Abstract
The pill burden of patients with hypertension and dyslipidemia can result in poor medication compliance. This study aimed to evaluate the efficacy and safety of fixed-dose combination (FDC) therapy with olmesartan medoxomil (40 mg) and rosuvastatin (20 mg) in Korean patients with mild to moderate hypertension and dyslipidemia. This multicenter, randomized, double-blind, factorial-design study included patients aged ≥20 years with mild to moderate essential hypertension and dyslipidemia. Patients were randomly assigned to receive FDC therapy (40 mg olmesartan medoxomil, 20 mg rosuvastatin), 40 mg olmesartan medoxomil, 20 mg rosuvastatin, or a placebo. The percentage change from baseline in low-density lipoprotein cholesterol levels was compared between FDC therapy and olmesartan medoxomil, and the change from baseline in diastolic blood pressure was compared between FDC therapy and rosuvastatin 8 weeks after treatment. A total of 162 patients were included. The least square mean percentage change (standard error) from baseline in low-density lipoprotein cholesterol levels 8 weeks after treatment was significantly greater in the FDC than in the olmesartan medoxomil group (−52.3% [2.8%] vs −0.6% [3.5%], P<0.0001), and the difference was −51.7% (4.1%) (95% confidence interval: −59.8% to −43.6%). The least square mean change (standard error) from baseline in diastolic blood pressure 8 weeks after treatment was significantly greater in the FDC group than in the rosuvastatin group (−10.4 [1.2] mmHg vs 0.1 [1.6] mmHg, P<0.0001), and the difference was −10.5 (1.8) mmHg (95% confidence interval: −14.1 to −6.9 mmHg). There were 50 adverse events in 41 patients (22.7%) and eight adverse drug reactions in five patients (2.8%). The study found that FDC therapy with olmesartan medoxomil and rosuvastatin is an effective, safe treatment for patients with hypertension and dyslipidemia. This combination may improve medication compliance in patients with a large pill burden.
Introduction
The coexistence of hypertension and dyslipidemia, which are central to the pathogenesis of coronary heart disease, has been reported to be prevalent.Citation1–Citation4 The risk of coronary heart disease with the coexistence of hypertension and dyslipidemia has been reported to be higher than the sum of the risks of coronary heart disease with each of the component factors.Citation4–Citation6 As cardiovascular risk factors interact with each other, comprehensive control of both blood pressure (BP) and blood cholesterol level is effective for reducing the risk of future cardiovascular events.Citation6,Citation7
In clinical practice, the pill burden in patients with both hypertension and dyslipidemia can result in poor adherence and persistence with the prescribed drugs.Citation8 A fixed-dose combination (FDC) of a BP-lowering agent and statin could improve adherence and persistence in patients with multiple risk factors, resulting in a reduction of the risks of future cardiovascular events.
In our previous study, the coadministration of olmesartan medoxomil (40 mg) and rosuvastatin (20 mg) did not significantly influence each other’s pharmacokinetics without adverse events (AEs).Citation9 In healthy volunteers, FDC therapy with olmesartan medoxomil (40 mg) and rosuvastatin (20 mg) had a similar pharmacokinetic profile to that of coadministration of each drug as individual tablets.Citation10 The present study aimed to evaluate the efficacy and safety of FDC therapy with olmesartan medoxomil (40 mg) and rosuvastatin (20 mg) in Korean patients with mild to moderate hypertension and dyslipidemia.
Materials and methods
Study design
This was a randomized, double-blind, factorial-design study performed at 25 locations in Korea between September 2012 and May 2013 (). This study was designed to adhere to the Korean Good Clinical Practice guidelines, related regulations in Korea, and the Declaration of Helsinki, and it was approved by the Ministry of Food and Drug Safety, and the institutional review boards of each of the participating institutions () (ClinicalTrials.gov Identifier: NCT01764295).
