Abstract
The effects of misoprostol use on cervical priming prior to hysteroscopy have been controversial. Therefore, a systematic literature review and meta-analysis of studies were conducted to assess the effect of misoprostol on cervical priming prior to hysteroscopy. All studies published before July 2014 with data related to the use of misoprostol for cervical priming compared with placebo or no medication prior to hysteroscopy, were identified. Twenty-five randomized controlled trials involving 2,203 females were systematically analyzed. The results showed that, compared with placebo or no medication, the use of misoprostol prior to hysteroscopy led to a significant relief of the need for cervical dilatation, resulted in a significantly greater cervical width, had fewer hysteroscopy complications, and mild and insignificant side effects. Subgroup analyses revealed that the regimen of 200 or 400 μg vaginal misoprostol may be a simple and effective method for cervical priming, especially prior to operative hysteroscopy.
Introduction
Hysteroscopy is a minimally invasive approach for observing the uterine cavity for a variety of gynecological problems, and has become a valuable diagnostic and therapeutic procedure.Citation1,Citation2 Also, hysteroscopy is potentially useful for female sterilization and offers promise as an investigative tool for studying the intratubal milieu.Citation3 However, many patients undergoing the procedure are at risk for cervical dilatation complications, such as cervical laceration, uterine perforation, and creation of false passages.Citation4 Fortunately, the incidence of these complications may be reduced if the cervix is ripened beforehand.
Misoprostol, a prostaglandin E1 analog, which was initially used for the treatment of peptic ulcers, has been widely applied in obstetrics and gynecology because of its ripening effect on cervix during the induction of abortion or labor.Citation5,Citation6 The primary advantages of the drug include its thermostability, low cost, and the ease of administration.Citation7 Moreover, misoprostol is available in many formulations: tablets or gelcaps, at doses of 200, 400, 800, and 1,000 μg, and can be administered by mouth or sublingually, as well as via the rectal or vaginal route.Citation8–Citation10 Because of its effect on cervical ripening in pregnant females, misoprostol has also been used for cervical priming prior to hysteroscopy by surgeons. While numerous studies indicated the efficacy of misoprostol for achieving cervical dilatation in patients undergoing hysteroscopy,Citation10–Citation15 some reports concluded that the use of misoprostol before hysteroscopy did not facilitate cervical dilatation.Citation8,Citation9,Citation16–Citation18 The discrepancy may be due to small sample sizes, differences in the route of administration of misoprostol, the types of hysteroscopy (operative or diagnostic hysteroscopy), and/or different populations under study.
To more systematically evaluate the efficacy and safety of misoprostol for cervical priming prior to hysteroscopy, we conducted a meta-analysis on randomized controlled trials (RCTs) comparing misoprostol versus placebo or no medication before diagnostic or operative hysteroscopy in females receiving hysteroscopy. In addition, we hope that such analyses would help in determining the optimal dose and route of administration for the application of misoprostol in hysteroscopy.
Methods
Search strategy
We searched (published up to July 2014) the three most popular databases – MEDLINE (via PubMed), EMBASE (via embase.com), and Cochrane – for articles in any language. The search strategy used the terms “hysteroscopy” AND “misoprostol”. In addition, the references of the relevant articles and previous systematic reviews were checked to identify potentially eligible trials.
Selection criteria
We included RCTs for cervical priming using misoprostol prior to diagnostic or operative hysteroscopy in females regardless of age, parity, or other characteristics. The intervention in the trials was the use of misoprostol compared with placebo or no medication before hysteroscopy. No restriction was placed on dose, route, or timing of misoprostol administration. We excluded studies without a placebo or no medication group, as well as those comparing misoprostol to another method (laminaria tents or dinoprostone). Nonrandomized trials such as case–control studies were also excluded.
