-FU-ME. A heating–cooling cycle test was also conducted, and the results were in consistent with that of the formulation stability study (data not shown). The improved stability of this system might be related to the protective effect of the gel in its action against droplet merge and phase transfer of the 5FU microemulsion.
Table 3 Stability of 5FU microemulsion
Table 4 Stability of TG-5FU-ME
Figure 2 Appearance of 5FU microemulsion.
Abbreviation: 5FU, 5-fluorouracil.
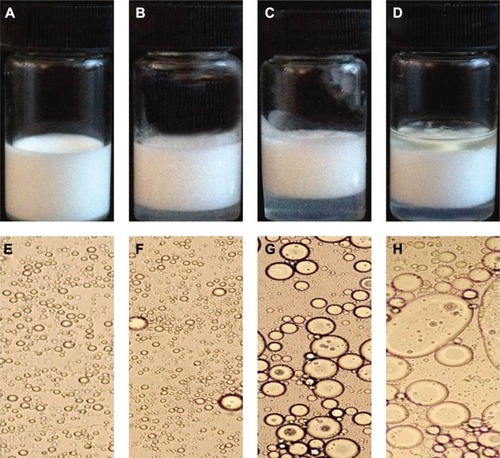
Figure 3 Appearances of TG-5FU-ME.
Abbreviations: RT, room temperature; TG-5FU-ME, thermosensitive gel-mediated 5FU water-in-oil microemulsion.
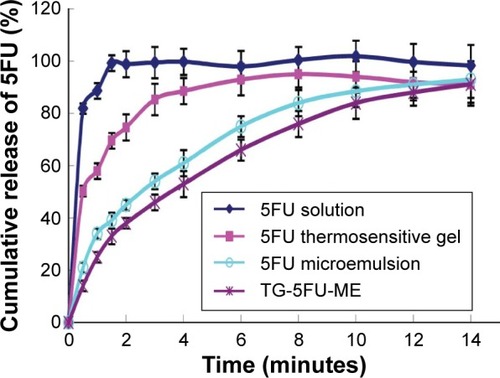
In vitro release
The in vitro release profiles of the different formulations are shown in . The 5FU solution was completely released within 30 minutes, and 5FU thermosensitive gel released within 2 hours. The 5FU microemulsion prolonged the release to 8 hours, and TG-5FU-ME increased the releasing time to 10 hours.
Figure 4 Release profiles of 5FU formulations in vitro.
Abbreviations: 5FU, 5-fluorouracil; TG-5FU-ME, thermosensitive gel-mediated 5FU water-in-oil microemulsion.
It has been proven that microemulsion system possesses sustained drug release property due to the resistance rendered by the oil phase which blocks the diffusion of the drug from inner phase into the release medium.Citation27 Furthermore, studies demonstrate that the drug release from thermosensitive gels follows the Higuchi square root law, and the diffusion coefficient of the drug in the gel declines with increasing levels of both poloxamers and bioadhesive material and is consistent with a consequent increase in bulk viscosity and gel rigidity.Citation36–Citation39 We assume that when the microemulsion system was entrapped in the thermosensitive gel, both of those systems would influence the releasing character of the drug. Further studies are needed to clarify the releasing properties and mechanisms of the microemulsion entrapped thermosensitive gel system.
Trans Caco-2 cell test
TEER measurements and mannitol transport were used to test cellular integrity. The results showed that there was no significant variation in the TEER values for all the samples in the test, and the average transport of mannitol (0.91±0.06×10−6 cm/s) did not vary significantly after exposure to 5FU thermosensitive gel (0.88±0.03×10−6 cm/s), 5FU microemulsion (0.92±0.05×10−6 cm/s), and TG-5FU-ME (0.94±0.07×10−6 cm/s), indicating that the cell monolayers remained intact after treatment.
