Abstract
Aim
The aim of this study was to investigate the possibility of using 99mtechnetium (99mTc)-labeled tirofiban (a reversible antagonist of glycoprotein IIb/IIIa) for detection of deep venous thrombosis (DVT) in rats without causing an antiplatelet effect.
Methods
The ability of in vitro tirofiban to inhibit adenosine 5′-diphosphate (ADP)-induced platelet aggregation was evaluated using optical aggregometer. Binding of 99mTc-tirofiban to platelets was evaluated. Serum levels of unlabeled (a validated high performance liquid chromatography method) and 99mTc-tirofiban after single intravenous injection were evaluated in male Wistar rats with or without induced DVT (femoral vein ligation model), and the rats were also subjected to whole body scintigraphy.
Results
Tirofiban in vitro inhibits ADP-induced aggregation of human platelets in a dose- and concentration-dependent manner (10 nM to 2 μM), but only if it is added before ADP and not after ADP. 99mTc labeling did not affect the ability of tirofiban to bind to either human or rat platelets, nor did it affect tirofiban pharmacokinetics in intact rats or in animals with induced DVT. When 99mTc-tirofiban was injected to rats after induction of DVT, at a molar dose lower than the one showing only a weak antiaggregatory effect in vitro, whole body scintigraphy indicated localization of 99mTc-tirofiban around the place of the induced DVT.
Conclusion
99mTc labeling of tirofiban does not affect its ability to bind to glycoprotein IIb/IIIa or its in vivo pharmacokinetics in rats, either intact or with DVT. A low, nonantiaggregatory dose of 99mTc-tirofiban may be used to visualize DVT at an early stage.
Introduction
Platelets play an important role in pathological thrombus formation, particularly within atherosclerotic arteries subjected to a high shear stress,Citation1 and in the regulation of immune responses, cancer metastasis, vascular development, and angiogenesis.Citation2–Citation4 Binding of plasma fibrinogen to activated platelet glycoprotein (GP) IIb/IIIa receptors is a prerequisite and an important event in platelet aggregation and therefore in thrombus formation, regardless of the type of platelet stimulus. One way to inhibit platelets is to block the platelet membrane GPIIb/IIIa receptor, which binds circulating fibrinogen or von Willebrand factor and cross-links platelets at the final common pathway to platelet aggregation.Citation5–Citation7
Tirofiban (N-(methylsulfonyl)-4-O-(4-(4-piperidinyl)-L-tyrosine) is a nonpeptide derivative of tyrosine, highly selective, short-acting inhibitor of fibrinogen binding to the platelet GPIIb/IIIa receptor. As a molecule with a small molecular weight and simple chemical structure, tirofiban provides the possibility to be used as a radiolabeled potential radiopharmaceutical to inject in the patient without the risk of the occurrence of immune reactions after administration. The size and structure are also important parameters for its fast elimination from the body. Tirofiban binds GPIIb/IIIa receptors in the same way as fibrinogenCitation8,Citation9 and can be seen only in the active clot. This phenomenon indicates the difference between acute and chronic blood clot and can be used as an advantage for vizualization.
When administered intravenously, tirofiban inhibits ex vivo platelet aggregation in a dose- and concentration- dependent manner. The extent of this inhibition is proportional to the concentration of tirofiban in plasma.Citation10–Citation12 In combination with heparin, it is used for treatment of acute coronary syndrome (in patients who are to be managed medically or those undergoing angiography/coronary intervention).Citation13,Citation14 Platelet aggregation inhibitionCitation6 is reversible following cessation of the infusion of tirofiban. Tirofiban also activates growth-stimulatory signals in the endothelium.Citation8 As a nonpeptide tyrosine derivative, tirofiban has a rapid onset and short duration of action when administered intravenously, with a half-life of ~2 hours. This qualifies it as a potential radiopharmaceutical for the detection of deep venous thrombosis (DVT) with more desirable properties compared to other platelet aggregation inhibitors, eg, monoclonal antibodies (abciximab) and peptides containing Arg–Gly–Asp (RGD) or Lys–Gly–Asp (KGD) sequences (eptifibatide), which have been developed in the recent years. With tirofiban, 90% inhibition of platelet aggregation is achieved by the end of a 30-minute infusion.Citation15,Citation16 Tirofiban is cleared from the plasma largely via kidneys. The plasma protein binding is concentration independent (over the range of 0.01–25 μg/mL), with the unbound fraction of ~35%. The steady-state volume of distribution ranges from 22 L to 42 L.Citation15–Citation17
The idea to formulate radioactive tirofiban using 99mtechnetium (99mTc) as a radioisotope for labeling and for diagnostic purpose was generated following the demand to detect primary pathological deviations in the blood vessels, to identify the localization of the new fresh thrombus, and to define its morphological characteristics.
