Abstract
Purpose
Urocortin 3 is a key neuromodulator in the regulation of stress, anxiety, food intake, gut motility, and energy homeostasis, while ghrelin elicits feeding behavior and enhances gastric emptying, adiposity, and positive energy balance. However, the interplays between urocortin 3 and ghrelin on food intake and gastric emptying remain uninvestigated.
Methods
We examined the differential effects of central O-n-octanoylated ghrelin, des-Gln14-ghrelin, and urocortin 3 on food intake, as well as on charcoal nonnutrient semiliquid gastric emptying in conscious rats that were chronically implanted with intracerebroventricular (ICV) catheters. The functional importance of corticotropin-releasing factor (CRF) receptor 2 in urocortin 3-induced responses was examined by ICV injection of the selective CRF receptor 2 antagonist, astressin2-B.
Results
ICV infusion of urocortin 3 opposed central acyl ghrelin-elicited hyperphagia via CRF receptor 2 in satiated rats. ICV injection of O-n-octanoylated ghrelin and des-Gln14-ghrelin were equally potent in accelerating gastric emptying in fasted rats, whereas ICV administration of urocortin 3 delayed gastric emptying. In addition, ICV infusion of urocortin 3 counteracted central acyl ghrelin-induced gastroprokinetic effects via CRF receptor 2 pathway.
Conclusion
ICV-infused urocortin 3 counteracts central acyl ghrelin-induced hyperphagic and gastroprokinetic effects via CRF receptor 2 in rats. Our results clearly showed that enhancing ghrelin and blocking CRF receptor 2 signaling in the brain accelerated gastric emptying, which provided important clues for a new therapeutic avenue in ameliorating anorexia and gastric ileus found in various chronic wasting disorders.
Introduction
The anorexia–cachexia syndrome often occurs in human chronic inflammatory diseases, such as cancer, cardiovascular disease, uremia, and rheumatoid arthritis,Citation1,Citation2 in which central nervous mechanisms are deeply engaged. Emerging evidence indicates that the corticotropin-releasing factor (CRF) family of peptides play key roles in the activity and plasticity of neurons in enduring changes in anorexigenic effects in feeding behavior.Citation3–Citation5 Urocortin 3, the latest identified member of the CRF family, has been shown to be involved in feeding and metabolic control under stress conditions. Increased messenger RNA expression of urocortin 3 in the hypothalamus has been observed in rat models with acute restraintCitation6 and chronic psychologicalCitation7 stress. Central urocortin 3 has also been demonstrated to be responsible for anxiety-like behaviors and metabolic functions.Citation8 Taken together, urocortin 3 appears to be a key neuromodulator linking stress-induced anxiety and energy homeostasis under anxious conditions.
Meanwhile, ghrelin, exhibiting appetite- and adiposity-enhancing properties, is useful in improving appetite.Citation9 Plasma levels of acyl ghrelin have been shown to be compensatory but insufficiently increased in tumor-bearing rats compared to paired-fed controls, which may be the consequence of hyperactivation of the CRF family.Citation10 To overcome insufficient ghrelin levels and response, administration of exogenous ghrelin and its mimetics have been considered to be effective in treating eating/wasting disorders and cachexia.Citation11 In addition, potentiation of ghrelin receptor signaling by modulating the CRF family is another attractive strategy.Citation10 Intracerebroventricular (ICV) injection of urocortin 3 but not CRF increased plasma ghrelin concentration in chicks, suggesting that urocortin 3/CRF receptor 2 is the main regulator in the reciprocal interactions with the ghrelin/growth hormone secretagogue receptor 1a system.Citation12 However, no information is available at present regarding the interplays between urocortin 3 and acyl ghrelin on feeding.
In this study, we investigated the differential effects of central urocortin 3 and two splice variants of acyl ghrelin on food intake and evaluated peptide–peptide interactions in conscious rats. As appetite and gut motility are inextricably linked,Citation13,Citation14 we appraised the differential influences of central urocortin 3 and two splice variants of acyl ghrelin, as well as their cross talks, on gastric emptying. Finally, the functional importance of CRF receptor 2 in mediating these biological actions on food intake and gastric emptying was explored.