Screening was performed after patients signed a written informed consent form for participation in this study. After assessing the screening results of the patients, those who satisfied the inclusion criteria underwent therapeutic lifestyle change for a period of >4 weeks. After the therapeutic lifestyle change period, central laboratory tests and BP measurements for final decisions were performed at the baseline visit. After a qualification period of <1 week, the selected patients were randomly allocated to the following four groups: the FDC therapy group (olmesartan medoxomil [40 mg] and rosuvastatin [20 mg], DWJ1276, Daewoong Pharmaceuticals, Seoul, Korea); olmesartan medoxomil group (olmesartan medoxomil [40 mg], Olmetec®, Daiichi Sankyo, Tokyo, Japan); rosuvastatin group (rosuvastatin [20 mg], Crestor®, AstraZeneca plc, London, UK); and placebo group. Each placebo tablet had an appearance and an odor identical to that of the active tablets. The pills were completely indistinguishable. All randomly assigned subjects took three tablets of investigational drugs orally once a day for 8 weeks at the same time each day. For randomization, this study used a stratified block randomization method stratified according to the low-density lipoprotein cholesterol (LDL-C) (100 mg/dL ≤ LDL-C <130 mg/dL, 130 mg/dL ≤ LDL-C <160 mg/dL, LDL-C ≥160 mg/dL) level and diastolic blood pressure (DBP) (90 mmHg ≤ DBP <100 mmHg, DBP ≥100 mmHg, in case of subjects with diabetes or chronic renal disease, 80 mmHg ≤ DBP <90 mmHg, DBP ≥90 mmHg). The randomization code was generated with the proc plan procedure using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA) by an independent statistician of the contract research organization. The independent statistician made an extra “randomization list with investigational drug number”. The investigators and the pharmacists used this list for prescription of investigational drugs. All the investigators, participants, and study staffs remained blinded to treatment group until study completion.
Patients stopped taking any antihypertensive drugs at least 2 weeks before randomization and stopped taking lipid-lowering drugs during the entire therapeutic lifestyle change period. In addition, antihypertensive and lipid-lowering drugs that could interact with the study drugs were discontinued during the treatment period. During the study period, the patients visited the participating institutions five times as follows: screening visit, baseline visit, randomization visit, and visits at weeks 4 and 8 after starting treatment. The following procedures were carried out at each visit: physical examination, vital signs (DBP/systolic blood pressure [SBP], temperature, and pulse), laboratory tests (hematology, chemistry, and urinalysis), assessment of compliance, and AEs.
When the subject showed signs or symptoms of hypotension with SBP <90 mmHg or DBP <60 mmHg, hypertension with SBP ≥180 mmHg or DBP ≥110 mmHg, and abnormal results values of liver function (aspartate aminotransferase and alanine aminotransferase three times greater than upper limit of normal level), the subject had to discontinue this study for his or her safety.
Inclusion and exclusion criteria
The study recruited patients aged ≥20 years with mild to moderate essential hypertension and dyslipidemia, as defined by the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC VII) and the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) ().
Table 1 Inclusion criteria according to risk factors and a 10-year risk assessment
Patients were excluded if they had secondary hypertension (medical history of secondary hypertension or suspected secondary hypertension by physician) or dyslipidemia; hypersensitivity to olmesartan medoxomil or rosuvastatin; uncontrolled diabetes mellitus (hemoglobin A1c ≥9% or fasting plasma glucose level ≥160 mg/dL); myocardial infarction, transient ischemic attack, percutaneous transluminal coronary angioplasty, or unstable angina within the previous 6 months; severe heart failure (New York Heart Association class 3 and 4); thyroid stimulating hormone levels ≥1.5 times the upper normal limit; creatinine level ≥1.5 times the upper normal limit; creatinine kinase, aspartate aminotransferase, and alanine aminotransferase levels ≥2 times the upper normal limits; triglyceride levels ≥400 mg/dL; or any disease that could influence the study results.
Objectives and outcome measures
The primary objectives were to determine the superiority of FDC therapy over olmesartan medoxomil (40 mg) for the percentage change from baseline in the LDL-C level and the superiority of FDC therapy over rosuvastatin (20 mg) for the change from baseline in DBP at week 8.
The secondary objectives were to compare the FDC therapy to olmesartan medoxomil (40 mg) for the change from baseline in DBP and the FDC therapy to rosuvastatin (20 mg) for the percentage change from baseline in the LDL-C level at week 8. The additional secondary objectives were to compare the FDC therapy to olmesartan medoxomil (40 mg) and rosuvastatin (20 mg) for the percentage change from baseline in the total cholesterol, triglyceride, and high-density lipoprotein cholesterol levels at weeks 4 and 8; the change from baseline in SBP at weeks 4 and 8; and the percentage of patients who achieved the treatment goals (LDL-C <160 mg/dL, 130 mg/dL, 100 mg/dL each category according to the risk factors and a 10-year risk assessment, SBP/DBP <140/90 mmHg, in case of subjects with diabetes or chronic renal disease, 130/80 mmHg) defined by the NCEP ATP III and JNC VII at week 8.