Tirofiban antiplatelet action is of a great potential value in experimental medicine, particularly its potential use as an imaging radiopharmaceutical. In a previous study, we developed and validated a high performance liquid chromatography (HPLC) method with ultraviolet (UV) detection for determination of tirofiban in serum.Citation18 In the present study, we aimed to verify whether the introduction of radioactive technetium in the molecule of tirofiban will lead to changes in the affinity of tirofiban to bind the GPIIb/IIIa receptors and to identify the in vitro concentration of tirofiban with minimal antiplatelet activity that could be converted into an in vivo dose of 99mTc-tirofiban and use it to visualize early DVT in the rat.
Methods
Ethics
This study was approved by the ethics committee of the Faculty of Natural Sciences and Mathematics, Ss Cyril and Methodius University in Skopje. All animal experiments were conducted in accordance with the law and guidelines on the protection and welfare of animals and the law for the ratification of the European Convention for the Protection of Vertebrate Animals used for experimental and other scientific purposes. Human blood used for in vitro analysis was obtained from healthy volunteers who provided a signed informed consent.
Experimental animals
We used healthy male Wistar rats (six per experimental group), because of their sensitivity and behavioral similarities as in humans, weighing 225–250 g, and kept six per cage, at 22°C, under a 12:12 hours light–dark schedule with food and water ad libitum.
Chemicals
Tirofiban hydrochloride was purchased from Merck Sharpe&Dhome (USA) (Aggrastat®, batch no L-000700462-006X027), adenosine 5′-diphosphate (ADP) was purchased from Sigma-Aldrich Co. (St Louis, MO, USA), methanol and acetonitrile of HPLC grade were obtained from Sigma-Aldrich Co., and all other reagents were of analytical grade. Redistilled water was used to prepare the HPLC mobile phase solutions. 99mTc was obtained from a Mo99/Tc99m generator system ELUMATIC III-CIS-bio (IBA Molecular, France) 16 GBq as a solution of sodium pertechnetate in 0.9% sodium chloride with a specific activity of 370–555 MBq/5 mL.
Preparation of human and rat platelets
Rat blood samples were collected from the carotid vein using a syringe containing 3.3% (w/v) sodium citrate. The ratio of blood and sodium citrate was 10:1 in order to obtain effective separation of the platelets and to prevent coagulation. Following the incubation period, blood was mounted on a hemacytometer. The cells were allowed to settle and were counted in a specific area of the hemacytometer chamber under a microscope. Platelet numbers varied from 3.6×108/mL to 5.4×108/mL. Blood from five healthy volunteers was obtained from the cubital vein using a syringe containing 3.3% (w/v) sodium citrate. The ratio of blood, sodium citrate, and hydroxyethyl starch was 5:1:0.5 in order to obtain effective separation of the platelets and to prevent coagulation. To prepare washed platelets, platelet-rich plasma (PRP) suspension was acidified to pH 6.5 with 1 M citric acid; the sample was centrifuged at 1,500× g for 20 minutes to obtain pellets, which were resuspended in a Ca2+-free Tyrode Hepes buffer (152 mM NaCl, 2.8 mM KCl, 8.9 mM NaHCO3, 0.8 mM KH2PO4, 0.8 mM MgCl2, 5.6 mM glucose, apyrase [2 U/mL], 10 μM EDTA, BSA [3.5 mg/mL], and 10 mM Hepes, pH 6.5). Platelets were washed once with the above buffer and finally suspended in the same buffer with the exception that, in the final suspension medium, apyrase and EDTA were omitted, and pH was adjusted to 7.4. Platelet concentration was standardized to 2–3×108 cells/mL by dilution with Tyrode Hepes buffer.