The apparent permeability coefficient (Papp) evaluates the velocity of a drug crossing the cell monolayer.Citation40 As shown in , for 5FU thermosensitive gel, 5FU microemulsion, or TG-5FU-ME, the amount of 5FU transported from A→B or from B→A across the Caco-2 monolayer was in an identical pattern, which was proportional to time in 150 minutes, with no significant difference of the Papp value between the A→B and B→A. The Papp values of 5FU microemulsion and TG-5FU-ME were 1.24×10−5 cm/s and 1.25×10−5 cm/s, respectively, approximately six times higher than that of 5FU thermosensitive gel (1.99×10−6 cm/s; as shown in ). Furthermore, in the A→B direction, 5FU intracellular accumulation of the 5FU microemulsion and TG-5FU-ME was 17.45% and 18.41% of the given amount, respectively, which were greater than that of the 5FU thermosensitive gel (3.41%; as shown in ). As the cell monolayers remained intact after treatment as described earlier, the increased intracellular accumulation and transportation of 5FU for 5FU microemulsion or TG-5FU-ME appeared to be attributable to the improved lipophilicity of the formulation.
Figure 5 AP to BL (A→B) and BL to AP (B→A) transport of 5FU.
Abbreviations: AP, apical; BL, basolateral; 5FU, 5-fluorouracil; SD, standard deviation.
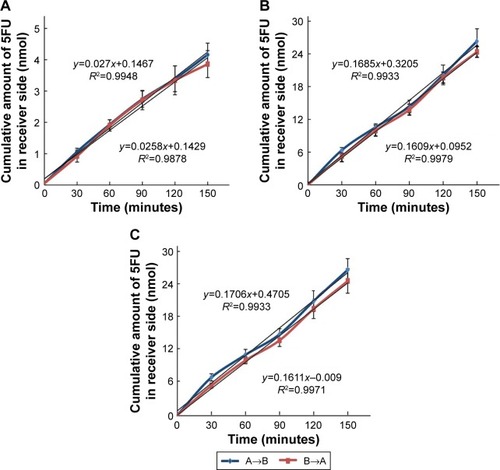
Figure 6 Formulation change affects transportation efficacy of 5FU crossing the Caco-2 cell monolayers.
Abbreviations: 5FU, 5-fluorouracil; HBSS, Hank’s buffered salt solution; HPLC, high-performance liquid chromatography; TG-5FU-ME, thermosensitive gel-mediated 5FU water-in-oil microemulsion; SD, standard deviation.
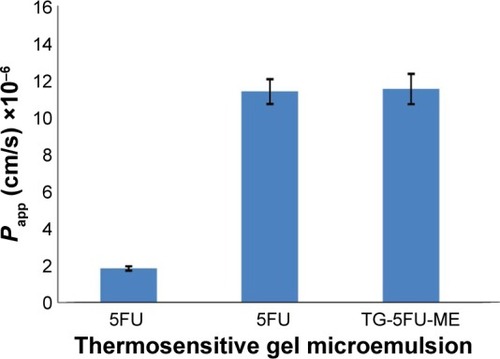
Figure 7 Absorption of 5FU in vitro.
Abbreviations: AP, apical; BL, basolateral; 5FU, 5-fluorouracil; TG-5FU-ME, thermosensitive gel-mediated 5FU water-in-oil microemulsion; SD, standard deviation.
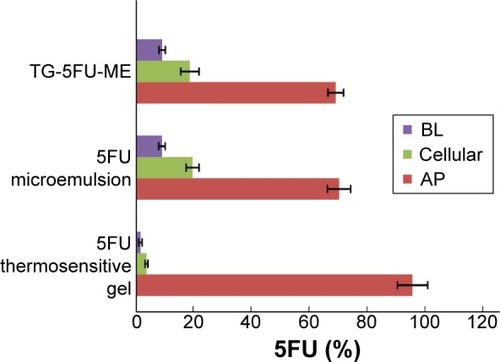
5FU is a hydrophilic drug and crosses the rectal epithelium primarily via passive transport through the water-filled pores. Tight junction between cells greatly restricts the transport and consequently reduces the absorption of drug. The introduction of a microemulsion system increased the lipophilicity of the formulation which facilitated passive diffusion or/and clathrin-mediated endocytosis.Citation41
Measurement of rectal retention time
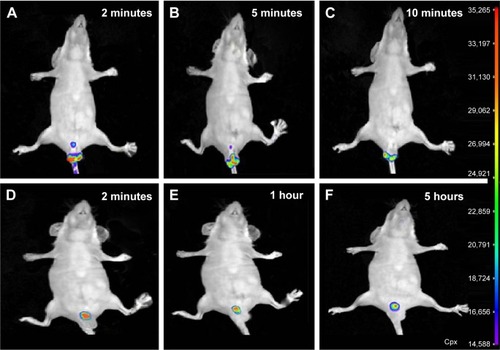
5FU microemulsion and TG-5FU-ME with 0.1%Cy7NHS ester added in the water phase were administered (20 mg kg−1 of 5FU) into the rectum of mouse 0.5 cm above the anus using a stomach probe needle. As shown in , the 5FU microemulsion flew out within 10 minutes after administration, while TG-5FU-ME sustained at the applied site for >5 hours. This phenomenon suggested that the bioadhesive force and gel strength of TG-5FU-ME were sufficient to hold the formulation in the rectum for a time period much longer than that of the microemulsion.