For reliability evaluations, the percentage change from baseline in the LDL-C level and the change from baseline in DBP were compared between FDC therapy and placebo at week 8.
For safety evaluations, the dates of onset of AEs and termination, severity of AEs, actions taken for the AEs, and relationships of the AEs with the study products were assessed at each visit. In addition, abnormal vital signs, laboratory test results (including hematology, biochemistry, and urinalysis), physical examination results, and echocardiography results were recorded.
Statistical analysis
The hypotheses being tested were that the FDC therapy was superior to olmesartan medoxomil (40 mg) in reducing the LDL-C level and superior to rosuvastatin (20 mg) in reducing DBP. The expected difference of the mean percentage change from baseline in the LDL-C level between FDC therapy and olmesartan medoxomil (40 mg) was −53.8% (standard deviation: 20%) and the expected difference of the mean change from baseline in DBP between FDC therapy and rosuvastatin (20 mg) was −6 mmHg (standard deviation: 8.7 mmHg). Finally, the sample sizes were calculated using the percent change of LDL-C level and the change in DBP. The sample size identified for assessing the change in DBP, which was larger, was selected. For collecting more safety data of FDC therapy, the randomization ratio was set into 2:1:1:1. According to the randomization ratio of 2:1:1:1 and a 20% drop-out rate, a sample size of 150 patients was calculated (60 patients in the FDC therapy group and 30 patients each in the olmesartan medoxomil, rosuvastatin, and placebo groups). As both the hypotheses required significant findings for acceptance, each individual significance level was set at 5% for the entire hypothesis and the statistical power for each hypothesis was set at 80%.
Continuous data were summarized using descriptive statistics, and the treatment groups were compared using analysis of covariance, with baseline values, stratification factors (risk factors and BP), and drug interaction variables as covariates. Categorical data were analyzed using logistic regression models, with stratification factors and drug interaction variables as covariates. All analyses were two-sided, and the significance level was α=0.05. The analyses were performed using SAS version 9.2 (SAS Institute Inc.).
Results
Patients’ characteristics
A total of 423 patients underwent the screening examination, and of these patients, 183 who were found to be suitable for this study were randomized. Of these 183 patients, 181 patients were administered the investigational products. However, 19 patients were excluded based on the inclusion/exclusion criteria. Therefore, 162 patients completed the treatment and were included in the full analysis set ().
Figure 1 Study flowchart.
The demographics of the full analysis set according to the treatment group are presented in . The mean (standard deviation) age of the patients was 61.4 (7.8) years, and the mean body mass index was 25.4 (2.7) kg/m2. The mean SBP was 150.5 (13.5) mmHg, and the mean DBP was 92.6 (6.6) mmHg. The mean LDL-C level was 154.5 (31.7) mg/dL, and the mean high-density lipoprotein cholesterol, triglyceride, and total cholesterol levels were 50.0 (11.4) mg/dL, 147.7 (67.2) mg/dL, and 230.2 (36.3) mg/dL, respectively. There were no significant differences in demographic characteristics, except for family history of premature coronary heart disease, among the treatment groups (P=0.0118).
Table 2 Demographics and baseline characteristics prior to randomization in the full analysis set
Lipid parameters
The least square mean percentage changes (standard error) from baseline in the LDL-C level 8 weeks after treatment were −52.3% (2.8%) in the FDC therapy group, −0.6% (3.5%) in the olmesartan medoxomil group, and −46.9% (3.5%) in the rosuvastatin group. The difference between the FDC and olmesartan medoxomil groups was −51.7% (4.1%) (95% confidence interval [CI]: −59.8% to −43.6%), and the percentage change was significantly higher in the FDC therapy group than in the olmesartan medoxomil group (P<0.0001). The difference between the FDC therapy and rosuvastatin groups was −5.4% (4.1%) (95% CI: −13.5% to 2.7%), and the percentage change was not significantly different between the FDC and rosuvastatin groups (P=0.1864). The percentage changes in LDL-C levels at weeks 4 and 8 are presented in , and the percentage changes in other lipid parameters at weeks 4 and 8 are presented in .