In vitro evaluation of platelet aggregation
Platelet aggregation was assessed using a Carat TX4 optical platelet aggregometer (Carat Diagnostic Ltd, Hungary) as the percent change in light transmission (over 5 minutes, maximum voltage 25 mA). Platelet mass (450–475 μL per assay) was prepared from saline-washed PRP (to preserve isotonicity and prevent interference of cell-free plasma) using acid citrate dextrose (ACD; ACD-to-PRP ratio 1%:10%) with the addition of 10% calcium chloride (final Ca2+ concentration in the medium 100 mM) to immobilize ACD. Spontaneous aggregation was assessed using platelet poor plasma as a control without platelet count adjustments. Induced aggregation was assessed by adding ADP (in a volume of 25 μL) at concentrations of 1.0 μM, 10 μM, or 100 μM. The procedure was carried out in three phases: 1) preparation of the baseline by determination of the “0” point voltage measuring cell-free plasma, 2) voltage measurement of PRP in order to get the maximum voltage, and 3) determination of the stimulated aggregation.Citation19 Inhibition by tirofiban (concentration range 10 nM to 2 μM) of platelet aggregation induced by 10 μM ADP was performed with cell-free plasma as a control.Citation20–Citation22 The process of aggregation was assessed by an optical aggregometer as voltage change (%) indicating the presence of formed aggregates as a function of time.
Freeze drying preparation of ready to use kit of the radiopharmaceutical formulation
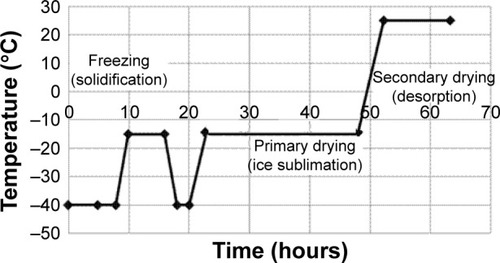
Freeze drying preparation of ready to use kit of tirofiban formulation was performed in a vacuum and nitrogen atmosphere (Labconco Freezone Stoppering Tray Dryer, USA). Tirofiban makes a stable complex with diethylene triamine pentaacetic acid.Citation23,Citation24 Tin chloride was used as a reducing agent for 99mTc. The ratio of tirofiban and tin chloride was 1:2. Freeze drying process started with direct freezing at an initial temperature of −40°C. The whole process lasted 23 hours, including a diagram of the program (), which had a primary freezing for 1 hour at −4°C, and then the temperature was increased from −40°C to −15°C by 0.20°C/min, once again cooled to −40°C and kept for 2 hours. Primary drying was conducted at −10°C with a heating speed of 0.15°C/min, while secondary drying was done at 25°C with a heating speed of 0.15°C/min and a vacuum of 0.280 Torr. The finished product was stored well closed at 2°C–6°C and used for labeling with 99mTc.
Labeling of tirofiban with 99mTc
Labeling of tirofiban with technetium in the form of pertechnetate (99mTcO4−) with the activity of 10–15 mCi (370–555 MBq) was carried out using the method of direct labeling under nitrogen, in sterile conditions, on the freeze-dried preparation. The incubation mixture was left over 15 minutes at room temperature with occasional stirring. The quality of the labeled product was tested using instant thin layer chromatography, and the percentage of the yield was always >95. Specific activity of the final product was 370–555 MBq/mL.