Data extraction and quality assessment
Two reviewers, YH and WWZ, independently extracted the data that were retrieved from the search. The results were then compared and disagreements were resolved by discussion. If the two primary reviews could not reach a consensus the third reviewer (XLH) was be consulted. Information of the authorship, publication year, patient demographics, type of intervention, and outcomes were extracted. To assess the validity of the included trials, two investigators (YH and WWZ) independently examined the study quality using the Cochrane Handbook for Systematic Reviews of Interventions with respect to the generation of random sequences, allocation concealment, blinding, incomplete outcome data, and selective reporting.Citation19 The risk of publication bias was assessed using funnel plots.
Outcomes
The outcomes of interest for this article included the following variables: number of females who required cervical dilatation, cervical width at the start of hysteroscopy, hysteroscopy complications such as cervical tears and uterine perforation, and the incidence of misoprostol side effects such as abdominal pain, nausea, diarrhea, genital bleeding, and fever.
Data synthesis
Statistical analyses were performed with the use of Review Manager (RevMan), Version 5.1 (The Nordic Cochrane Centre, the Cochrane Collaboration, Copenhagen, Denmark). To calculate the risk ratio (RR) for dichotomous data and the mean differences (MD) for continuous data with 95% confidence intervals (CIs), the fixed effects model was used. Statistical significance was set at a P-value of <0.05. We evaluated statistical heterogeneity by employing P-values, chi-square, and I2 tests.Citation20 If significant heterogeneity was found (P≤0.10, I2>50%), a random effects model was applied to limit the effects of heterogeneity. A subgroup analysis was also performed to reveal the possible reasons for the heterogeneity.
Results
Description of studies
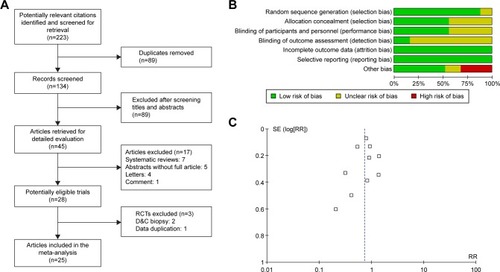
A total of 2,203 females requiring hysteroscopy from 25 RCTs were included in this meta-analysis. A flow diagram for the literature search is presented in .
Figure 1 Flow diagram and quality of the selected study.
Abbreviations: D&C, dilatation and curettage; RCTs, randomized controlled trials; RR, risk ratio; SE, standard error.
We identified 25 randomized studies comparing misoprostol versus placebo or no medication prior to hysteroscopy. summarizes the characteristics of these studies, which include seven studies of operative hysteroscopy,Citation8–Citation10,Citation13,Citation14,Citation21,Citation22 13 studies of diagnostic hysteroscopy,Citation16–Citation18,Citation23–Citation32 and five studies on both diagnostic and operative hysteroscopy.Citation11,Citation12,Citation15,Citation33,Citation34 Additionally, the route of misoprostol administration was oral (four studies), vaginal (18 studies), sublingual (four studies), or rectal (one study). shows that the dose of misoprostol administration prior to hysteroscopy differed considerably among the available trials and the outcomes.
Table 1 Main characteristics of the included RCT studies
Quality of trials and assessment of publication bias
Two investigators independently assessed the risk of bias of the eligible trials by using the Cochrane Handbook for Systematic Reviews of Interventions,Citation19 and a consensus was reached after discussion. As demonstrated in , most of the included trials had properly randomized their participants and 60% had adequate randomization allocations. With regard to performance bias, 60% had adequate blinding. All papers were judged to be free of attrition and reporting biases.
As shown in , the funnel plots appeared to be symmetrical, which indicated that there was no obvious publication bias.