Figure 8 Rectal retention time.
Abbreviations: 5FU, 5-fluorouracil; TG-5FU-ME, thermosensitive gel-mediated 5FU water-in-oil microemulsion; cpx, count per second.
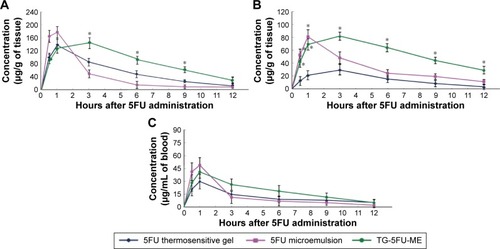
5FU distribution in vivo
As compared to the 5FU thermosensitive gel group, the 5FU concentration in rectal tissue was significantly higher in the TG-5FU-ME group after 3 hours than that in the 5FU thermosensitive gel group (; P<0.05). This result is consistent with that achieved in the Caco-2 transport study () and in vitro release study (), and could be explained by the sustained drug release, increased rectal mucous membrane permeation, and enhanced intracellular uptake in TG-5FU-ME treatment. In the regional lymph nodes, the 5FU concentration in the TG-5FU-ME group and 5FU microemulsion group was found to be significantly higher () than that in the 5FU thermosensitive gel group (P<0.05 for both), which agrees with the results of other researchers.Citation17 It is worthy to note that the high rectal and regional lymph node concentrations lasted for up to 9 hours after TG-5FU-ME administration, whereas in the 5FU microemulsion group drug concentrations remained high only for 2 hours. TG-5FU-ME seemed to acquire the advantage of both the 5FU microemulsion and 5FU thermosensitive gel, showing an increased transportation and rectal retention time. Blood level of 5FU in the 5FU microemulsion group as well as the TG-5FU-ME group was low and showed no significant difference from that of the 5FU thermosensitive gel group (P>0.05 for both), suggesting a reduced chance of causing side effect.
Figure 9 Tissue distribution of 5FU.
Abbreviations: 5FU, 5-fluorouracil; TG-5FU-ME, thermosensitive gel-mediated 5FU water-in-oil microemulsion; SD, standard deviation.
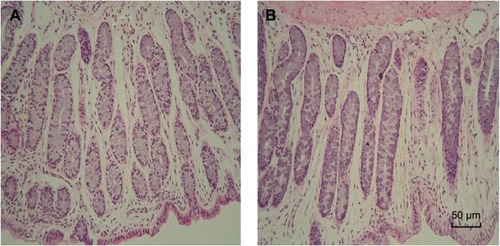
Rectal irritation
Rectal tissue morphological test showed that neither 5FU thermosensitive gel nor TG-5FU-ME caused irritation or damage on rectal tissues during the treatment course (). This is at least partially due to their good spreading property, which leads to a relatively low drug concentration per unit square of the rectal mucous membrane. In addition, the encapsulation of the drug in the emulsion minimized the direct contact of the drug with the rectal mucous.
Figure 10 The morphology of rectal tissues after exposure to 5FU thermosensitive gel and TG-5FU-ME.
Abbreviations: 5FU, 5-fluorouracil; H&E, hematoxylin and eosin; TG-5FU-ME, thermosensitive gel-mediated 5FU water-in-oil microemulsion.