In vitro binding of 99mTc-tirofiban to rat and human platelets
Apart from citrate contained in the blood-withdrawal tubes, no other anticoagulant was used. Blood was centrifuged for 10 minutes at 1,500× g, and PRP was separated. 99mTc-tirofiban with the activity of 1.7×105 cpm (in a volume of 40 μL) and 0.9% NaCl (810 μL) were added to 1 mL of PRP (2–3×108 cells/mL; counted manually as described), and the mixture was incubated over 10 minutes at room temperature and centrifuged again to remove the unbound radioactivity. Unbound radioactivity was further removed by washing the platelets twice with 0.9% NaCl (centrifugation after each washing), and platelet-bound radioactivity was measured using a dose calibrator. All experiments were done in triplicate.
Experimental model of DVT
Rats were randomly assigned to undergo venous thrombosis induction by ligation of the femoral vein (n=18)Citation13,Citation18 under anesthesia (thiopental sodium 20 mg/kg bw, intraperitoneally) or to serve as controls (n=18). Hypercoagulability of blood was achieved by intravenous administration of 0.2 mL of tissue thrombin.Citation25,Citation26
Serum concentrations of unlabeled and 99mTc-tirofiban in rats with and without DVT
Animals were anesthetized throughout these 60-minute experiments. Unlabeled tirofiban hydrochloride (in 0.9% NaCl) was injected as iv (tail vein) bolus dose (0.6 mg/kg), and blood was withdrawn (0.3 mL into heparinized tubes) in 15-minute intervals. Samples were centrifuged at 3,500 rpm for 5 minutes, methanol was added (serum:methanol 1:3, v/v) for protein precipitation, and samples were centrifuged once again at 3,500 rpm over 5 minutes. The obtained serum was analyzed for tirofiban concentrations using a previously developed HPLC method with UV detection.Citation18,Citation27 In brief, we used a Perkin Elmer Series 200 chromatographic system. The analyte separation was carried out at ambient temperature on a reversed-phase LiChrospher® 100 RP-18 column (4.0×250 mm, 5 μm particle size) (Merck Millipore, Billerica, MA, USA) using the isocratic mode. The mobile phase consisted of a mixture of 0.1 M KH2PO4 (pH 5.0, adjusted with 1.0 N sodium hydroxide solution) and acetonitrile in the ratio of 80%:20% (v/v) with a flow rate of 1.0 mL/min. The UV detector was set at a wavelength of 274 nm.18,27 99mTc-tirofiban was injected as a bolus iv dose (3–4×104 cpm in a volume of 50–100 μL; corresponding to 2.0 nmol of tirofiban), and blood samples were taken in the same way as with unlabeled tirofiban.
Imaging studies
Imaging studies were completed after iv administration of 99mTc-tirofiban (3–4×104 cpm in a volume of 50–100 μL, corresponding to 2.0 nmol of tirofiban) using a gamma camera with high resolution (“Sopha Medical” SPECT camera with a single detector, 64 positions, 30 steps, 64×64 bits, and rotation of 360°)Citation28 at 1 hour and 6 hours after injection. Rats were anesthetized (thiopental sodium) throughout the study.
Data analysis
Data were expressed as mean ± standard deviation. Kolmogorov–Smirnov test and Shapiro–Wilk’s W test were used to identify the distribution of variables. Nonparametric statistical methods were used for the heterogeneous variables. Mann–Whitney U-test was used to compare data on 99mTc-tirofiban binding to human and rat platelets. Friedman ANOVA and Wilcoxon matched pairs test for dependent samples and Mann–Whitney U-test for independent samples were used to compare data on the mean serum concentrations of unlabeled and of 99mTc-tirofiban over 60 minutes after intravenous injection in rats without or with induced DVT. A P-value of <0.05 was considered statistically significant.
Kinetica 4.1 (InnaPhase Corp) was used to calculate elimination half-lives (by noncompartmental method) of unlabeled and 99mTc-tirofiban. Scintigraphic images were processed by computer program “Sopha Medical,” which is a part of the gamma camera.