Outcomes
Need for cervical dilatation
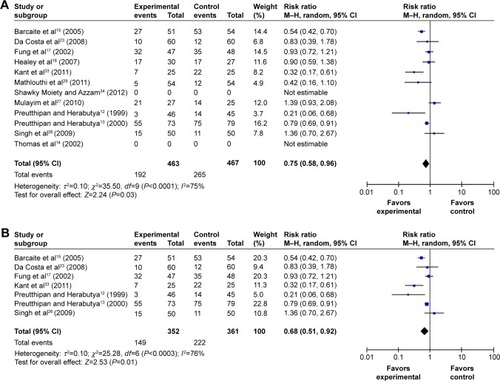
Data on the need for cervical dilatation before hysteroscopy were reported in ten studies that included a total of 930 females. Due to the high statistical heterogeneity, results were pooled using the random effects model. Compared with placebo or no medication, misoprostol administration prior to hysteroscopy reduced the need for cervical dilatation to a statistically significant degree (RR 0.75; 95% CI 0.58–0.96; I2=75% ).
Figure 2 Comparison of the need for cervical dilatation between the misoprostol group and the placebo or no medication group, including both operative and diagnostic hysteroscopy studies.
Abbreviations: CI, confidence interval; df, degrees of freedom; M–H, Mantel–Haenszel.
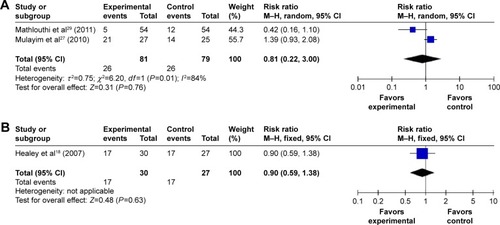
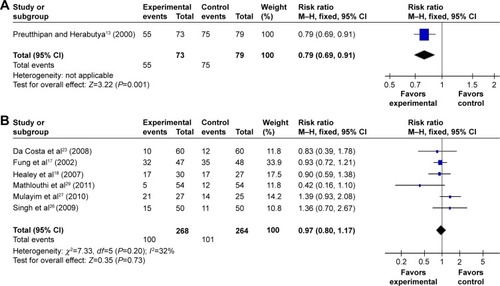
By subgroup analysis, when only operative hysteroscopy was examined, the need for cervical dilatation in the misoprostol group was significantly decreased compared to the placebo or no medication groups (RR 0.79; 95% CI 0.69–0.91 ), while the need for cervical dilatation was not significantly decreased before diagnostic hysteroscopy (RR 0.97; 95% CI 0.80–1.17; I2=32% ). The need for cervical dilatation after vaginal misoprostol administration was significantly decreased compared to placebo or no medication (RR 0.68; 95% CI 0.51–0.92; I2=76% ), while after sublingual (RR 0.81; 95% CI 0.22–3.00; I2=84% ) and oral (RR 0.90; 95% CI 0.59–1.38; ) misoprostol administration, the need for cervical dilatation was not significantly decreased.
Figure 3 Comparison of the need for cervical dilatation between the misoprostol group and the placebo or no medication group, including vaginal, oral, sublingual administration routes.
Abbreviations: CI, confidence interval; df, degrees of freedom; M–H, Mantel–Haenszel.
Cervical width
Fourteen trials provided data on the MD in the cervical width before the hysteroscopy. Patients receiving misoprostol appeared to have a significantly greater cervical width compared with placebo or no medication (MD 1.34 mm; 95% CI 0.55–2.14; I2=98% ). The cervical width after vaginal misoprostol administration was significantly greater than that in the placebo or no medication group (MD 1.64 mm; 95% CI 0.93–2.35; I2=95% ), but after sublingual (MD 0.40 mm; 95% CI −0.80 to 1.61; I2=98% ) or oral (MD −0.20 mm; 95% CI −1.31 to 0.91; ) misoprostol administration cervical width was not significantly greater. In addition, in the 200 μg subgroup (MD 2.20 mm; 95% CI 1.21–3.19; I2=94% ) or the 400 μg subgroup (MD 2.20 mm; 95% CI 1.14–3.26; I2=92% ), the cervical width was significantly greater than that in the placebo or no medication group, while it was not in the 800 μg subgroup (MD 0.16 mm; 95% CI −0.33 to 0.66; I2=0% ) or the 1,000 μg subgroup (MD 0.60 mm; 95% CI −0.73 to 1.94; I2=76% ).