Conclusion
The TG-5FU-ME formulation was designed to improve the in situ delivery of 5FU for the treatment of colon cancer. In this study, we showed that the microemulsion facilitated 5FU transportation into target tissue and the thermosensitive gel increased the stability of the 5FU microemulsion as well as the retention time in rectal membrane. The formulation is in a free-flowing liquid form when the temperature is below 10°C. After applying to the rectum, a gel layer containing 5FU microemulsion forms and spreads broadly on rectal mucous membrane with appropriate gel strength and bioadhesive force, which could improve the patient’s compliance and ameliorate their alienation. For chemotherapy, this formulation might be able to efficiently prolong the drug’s rectal retention time, enlarge the drug’s contact area with diseased tissue, and improve the drug’s permeability. With regard to safety concern, it could reduce the direct contact of the drug with the rectum site. Our study validated the advantages of this formulation, and therefore, TG-5FU-ME could be considered a promising rectal delivery system for the treatment of rectal cancer. Furthermore, this dosage form would be a promising alternative applied in other mucosal drug delivery systems such as ocular, nasal, buccal, and vaginal.
Authors contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- AllemaniCWeirHKCarreiraHGlobal surveillance of cancer survival 1995–2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2)Lancet20153859972977101025467588
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal Cancer Statistics, 2012CA Cancer J Clin20156528710825651787
- SiegelRMaJZouZJemalACancer Statistics, 2014CA Cancer J Clin201464192924399786
- PhillipsTAHowellAGrieveRJWellingPGPharmacokinetics of oral and intravenous fluorouracil in humansJ Pharm Sci19806912142814317463330
- FraileRJBakerLHBurokerTRHorwitzJVaitkeviciusVKPharmacokinetics of 5-fluorouracil administered orally, by rapid intravenous and by slow infusionCancer Res1980407222322287388790
- LongleyDBHarkinDPJohnstonPG5-Fluorouracil: mechanisms of action and clinical strategiesNat Rev Cancer20033533033812724731
- ParkerWBChengYCMetabolism and mechanism of action of 5-fluorouracilPharmacol Ther19904833813951707544
- GamelinECDanquechinDorvalEMDumesnilYFRelationship between 5-fluorouracil (5-FU) dose intensity and therapeutic response in patients with advanced colorectal cancer receiving infusional therapy containing 5-FUCancer19967734414518630950
- Di PaoloADanesiRFalconeARelationship between 5-fluorouracil disposition, toxicity and dihydropyrimidine dehydrogenase activity in cancer patientsAnn Oncol20011291301130611697844
- KuropkatCGriemKClarkJRodriguezERHutchinsonJTaylorSGSevere cardiotoxicity during 5-fluorouracil chemotherapy – a case and literature reportAm J Clin Oncol199922546647010521060
- AnithaAMayaSSivaramAJMonyUJayakumarRCombinatorial nanomedicines for colon cancer therapyWiley Interdiscip Rev Nanomed Nanobiotechnol20168115115926061225
- JainSKChaurasiyaAGuptaYDevelopment and characterization of 5-FU bearing ferritin appended solid lipid nanoparticles for tumour targetingJ Microencapsul200825528929718608808
- WangLLZhengWSChenSHFangXQDevelopment of in situ gelling and bio adhesive 5-fluorouracil enemaPLoS One201388e7103723976976
- PokornyRMWrightsonWRLewisRKSuppository administration of chemotherapeutic drugs with concomitant radiation for rectal cancerDis Colon Rectum19974012141414209407977
- TakahashiTKohnoKYamaguchiTNarisawaTPreoperative use of 5-fluorouracil suppository for carcinoma of the rectumAm J Surg198214321831857058985
- GalandiukSWrightsonWMarrLMyersSLaRoccaRVSuppository delivery of 5-fluorouracil in rectal cancerAnn Surg Oncol1996332702768726182
- IchikawaDYamaguchiTYoshiokaYJSawaiKTakahashiTPrognostic evaluation of preoperative combined treatment for advanced cancer in the lower rectum with radiation, intraluminal hyperthermia, and 5-fluorouracil suppositoryAm J Surg199617133463508615470
- HorieHKashiwagiHKonishiFFurutaKOzawaAKanazawaKImproved outcome following preoperative radiochemotherapy: 40.5 Gy accelerated hyperfractionation and 5-fluorouracil suppositories for patients with carcinoma of the lower rectumSurg Today1999291099299810554320