Results
Effect of tirofiban in vitro on ADP-induced aggregation of human platelets
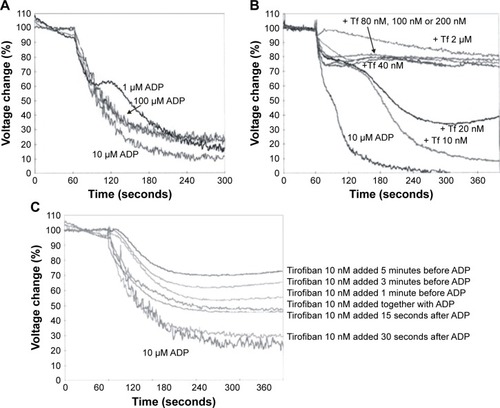
Addition of ADP (1 μM, 10 μM, or 100 μM) to human platelets in vitro (2.41–2.48×108 cells/mL) induced platelet aggregation, most profoundly at 10 μM (). Pretreatment of the platelets with tirofiban (10 nM to 2 μM) concentration dependently inhibited the effect of 10 μM of ADP (), with the lowest concentration of tirofiban (10 nM) producing only a slight effect. Moreover, the inhibitory effect of 10 nM of tirofiban existed only when it was added before ADP (1–5 minutes before) or at the same time with ADP, but not when it was added after ADP (practically no inhibition was observed when added 30 seconds after ADP) (). This indicated that an in vivo dose of tirofiban that would yield systemic concentrations ≤10 nM and particularly if injected after induction of DVT would not produce any relevant antiaggregatory effect.
Figure 2 Effect of in vitro Tf on ADP-induced aggregation of human platelets.
Abbreviations: ADP, adenosine diphosphate; Tf, tirofiban.
99mTc labeling does not prevent binding of tirofiban to human or rat platelets in vitro
After 10 minutes incubation of the rat or human platelets (2–3×108 cells/mL) with 99mTc-labeled tirofiban (nominal activity 1.7×105 cpm; actually added to rat platelets [n=6] 173,785–180,991 cpm; and actually added to human platelets [n=6] 171,349–182,006 cpm) and after thorough washing (twice) of the platelets with 0.9% NaCl, 59.6%±2.1% and 57.4%±1.6% of the added radioactivity, respectively (P=0.065 for the difference between human and rat platelets), were recovered bound to platelets indicating that 99mTc labeling did not prevent binding of tirofiban to platelets.
99mTc labeling does not affect the decline of the systemic tirofiban concentrations
Serum concentrations of both the unlabeled tirofiban (0.6 mg/kg) () and 99mTc-tirofiban (3–4×104 cpm in a volume of 50–100 μL, corresponding to 2.0 nmol of tirofiban) () similarly rapidly declined upon intravenous injections, comparably so in animals with DVT and without DVT.
Figure 3 Mean serum concentrations of unlabeled (A) and of 99mTc-tirofiban (B) over 60 minutes after intravenous injection in rats without or with induced DVT.
Abbreviation: DVT, deep venous thrombosis.
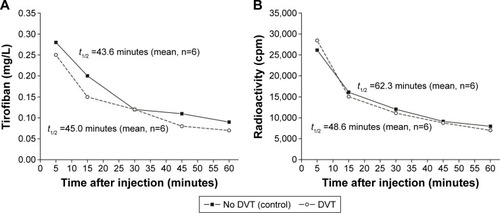
There are statistically significant differences between mean concentrations of tirofiban in serum (mg/L), in the group of rats with DVT (N1) (Friedman ANOVA chi-square =30.06, P<0.001) and in the group of rats without DVT (N2) (Friedman ANOVA chi-square =28.38, P<0,001), within the specified time intervals, after injected dose of 0.6 mg/kg. In the studied group of rats without DVT (N2), no significant difference between the concentrations of tirofiban in serum was observed only after 45 minutes and 60 minutes (Wilcoxon matched pairs test: P=0.2736). According to Mann–Whitney U-test (between groups N1 and N2), the difference is not significant between the measured concentrations, only after 30 minutes (P=0.6359) ().