Figure 5 Comparison of the cervical width prior to hysteroscopy between the misoprostol group and the placebo or no medication group.
Abbreviations: CI, confidence interval; df, degrees of freedom; IV, independent variable; SD, standard deviation.
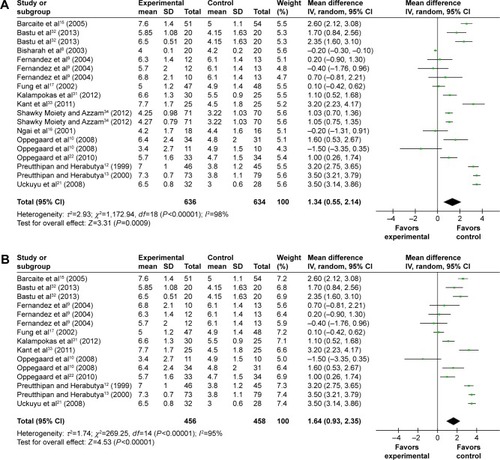
Figure 6 Comparison of the cervical width prior to hysteroscopy between the misoprostol group and the placebo or no medication group.
Abbreviations: CI, confidence interval; df, degrees of freedom; IV, independent variable; SD, standard deviation.
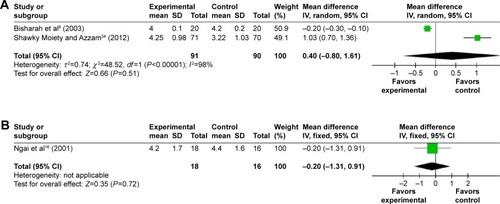
Figure 7 Comparison of the cervical width prior to hysteroscopy between the misoprostol group and the placebo or no medication group.
Abbreviations: CI, confidence interval; df, degrees of freedom; IV, independent variable; SD, standard deviation.
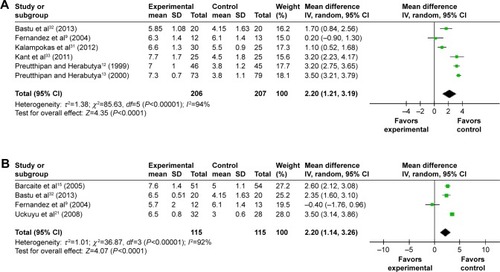
Figure 8 Comparison of the cervical width prior to hysteroscopy between the misoprostol group and the placebo or no medication group.
Abbreviations: CI, confidence interval; df, degrees of freedom; IV, independent variable; SD, standard deviation.
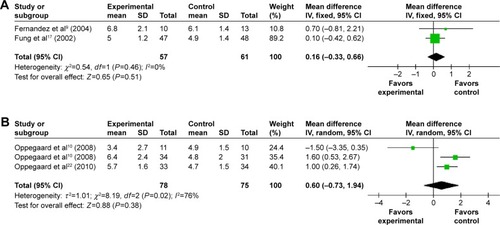
Complication of hysteroscopy
There was no significant difference between the misoprostol group and the placebo or no medication group when assessing the uterine perforation rate. However, the analysis of 14 trials, including 1,358 females, showed that the use of misoprostol prior to hysteroscopy resulted in a statistically significant decrease in the rate of cervical lacerations compared to placebo or no medication. When analyzing false passage, the risk was also significantly lower in the misoprostol group. All effect estimates for the above hysteroscopy complications with 95% CIs and P-values are shown in .