- PaekSHXuanJJChoiHGPoloxamer 188 and propylene glycol-based rectal suppository enhances anticancer effect of 5-fluorouracil in miceBiol Pharm Bull20062951060106316651748
- NishiokaBWatanabeSFijitaYClinical studies of intrarectal administration of 5FU emulsion as an adjunct to surgical treatment for rectal cancerJpn J Surg19801021101147431683
- WatanabeSNishiokaBFujitaYExperimental studies of intrarectal administration of emulsified 5FU as an adjuvant to the surgical treatment of rectal cancerJpn J Surg19801021721
- RougierPNordlingerBLarge-scale trial for adjuvant treatment in high-risk resected colorectal cancers – rationale to test the combination of loco-regional and systemic chemotherapy and to compare l-leucovorin plus 5-FU to levamisole plus 5-FUAnn Oncol19934suppl 2S21S28
- AnithaAMayaSChennazhiKPLakshmananVJayakumarRIn vitro combinatorial anticancer effects of 5-fluorouracil and curcumin loaded N, O-carboxymethyl chitosan nanoparticles towards colon cancer and in vivo pharmacokinetic studiesEur J Pharm Biopharm201488123825124815764
- AnithaADeepaNChennazhiKPLakshmananVJayakumarRCombinatorial anticancer effects of curcumin and 5-fluorouracil loaded thiolated chitosan nanoparticles towards colon cancer treatmentBiochim Biophys Acta2014184092730274324946270
- SaeedniaLUstaAAsmatuluRPreparation and Characterization of Drug-loaded Thermosensitive HydrogelsSan Diego, CAASME International Mechanical Engineering Congress and Exposition20131521
- ShishuKamalpreetMaheshwariMDevelopment and evaluation of novel microemulsion based oral formulations of 5-fluorouracil using non-everted rat intestine sac modelDrug Dev Ind Pharm201238329430021864111
- TenjarlaSMicroemulsions: an overview and pharmaceutical applicationsCrit Rev Ther Drug Carrier Syst199916546152110635455
- HaussDJFogalSEFicorilliJVLipid-based delivery systems for improving the bioavailability and lymphatic transport of a poorly water-soluble LTB4 inhibitorJ Pharm Sci19988721641699519148
- ChenHBChangXLDuDRLiJXuHBYangXLMicroemulsion-based hydrogel formulation of ibuprofen for topical deliveryInt J Pharm20063151–2525816600540
- ChoiHGOhYKKimCKIn situ gelling and mucoadhesive liquid suppository containing acetaminophen: enhanced bioavailabilityInt J Pharm199816512332
- KimCKLeeSWChoiHGTrials of in situ gelling and mucoadhesive acetaminophen liquid suppository in human subjectsInt J Pharm19981741–2201207
- ParkYJYongCSKimHMEffect of sodium chloride on the release, absorption and safety of diclofenac sodium delivered by poloxamer gelInt J Pharm20032631–210511112954185
- RyuJMChungSJLeeMHKimCKShimCKIncreased bioavailability of propranolol in rats by retaining thermally gelling liquid suppositories in the rectumJ Control Release199959216317210332051
- KlimaszewskaKBernier-CardouMCyrDRSuttonBCSInfluence of gelling agents on culture medium gel strength, water availability, tissue water potential, and maturation response in embryogenic cultures of Pinus strobus LIn Vitro Cell Dev Biol Plant2000364279286
- KoffiAAAgnelyFPonchelGGrossiordJLModulation of the rheological and mucoadhesive properties of thermosensitive poloxamer-based hydrogels intended for the rectal administration of quinineEur J Pharm Sci200627432833516356700
- KoffiAAAgnelyFBesnardMBrouJKGrossiordJLPonchelGIn vitro and in vivo characteristics of a thermogelling and bioadhesive delivery system intended for rectal administration of quinine in childrenEur J Pharm Biopharm200869116717518023982
- GangulySDashAKA novel in situ gel for sustained drug delivery and targetingInt J Pharm20042761–2839215113617
- MooreTCroySMallapragadaSPanditNExperimental investigation and mathematical modeling of Pluronic (R) F127 gel dissolution: drug release in stirred systemsJ Control Release2000672–319120210825553
- AndersonBCPanditNKMallapragadaSKUnderstanding drug release from poly(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide) gelsJ Control Release2001701–215716711166416
- ChenMJChengYMLaiPHWuJFHsuYCIn vitro biocompatibility of thermally gelling liquid mucoadhesive loaded curcuminoids in colorectal cancer chemopreventionInt J Colorectal Dis201227786987822222465
- JeongBKimSWBaeYHThermosensitive sol-gel reversible hydrogelsAdv Drug Deliv Rev2002541375111755705