Regarding the elimination of nonradioactive tirofiban between the groups (N1 and N2), there is no significant difference (P=0.1370). Regarding the elimination of radioactive tirofiban between the groups (N1 and N2), the differences are significant for P=0.0001 (significantly longer is the time of radioactivity in the group without DVT) ().
Whole body scintigraphy
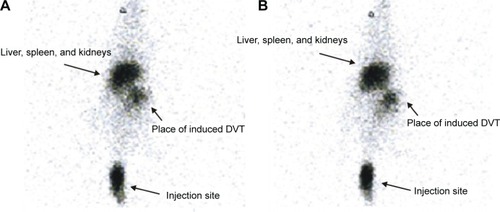
At 1 hour and 6 hours after an iv injection of 99mTc-tirofiban (tail vein) (3–4×104 cpm in a volume of 50–100 μL, corresponding to 2.0 nmol of tirofiban), radioactivity accumulated at the site of the induced DVT ().
Figure 4 Whole body scintigraphy at 30 minutes (A) and at 60 minutes (B) after injection (tail vein) of 99mTc-tirofiban (3–4×105 cpm, corresponding to 2 nmol of tirofiban) to rats with induced DVT.
Abbreviation: DVT, deep venous thrombosis.
Discussion
In a strict sense, DVT refers specifically to thrombosis of the deep veins of the lower extremities, where thrombosis of the proximal veins is of a particular interest as a risk factor for the development of pulmonary embolism. Therefore, timely diagnosis and treatment is essential.Citation29,Citation30 Since ascending contrast venography, a gold standard for DVT diagnosis, is technically demanding, rather expensive and associated with complications, alternative strategies have been developed that combine clinical judgment (Wells’ score), laboratory findings (d-dimer), and noninvasive imaging (ultrasound). However, the diagnostic algorithm is rather complex with still a considerable level of uncertainty.Citation31 Imaging methods based on radiolabeled peptides or peptidomimetics that bind to specific platelet receptors have long been suggested as a potentially feasible approach to the early diagnosis of thromboembolic events.Citation32,Citation33 The first peptide labeled with radioactive 99mTc was apticid, and attempts have been made to label abciximab as well as small peptidomimetics that recognize a specific fibrinogen-binding site through the RGD sequence (Arg-Gly-Asp), such as tirofiban and eptifibatide.Citation34,Citation35 For several reasons, in this study, we focused on DVT, tirofiban, and 99mTc: 1) DVT is the third most common cardiovascular disease with an annual incidence estimated at ~100–200/100,000 inhabitants and with a high recurrence rate;Citation29 2) tirofiban is a high-affinity platelet GPIIb/IIIa receptor antagonist with a convenient property for a potential diagnostic tool – it rapidly dissociates from its binding site and is rapidly eliminated, and its antiaggregation effect diminishes rapidly upon ending an infusion. Consequently, a bolus iv injection is not likely to affect platelet aggregation and thus compromises the diagnostic process and 3) 99mTc is an inexpensive, readily available radionuclide with appropriate gamma decay efficiently detected by a gamma camera. The present results demonstrate that 99mTc-labeled tirofiban retained two important features – the ability to bind to platelets (rat and human) and a rapid elimination after an iv bolus injection comparable to that of the unlabeled molecule. The present results are in-line with dose identified in rats and dogs during preclinical development of tirofiban.Citation36 Moreover, elimination kinetics apparently was not affected by the existence of DVT (a condition with “activated platelets” as opposed to intact animals). A certain limitation of the present observations lies with the fact that the potential effect of 99mTc labeling on the in vivo antiplatelet effect of tirofiban was not directly investigated. Also, the antiplatelet effect of the dose selected for imaging studies (2.0 nmol/rat) was