Table 2 Effect estimates on complications of hysteroscopy and side effects of misoprostol
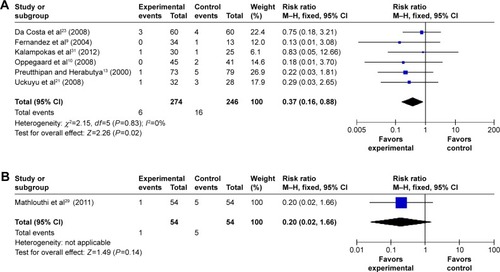
In addition, compared with placebo or no medication, hysteroscopy complications (cervical lacerations and false passage) after vaginal misoprostol (RR 0.36; 95% CI, 0.19–0.66; ten trials, 848 patients in ; RR 0.37; 95% CI, 0.16–0.88; six trials, 520 patients in ) administration were significantly decreased, but not after sublingual and oral (RR 0.48; 95% CI, 0.22–1.03; four trials, 381 patients in ; RR 0.2; 95% CI, 0.02–1.66; one trial, 54 patients in ) misoprostol administration.
Figure 9 The complication of hysteroscopy: cervical laceration in the misoprostol group compared to the placebo or no medication group.
Abbreviations: CI, confidence interval; df, degrees of freedom; M–H, Mantel–Haenszel.
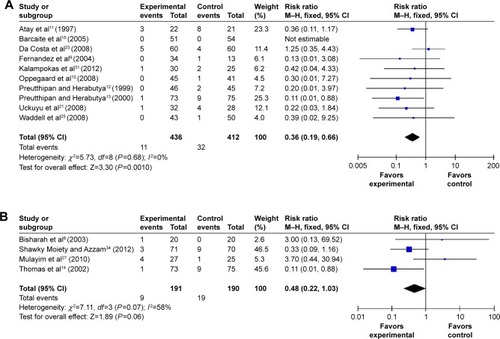
Figure 10 The complication of hysteroscopy: false passage in the misoprostol group compared to the placebo or no medication group.
Abbreviations: CI, confidence interval; df, degrees of freedom; M–H, Mantel–Haenszel.
Side effects of misoprostol
The pooled analysis ruled out that misoprostol side effects such as mild abdominal pain, bleeding, nausea, diarrhea, and fever were significantly more frequent in the misoprostol group compared with placebo or no medication. These side effects were generally minor, transient, and tolerable without the need for further treatment. All the patients were discharged on the day of the procedure.
All effect estimates for misoprostol side effects with 95% CIs and P-values are shown in .
Discussion
This meta-analysis indicates that misoprostol prior to hysteroscopy may facilitate cervical dilatation. Misoprostol, when given vaginally, was more effective when compared with oral and sublingual administration. The mean cervical width was significantly larger in the misoprostol group. In addition, hysteroscopy complications such as cervical laceration and false passage were significantly less frequent in the misoprostol group with the exception of uterine perforation.
The main outcome such as cervical width has a high degree of heterogeneity (I2=98% ) that could not be explained by either subgroup analysis or sensitivity analysis because of clinical diversity, including different populations under study, different regimens, doses, time intervals, and administration routes of misoprostol. However, when only patients pretreated with misoprostol vaginally were examined, the cervical width was significantly larger in the misoprostol group. Furthermore, the subgroup analysis indicated that the lower doses of 200 or 400 μg vaginal misoprostol produced a more beneficial effect in the outcome of cervical width than the higher doses. Therefore, this statistical heterogeneity is mainly attributed to the different degree of beneficial effect of misoprostol on the final outcome, rather than the lack of effect of misoprostol in several of the trials.
Because the type of hysteroscopy is closely associated with the diameter of cervical dilatation, we conducted a subgroup analysis based on the type of hysteroscopy. When only diagnostic hysteroscopy was examined, there appeared to a lower need for cervical dilation, but this did not reach statistical significance. However, it appeared that females receiving misoprostol prior to operative hysteroscopy were more likely to avoid the need for cervical dilation. Thus, misoprostol appears to be more beneficial for operative hysteroscopy.