Table 3 Changes in the low-density lipoprotein cholesterol level at weeks 4 and 8 in the full analysis set
Table 4 Least square mean percentage change from baseline in the total cholesterol, triglyceride and high-density lipoprotein cholesterol levels at weeks 4 and 8 in the full analysis set
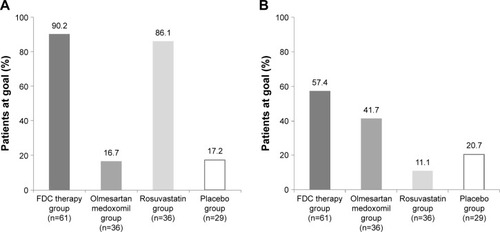
The treatment goal was achieved at 8 weeks in 90.2% (55/61) of patients from the FDC therapy group, 16.7% (6/36) of patients from the olmesartan medoxomil group, 86.1% (31/36) of patients from the rosuvastatin group, and 17.2% (5/29) of patients from the placebo group. The percentage of patients who achieved the treatment goal was significantly higher in the FDC therapy group than in the olmesartan medoxomil and placebo groups (both P<0.0001). There was no significant difference in the percentage of patients between the FDC therapy and rosuvastatin groups (P=0.5111, ).
Figure 2 Percentage of patients who achieved the treatment goals of low-density lipoprotein cholesterol levels and blood pressure at week 8 in the full analysis set.
Abbreviations: DBP, diastolic blood pressure; FDC, fixed-dose combination.
Blood pressure
The least square mean changes (standard error) from baseline in DBP at 8 weeks after treatment were −10.4 (1.2) mmHg in the FDC therapy group, 0.1 (1.6) mmHg in the rosuvastatin group, and −8.1 (1.5) mmHg in the olmesartan medoxomil group. The difference between the FDC therapy and rosuvastatin groups was −10.5 (1.8) mmHg (95% CI: −14.1 to −6.9 mmHg), and the change was significantly higher in the FDC therapy group than in the rosuvastatin group (P<0.0001). The difference between the FDC therapy and olmesartan medoxomil groups was −2.3 (1.8) mmHg (95% CI: −5.9 to 1.3 mmHg), and the change was not significantly different between the FDC therapy and olmesartan medoxomil groups (P=0.2096). The changes in DBP at 4 weeks and the changes in SBP at 4 and 8 weeks were similar to the changes in DBP at 8 weeks ().
Table 5 Changes in blood pressure at weeks 4 and 8 in the full analysis set
The treatment goal was achieved at 8 weeks in 57.4% (35/61) of patients from the FDC therapy group, 11.1% (4/36) of patients from the rosuvastatin group, 41.7% (15/36) of patients from the olmesartan medoxomil group, and 20.7% (6/29) of patients from the placebo group. The percentage of patients who achieved the treatment goal was significantly higher in the FDC therapy group than in the rosuvastatin and placebo groups (P<0.0001, P=0.0018, respectively). There was no significant difference in the percentage of patients between the FDC and olmesartan medoxomil groups (P=0.1360, ).
Safety
Safety analysis was performed in all the patients who were administered the investigational products one or more times. A total of 181 patients (71 patients in the FDC therapy group, 38 patients in the olmesartan medoxomil group, 38 patients in the rosuvastatin group, and 34 patients in the placebo group) were included in the safety set. The summary of AEs reported during the study period is presented in . Among the 181 patients, 41 (22.7%, 50 cases) experienced AEs during the study period. Every AE was reported regardless of the causal relationship.
Table 6 Summary of AEs and adverse drug reactions in the safety set
There were no significant differences in the incidences of AEs and adverse drug reactions (ADRs) among the treatment groups (P=0.9202, and P=0.5990, respectively). Most of the AEs were mild, and severe AEs were not reported in any of the treatment groups.
Serious AEs occurred in two patients (1.1%, two cases): one patient had myocardial infarction and was from the olmesartan medoxomil group, while the other patient who had subarachinoid hemorrhage was from the rosuvastatin group. The investigational products were immediately discontinued in these patients. However, all serious AEs were not likely related to investigational drugs and they were resolved without sequelae.