not directly evaluated. However, in vitro studiesCitation37 clearly indicated only a slight inhibitory effect of tirofiban at a concentration of 10 nM on ADP-induced platelet aggregation. Considering the steady state volume of distribution of tirofiban in rats of ~360 mL in a 220–250 g weighing rat,Citation38 the selected dose was reasonably expected to yield blood concentrations <10 nM. Moreover, considering the molecular weight of tirofiban hydrochloride (495.1), the selected in vivo dose corresponded to1.0 μg/animal or ~4–5 μg/kg in a 220–250 g weighing rats. In humans, the antiplatelet effect of tirofiban is achieved by a bolus iv dose (over 5 minutes) of 25 μg/kg, followed by a prolonged infusion.Citation16 By alometric scaling, this loading human dose corresponds to 155 μg/kg in a rat. Therefore, it is highly unlikely that the administered dose of 99mTc-tirofiban had any antiplatelet effect. This is further substantiated by the fact that the imaging findings were comparable at 30 minutes (time of the first onset of antiplatelet effect)Citation16 and at 60 minutes after administration of 99mTc-tirofiban.Citation37
Conclusion
The present results strongly indicate that 99mTc labeling of tirofiban hydrochloride has no impact on its essential pharmacodynamics and pharmacokinetic properties (radioactive tirofiban has a similar pharmacokinetic profile as nonradioactive tirofiban) and that a low, “sub-therapeutic” dose of 99mTc-tirofiban could enable early diagnosis of DVT.
Disclosure
The authors report no conflicts of interest in this work.
References
- FusterVBadimonLBadimonJJChesebroJHThe pathogenesis of coronary artery disease and the acute coronary syndromesN Engl J Med199232642422501727977
- JagroopIAMikhailidisDPThe effect of Tirofiban on fibrinogen/agonist-induced platelet shape change and aggregationClin Appl Thromb Hemost200814329530218445610
- ChristieDJAvariTCarringtonLRPlatelet Function Testing by Aggregometry: Approved GuidelineCLSI Guideline H58-A1-56238-683-2Clinical and Laboratory Standards InstituteWayne, PA2008
- HaywardCPMoffatKARabADevelopment of North American consensus guidelines for medical laboratories that perform and interpret platelet function testing using light transmission aggregometryAm J Clin Pathol201013495596321088160
- LeslieMCell biology. Beyond clotting: the powers of plateletsScience2010328597856256420430990
- BrassLUnderstanding and evaluating platelet functionHematology Am Soc Hematol Educ Program2010201038739621239824
- PickerSMIn-vitro assessment of platelet functionTransfus Apheresis Sci2011443305319
- GiordanoAD’AngelilloARomanoSTirofiban induces VEGF production and stimulates migration and proliferation of endothelial cellsVascul Pharmacol2014612–3637124751361
- HashemzadehMFurukawaMGoldsberrySMovahedMRChemical structures and mode of action of intravenous glycoprotein IIb/IIIa receptor blockers: a reviewExp Clin Cardiol200813419219719343166
- JayasunderaTGFeldmanDNMarmurJDTirofiban-induced coronary thrombosisJ Invas Cardiol1999113138140
- LannettaLPudduPECuturelloDSaladiniAPellicanoMSchiaritiMIs there still a role for glycoprotein IIb/IIIa antagonists in acute coronary syndromes?Cardiol Res20134117
- KingSShortMHarmonCGlycoprotein IIb/IIIa inhibitors: the resurgence of tirofibanVascul Pharmacol201678101626187354
- BruntonLLLazoJSParkerKLGoodman and Gilman’s, The Pharmacological Basis of Therapeutics11th edNew YorkThe McGraw Hill Co2006
- KumarAHerrmannHCTirofiban: an investigational platelet glycoprotein IIb/IIIa receptor antagonistExpert Opin Investig Drugs19976912571267
- AnonTirofiban hydrochlorideDrugs Future199520897901
- http://www.accessdata.fda.gov/drugsatfda_docs/label/1998/20912lbl.pdfAccessed October 24, 2014