The route of misoprostol administration for cervical dilatation can be oral, vaginal, or sublingual. Among the three routes, vaginal administration has higher bioavailability,Citation35 less severe gastrointestinal side effects, and longer sustained effect.Citation36 Batukan et al found that vaginal administration was more effective than the oral route for preoperative cervical ripening,Citation37 while other studies found no difference between the two routes,Citation38 or among the three routes.Citation39 In the present study, compared with the placebo or no medication group, the need for cervical dilatation, the mean cervical width, and hysteroscopy complications (cervical laceration and false passage) after vaginal misoprostol administration reached statistical significance, but they did not after sublingual and oral misoprostol administration. Therefore, the vaginal route appeared to be superior to the oral or sublingual routes.
To determine the optimal doses of vaginal misoprostol administration, we performed another subgroup analysis. Compared with the placebo or no medication group, the mean cervical width after vaginal misoprostol administration was significantly greater in the 200 and 400 μg subgroups, while in the 800 and 1,000 μg subgroups, the mean cervical width was not significantly different. Therefore, 200 or 400 μg of vaginal misoprostol prior to hysteroscopy is the optimal regimen.
It should be pointed out that all the misoprostol side effects such as diarrhea, fever, nausea, mild abdominal pain, and bleeding are significantly increased after the use of misoprostol. However, these side effects are generally minor, transient, and well tolerated by patients. Misoprostol side effects are related to dosage, interval, and route of administration. Increasing the dose and interval of vaginal misoprostol does not improve the effect on cervical dilatation but does increase the side effects.Citation28 In addition, misoprostol, when administered vaginally, has fewer side effects compared with oral or sublingual administration.Citation15,Citation38,Citation40
Compared with the meta-analysis by Polyzos et alCitation41 and Gkrozou et alCitation42 our meta-analysis identified 25 eligible studies that included more RCT studies. They had different emphasis such as menopausal status. Polyzos et al concluded that misoprostol may have a role as a cervical-ripening agent prior to hysteroscopy, and the efficacy of misoprostol is related to the menopausal status of patients.Citation41 Whereas our meta-analysis shows that the efficacy of misoprostol is related to the type of hysteroscopy and route of administration. Although Gkrozou et al concluded that neither the need for cervical dilatation nor the complication of hysteroscopy was different between the misoprostol group and the placebo group.Citation42 Our meta-analysis shows that females may experience substantial benefits after pretreatment with misoprostol, especially prior to operative hysteroscopy and vaginal administration.
However, it is a fact that, although there have been 25 RCTs published to date, the heterogeneity among the regimens, doses, time intervals, and route of administration makes analysis of the data very difficult. Ultimately, it prevented us from providing a solid guideline regarding the optimal schedule of misoprostol administration, especially in patients who differ in terms of parity (nulliparous or parous), means of delivery (vaginal delivery or cesarean section), and estrogen status (pre- or postmenopausal period). Future RCTs covering more study subjects from carefully selected populations and a uniform administration route and dosage schedule of misoprostol should be performed to identify the ideal conditions for the use of misoprostol prior to hysteroscopy.
Conclusion
The use of misoprostol prior to hysteroscopy may facilitate cervical dilatation and decrease hysteroscopy complications (cervical laceration and false passage). On the other hand, the side effects of misoprostol were relatively mild and insignificant. Our meta-analysis recommends for obstetricians and therapists that the regimen of 200 or 400 μg vaginal misoprostol may be optimal, especially prior to operative hysteroscopy.