A total of five patients experienced eight ADRs during the study period. In the FDC therapy group, two patients reported ADRs. Of these two patients, one had increases in aspartate aminotransferase and alanine aminotransferase levels and the other had an increase in blood creatinine levels and a decrease in creatinine clearance. All the ADRs were expected side effects of the approved drugs.
Discussion
The present study demonstrated that FDC therapy with olmesartan medoxomil (40 mg) and rosuvastatin (20 mg) was highly effective for achieving the therapeutic goals of the LDL-C level and BP. In the reduction of the LDL-C level, the effectiveness of FDC therapy was not different from that of rosuvastatin (20 mg), and in the reduction of BP, the effectiveness of FDC therapy was not different from that of olmesartan medoxomil (40 mg). Additionally, we found that FDC therapy was generally safe and well tolerated.
Olmesartan medoxomil is a selective angiotensin II type 1 receptor blocker (ARB) with proven BP-lowering efficacy.Citation11,Citation12 The antihypertensive efficacy of ARBs has been shown to be at least equivalent to the efficacies of other major classes of antihypertensive agents but with a better tolerability profile.Citation13 Several studies have demonstrated that ARBs have positive effects on left ventricular hypertrophy, endothelial dysfunction, and atherosclerosis, suggesting that ARBs offer cardiovascular protective benefits in addition to their favorable effects on BP.Citation14,Citation15 Olmesartan medoxomil has a more rapid onset of action than that of other ARBs, with significant improvements in efficacy.Citation11 The ability of olmesartan medoxomil to effectively reduce BP suggests that it is a good therapeutic option for intensive treatment in patients with mild to moderate hypertension.Citation12
Statins are usually used to treat dyslipidemia and manage patients with ischemic heart disease. However, with the completion of many large clinical trials on statins over the past 10 years, their use has been extended to preventive treatment for a variety of cardiovascular diseases.Citation2 Rosuvastatin is more effective than other statins for achieving LDL-C goals and producing favorable changes in the atherogenic lipid profile.Citation16,Citation17 Previous studies have shown that statins have direct effects on plaque stability, nitric oxide metabolism, inflammation, endothelial function, and oxidative stress.Citation18,Citation19 Additionally, statins have been shown to significantly reduce cardiovascular mortality and morbidity in patients at risk for cardiovascular diseases.Citation18,Citation20
The interplay between hypertension and dyslipidemia acts through the renin–angiotensin system to increase cardiovascular risk. Hypertension and dyslipidemia result in the release of angiotensin II, which acts on angiotensin 1 receptors. Activation of angiotensin 1 receptors stimulates NADH oxidase production in endothelial cells, resulting in the generation of reactive oxygen species in vascular cells and eventually endothelial dysfunction and decreased nitric oxide production.Citation21,Citation22 Combinations of ARBs and statins could be atheroprotective and effective in improving endothelial function through their synergistic mode of action on angiotensin 1 receptors, resulting in the reduction of cardiovascular morbidity and mortality.Citation2 FDC therapy with olmesartan medoxomil (40 mg) and rosuvastatin (20 mg) could be highly effective for the prevention of cardiovascular events through cardiovascular protective benefits beyond comprehensive control of both BP and blood cholesterol and could potentially increase treatment adherence in patients prescribed long-term polymedication.
Olmesartan medoxomil is not metabolized by the cytochrome P450 system and has no effect on P450 enzymes. Rosuvastatin clearance is not dependent on metabolism by cytochrome P450 3A4 to a clinically significant extent. Thus, interactions with drugs that inhibit or induce those enzymes, or are metabolized by these enzymes are not expected. A previous study showed that FDC therapy with olmesartan medoxomil (40 mg) and rosuvastatin (20 mg) has a similar pharmacokinetic profile to that of coadministration of each drug as individual tablets, without serious AEs.Citation10 These results suggested that FDC therapy could be used inter-changeably with the conventional formulation of the coadministration of each drug separately. In the present study, we demonstrated that the efficacy and safety of FDC therapy with olmesartan medoxomil (40 mg) and rosuvastatin (20 mg) were similar to those of each drug in the combination in patients with both hypertension and dyslipidemia.
The relatively small sample size would be a limitation of the present study. Further investigation of a larger patient population over a longer period will be needed to confirm the clinical benefit.