- Medicure PharmaAggrastat (Tirofiban Hydrochloride) Injection Premixed and Injection Prescribing InformationSomerset, NJMedicure Pharma2007
- Darkovska SerafimovskaMJanevik-IvanovskaEArsova-SarafinovskaZDjorgoskiIUgresicNDevelopment and validation of reverse phase high performance liquid chromatographic method for determination of tirofiban in serumInt J Pharm201444115120
- KulkarniSDopheideSMYapCLA revised model of platelet aggregationJ Clin Invest2000105678379110727447
- SmithJWSteinhublSRLincoffAMRapid platelet-function assay – an automated and quantitative cartridge-based methodCirculation19999956206259950658
- HolmesMBKubbaniSTerrienCSobelBSchneiderDQuantification by flow cytometry of the efficacy and interindividual variation of platelet inhibition induced by treatment with tirofiban and abciximabCoron Artery Dis200112324525311352081
- SimonDILiuCBGanzPA comparative study of light transmission aggregometry and automated bedside platelet function assay in patients undergoing percutaneous coronary intervention and receiving abciximab, eptifibrate, or tirofibanCatheter Cardiovasc Interv200152442543211285593
- Lister-JamesJMauerAThrombus imaging with a technetium 99m labeled, activated platelet receptor binding peptideJ Nucl Med1996213207
- PearsonDALister-JamesJMcBrideWJThrombus imaging using technetium-99m-labeled high-potency GPIIb/IIIa receptor antagonists. Chemistry and initial biological studiesJ Med Chem1996397137213828691467
- CallasJWFareedDDJA survey of animal models to develop new and novel antithrombotic agentsSasaharaAALoscalzoJLNew Therapeutic Agents in Thrombosis and ThrombolysisNew YorkMarcel Dekker1997928
- HerbertJMBernatAMaffrandJPImportance of platelets in experimental venous thrombosis in the ratBlood1992809228122861421399
- BougieDWWilkerPRWuitschickEDAcute thrombocytopenia after treatment with Tirofiban or eptifibatide is associated with antibodies specific for ligandoccupied GPIIb/IIIaJ Am Soc Hematol2002100620712076
- HardoffRBraegelmannFZanzonicoPExternal imaging of atherosclerosis in rabbits using an 123 I-labeled syntetic peptide fragmentJ Clin Pharmacol19933311103910478300886
- KonstantinidesSTorbickiAAgnelliGTask Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC)ESC Guidelines on the diagnosis and management of acute pulmonary embolismEur Heart J201435433033306925173341
- KearonCAklEComerotaAJAntithrombotic therapy for VTE diseaseChest20121412 supple419Se494S22315268
- National Institute for Health and Clinical ExcellenceVenous Thromboembolic Disease: the Management of Venous Thromboembolic Disease of Thrombophilia Testing. Clinical GuidelineMethods Evidence and RecommendationsLondon2012
- JanevicETadzerISTc-99m labelling of platelets using diethyldithiocarbamate or 2,5-dihydroxy-benzoic acidPeriod Biol198991409411
- SchneiderDJAnti-platelet therapy: glycoprotein IIbIIIa antagonistsBr J Clin Pharmacol201172467268221906121
- TrajkovskiRMilenkovVDukovskiRJanevicETadžerISObeležavanje humanih trombocita sa dietill-ditiokarbamat Tc-99m (DDC)Bilten Hemat Transf1989172125
- van RensburgWJRoodtJPLamprechtSMeiringSMBadenhorstPNTirofiban versus abciximab: tirofiban is administered at suboptimal dosages when evaluated in an arterial thrombosis model in non-human primatesClin Exp Med201212425726322219002
- YeungJHolinstatMNewer agents in antiplatelet therapy: a reviewJ Blood Med20123334222792011
- DarkovskaSMJanevik-IvanovskaEUgresicNDjorgoskiIImaging of deep venous thrombosis using radioactive-labeled tirofibanBratisl Med J2015116106216266
- VickersSTheoharidesADArisonBIn vitro and in vivo studies on the metabolism of tirofibanDrug Metab Dispos199927111360136610534322