Acknowledgments
This work was sponsored by the Zhejiang Provincial Program for the Cultivation of High-Level Innovative Health Talents. The study sponsors had no involvement in the collection, analysis, and interpretation of data, or in the writing of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- BirinyiLDaragóPTörökPPredictive value of hysteroscopic examination in intrauterine abnormalitiesEur J Obstet Gynecol Reprod Biol20041151757915223169
- WortmanMDaggettABallCOperative hysteroscopy in an office-based surgical setting: review of patient safety and satisfaction in 414 casesJ Minim Invasive Gynecol2013201566323107759
- ValleRFSciarraJJCurrent status of hysteroscopy in gynecologic practiceFertil Steril1979326619632389675
- BradleyLDComplications in hysteroscopy: prevention, treatment and legal riskCurr Opin Obstet Gynecol200214440941512151831
- LinCJChienSCChenCPThe use of misoprostol in termination of second-trimester pregnancyTaiwan J Obstet Gynecol201150327528222030039
- ThaisomboonARussameecharoenKWanitpongpanPPhattanachindakunBChangnoiAComparison of the efficacy and safety of titrated oral misoprostol and a conventional oral regimen for cervical ripening and labor inductionInt J Gynaecol Obstet20121161131621959071
- BlanchardKClarkSWinikoffBGainesGKabaniGShannonCMisoprostol for women’s health: a reviewObstet Gynecol200299231633211814515
- BisharahMAl-FozanHTulandiTA randomized trial of sublingual misoprostol for cervical priming before hysteroscopyJ Am Assoc Gynecol Laparosc200310339039114567819
- FernandezHAlbyJDTournouxCVaginal misoprostol for cervical ripening before operative hysteroscopy in pre-menopausal women: a double-blind, placebo-controlled trial with three dose regimensHum Reprod20041971618162115155607
- OppegaardKSNesheimBIIstreOQvigstadEComparison of self-administered vaginal misoprostol versus placebo for cervical ripening prior to operative hysteroscopy using a sequential trial designBJOG20081155663e1e918201279
- AtayVDuruNKPabuccuRErgünATokacGAydinBAVaginal misoprostol for cervical dilatation before operative office hysteroscopyGynaecol Endosc1997614749
- PreutthipanSHerabutyaYA randomized controlled trial of vaginal misoprostol for cervical priming before hysteroscopyObstet Gynecol199994342743010472872
- PreutthipanSHerabutyaYVaginal misoprostol for cervical priming before operative hysteroscopy: a randomized controlled trialObstet Gynecol200096689089411084173
- ThomasJALeylandNDurandNWindrimRCThe use of oral misoprostol as a cervical ripening agent in operative hysteroscopy: a double-blind, placebo-controlled trialAm J Obstet Gynecol2002186587687912015500
- BarcaiteEBartuseviciusARailaiteDRNadisauskieneRVaginal misoprostol for cervical priming before hysteroscopy in perimenopausal and postmenopausal womenInt J Gynaecol Obstet200591214114516102766
- NgaiSWChanYMHoPCThe use of misoprostol prior to hysteroscopy in postmenopausal womenHum Reprod20011671486148811425834
- FungTMLamMHWongSFHoLCA randomized placebo-controlled trial of vaginal misoprostol for cervical priming before hysteroscopy in postmenopausal womenBJOG2002109556156512066947
- HealeySButlerBKumFNDunneJHutchensDCraneJMA randomized trial of oral misoprostol in premenopausal women before hysteroscopyJ Obstet Gynaecol Can200729864865217714618
- HigginsJPTDouglasGAAssessing risk of bias in included studiesHigginsJPTSallyGCochrane Handbook for Systematic Reviews of InterventionsChichesterJohn Wiley & Sons Ltd2008188235
- HigginsJPThompsonSGQuantifying heterogeneity in a meta-analysisStat Med200221111539155812111919
- UckuyuAOzcimenEESevincFCZeynelogluHBEfficacy of vaginal misoprostol before hysteroscopy for cervical priming in patients who have undergone cesarean section and no vaginal deliveriesJ Minim Invasive Gynecol200815447247518588848
- OppegaardKSLiengMBergAIstreOQvigstadENesheimBIA combination of misoprostol and estradiol for preoperative cervical ripening in postmenopausal women: a randomized controlled trialBJOG20101171536120002369