Conclusion
For patients who have hypertension and dyslipidemia concomitantly, FDC therapy with olmesartan medoxomil (40 mg) and rosuvastatin (20 mg) is a good therapeutic option with appropriate efficacy and safety. Such a combo-pill may help enhance the compliance of the patients with large pill burden due to comorbidities.
Acknowledgments
This study was supported by and monitored by Daewoong Corporation Co., Ltd.
Supplementary material
Table S1 Twenty-five participating institutions
Disclosure
The authors report no conflicts of interest in this work.
References
- KannelWBRisk stratification in hypertension: new insights from the Framingham studyAm J Hypertens2000131 Pt 23S10S10678282
- NickenigGShould angiotensin II receptor blockers and statins be combined?Circulation200411081013102015326080
- O’MearaJGKardiaSLArmonJJBrownCABoerwinkleETurnerSTEthnic and sex differences in the prevalence, treatment, and control of dyslipidemia among hypertensive adults in the GENOA studyArch Intern Med2004164121313131815226165
- StamlerJWentworthDNeatonJDPrevalence and prognostic significance of hypercholesterolemia in men with hypertension. Prospective data on the primary screenees of the multiple risk factor intervention trialAm J Med1986802A33393946459
- CastelliWPAndersonKA population at risk. Prevalence of high cholesterol levels in hypertensive patients in the Framingham studyAm J Med1986802A23323946458
- EmbersonJWhincupPMorrisRWalkerMEbrahimSEvaluating the impact of population and high-risk strategies for the primary prevention of cardiovascular diseaseEur Heart J200425648449115039128
- JacksonRLawesCMBennettDAMilneRJRodgersATreatment with drugs to lower blood pressure and blood cholesterol based on an individual’s absolute cardiovascular riskLancet2005365945743444115680460
- ChapmanRHBennerJSPetrillaAAPredictors of adherence with antihypertensive and lipid-lowering therapyArch Intern Med2005165101147115215911728
- RohHSonHLeeDChangHYunCParkKPharmacokinetic interaction between rosuvastatin and olmesartan: a randomized, open-label, 3-period, multiple-dose crossover study in healthy Korean male subjectsClin Ther20143681159117025017182
- SonHRohHLeeDChangHKimJYunCParkKPharmacokinetics of rosuvastatin/olmesartan fixed-dose combination: a single-dose, randomized, open-label, 2-period crossover study in healthy Korean subjectsClin Ther201335791592223810276
- FabiaMJAbdillaNOltraRFernandezCRedonJAntihypertensive activity of angiotensin II AT1 receptor antagonists: a systematic review of studies with 24 h ambulatory blood pressure monitoringJ Hypertens20072571327133617563549
- BrunnerHROlmesartan medoxomil: current status of its use in monotherapyVasc Health Risk Manag20062432734017323586
- BurnierMAngiotensin II type 1 receptor blockersCirculation2001103690491211171802
- PrasadATupas-HabibTSchenkeWHAcute and chronic angiotensin-1 receptor antagonism reverses endothelial dysfunction in atherosclerosisCirculation2000101202349235410821809
- KlingbeilAUJohnSSchneiderMPJacobiJHandrockRSchmiederREEffect of AT1 receptor blockade on endothelial function in essential hypertensionAm J Hypertens200316212312812559678
- JonesPHDavidsonMHSteinEAComparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial)Am J Cardiol200392215216012860216
- SchwartzGGBologneseMATremblayBPEfficacy and safety of rosuvastatin and atorvastatin in patients with hypercholesterolemia and a high risk of coronary heart disease: a randomized, controlled trialAm Heart J20041481e415215813
- WernerNNickenigGLaufsUPleiotropic effects of HMG-CoA reductase inhibitorsBasic Res Cardiol200297210511612002257
- WassmannSNickenigGInterrelationship of free oxygen radicals and endothelial dysfunction – modulation by statinsEndothelium2003101233312699074
- SowersJREffects of statins on the vasculature: Implications for aggressive lipid management in the cardiovascular metabolic syndromeAm J Cardiol2003914A14B22B
- NickenigGHarrisonDGThe AT(1)-type angiotensin receptor in oxidative stress and atherogenesis: part I: oxidative stress and atherogenesisCirculation2002105339339611804998
- NickenigGHarrisonDGThe AT(1)-type angiotensin receptor in oxidative stress and atherogenesis: Part II: AT(1) receptor regulationCirculation2002105453053611815439