- Da CostaARPinto-NetoAMAmorimMPaivaLHScavuzziASchettiniJUse of misoprostol prior to hysteroscopy in postmenopausal women: a randomized, placebo-controlled clinical trialJ Minim Invasive Gynecol2008151677318262147
- ValenteEPde AmorimMMCostaAAde MirandaDVVaginal misoprostol prior to diagnostic hysteroscopy in patients of reproductive age: a randomized clinical trialJ Minim Invasive Gynecol200815445245818539093
- WaddellGDesindesSTakserLBeaucheminMCBessettePCervical ripening using vaginal misoprostol before hysteroscopy: a double-blind randomized trialJ Minim Invasive Gynecol200815673974418971139
- SinghNGhoshBNahaMMittalSVaginal misoprostol for cervical priming prior to diagnostic hysteroscopy – efficacy, safety and patient satisfaction: a randomized controlled trialArch Gynecol Obstet20092791374018449549
- MulayimBCelikNYOnalanGBagisTZeynelogluHBSublingual misoprostol for cervical ripening before diagnostic hysteroscopy in premenopausal women: a randomized, double blind, placebo-controlled trialFertil Steril20109372400240419243750
- El-MaznyAAbou-SalemNA double-blind randomized controlled trial of vaginal misoprostol for cervical priming before outpatient hysteroscopyFertil Steril201196496296521575939
- MathlouthiNSaodiOBen TemimeRMakhloufTAttiaLChachiaASublingual misoprostol for cervical ripening before diagnostic hysteroscopy: a randomized and prospective study about 108 casesTunis Med2011891182582922179917
- Sordia-HernándezLHRosales-TristanEVazquez-MendezJEffectiveness of misoprostol for office hysteroscopy without anesthesia in infertile patientsFertil Steril201195275976120728083
- KalampokasESofoudisCAntonogeorgosGA randomized controlled trial for cervical priming using vaginal misoprostol prior to hysteroscopy in women who have only undergone cesarean sectionArch Gynecol Obstet2012286485385722592929
- BastuECelikCNehirADoganMYukselBErgunBCervical priming before diagnostic operative hysteroscopy in infertile women: a randomized, double-blind, controlled comparison of 2 vaginal misoprostol dosesInt Surg201398214014423701149
- KantADivyakumarPriyambadaUA randomized trial of vaginal misoprostol for cervical priming before hysteroscopy in postmenopausal womenJ Midlife Health201121252721897735
- Shawky MoietyFMAzzamAProstaglandins prior to hysteroscopyGynecol Surg201291169173
- TangOSGemzell-DanielssonKHoPCMisoprostol: pharmacokinetic profiles, effects on the uterus and side-effectsInt J Gynaecol Obstet2007992S160S16717963768
- TangOSSchweerHSeyberthHWLeeSWHoPCPharmacokinetics of different routes of administration of misoprostolHum Reprod200217233233611821273
- BatukanCOzgunMTOzcelikBAygenESahinYTurkyilmazCCervical ripening before operative hysteroscopy in premenopausal women: a randomized, double-blind, placebo-controlled comparison of vaginal and oral misoprostolFertil Steril200889496697317681307
- ChoksuchatCCheewadhanaraksSGetpookCWootipoomVDhanavoravibulKMisoprostol for cervical ripening in non-pregnant women: a randomized double-blind controlled trial of oral versus vaginal regimensHum Reprod20062182167217016585121
- LeeYYKimTJKangHThe use of misoprostol before hysteroscopic surgery in non-pregnant premenopausal women: a randomized comparison of sublingual, oral and vaginal administrationsHum Reprod20102581942194820542898
- TanhaFDSalimiSGhajarzadehMSublingual versus vaginal misoprostol for cervical ripening before hysteroscopy: a randomized clinical trialArch Gynecol Obstet2013287593794023208460
- PolyzosNPZavosAValachisAMisoprostol prior to hysteroscopy in premenopausal and post-menopausal women. A systematic review and meta-analysisHum Reprod Update201218439340422544173
- GkrozouFKoliopoulosGVrekoussisTA systematic review and meta-analysis of randomized studies comparing misoprostol versus placebo for cervical ripening prior to hysteroscopyEur J Obstet Gynecol Reprod Biol20111581172321621897