Materials and methods
Animals
Male Sprague–Dawley rats weighing 250–320 g (7–8 weeks old) were obtained from National Yang-Ming University Laboratory Animal Center, Taipei, Taiwan. Rats were kept in rooms with controlled illumination (light cycle: 8 am to 8 pm), humidity and temperature (22.5°C±1.5°C) with free access to water and laboratory chow pellets (BioLASCO Taiwan Co. Ltd., Taipei, Taiwan). All experiments were performed after 8 am in conscious rats, according to the guidelines approved by the Institutional Animal Care and Use Committee, Taipei Veterans General Hospital, Taiwan.
Surgery
Implantation of ICV catheter
For ICV implantation, rats were anesthetized with intraperitoneal injection of sodium pentobarbital (50 mg/kg, Nembutal; Abbott Laboratories, Abbott Park, IL, USA), placed in a stereotaxic apparatus (Benchmark™, myNeuroLab, St Louis, MO, USA), and implanted with a guide cannula (25-gauge; Eicom, Kyoto, Japan), which reached the right lateral ventricle. The coordination in stereotaxic apparatus were 0.8 mm posterior to the bregma, 1.4 mm right lateral to the midline, and 4.5 mm below the outer surface of the skull using a stereotaxic frame with the incisor bar set at the horizontal plane passing through the bregma and lambda.Citation15,Citation16 The secure of the guide cannula, insertion of a dummy cannula (Eicom) into the guide cannula, and placement of a screw cap (Eicom) were performed as in our previous study.Citation17 The rats were allowed at least 7 days for full recovery after the implantation of ICV catheters before starting the studies of food intake and gastric emptying. All ICV injections were done over 60 seconds injecting a total volume of 10 μL in each rat via the AMI-5 (Eicom).
Preparation of drugs
Rat O-n-octanoylated ghrelin (American Peptide Co, Sunnyvale, CA, USA), des-Gln14-ghrelin (Tocris Bioscience, Bristol, UK), the highly selective ligand for CRF receptor subtype 2, mouse urocortin 3 (American Peptide) and the highly selective CRF receptor 2 antagonist, astressin2-B (Polypeptide Laboratories, Torrance, CA, USA), were kept in powder form at −20°C, and dissolved in sterile, pyrogen-free 0.9% saline (Otsuka, Tokyo, Japan) immediately before use.
Food intake study
The measurement of food intake was performed as in our previous studies with a slight modification.Citation15,Citation16 In short, the cumulative food intake was recorded and calculated at 1, 2, 4, 8, 12, and 24 hours immediately after ICV injection. The total volume of ICV injection was 10 μL in each rat. Food intake was determined by measuring the difference between the preweighed standard chow and weight of chow at each time point. At least 7 days before the food intake tests, all rats were allowed to fully acclimatize to the environment. Light-phase experiments for freely fed rats were started at 8 am. Before the tests, the rats were given free access to food and water (freely fed). A standard diet (BioLASCO Taiwan Co. Ltd.) was applied.
Influences of two splice variants of acyl ghrelin and urocortin 3 on spontaneous food intake in freely fed rats
In the first experiment, a vehicle (10 μL of saline), O-n-octanoylated ghrelin (0.1 nmol), des-Gln14-ghrelin (0.1 nmol), or urocortin 3 (1.0 or 2.0 nmol) was injected via the ICV catheter. The cumulative food intake was recorded at 1, 2, 4, 8, 12, and 24 hours after the injection. The optimal dosages of O-n-octanoylated ghrelin and des-Gln14-ghrelin on food intake were chosen according to our previous study.Citation15,Citation16
Effects of ICV-infused urocortin 3 on central splice variants of acyl ghrelin-induced hyperphagia in freely fed rats
In the second experiment, a vehicle (5 μL of saline) or urocortin 3 (1.0 or 2.0 nmol) was ICV injected immediately before the injection of O-n-octanoylated ghrelin (0.1 nmol), or des-Gln14-ghrelin (0.1 nmol). Immediately after the ICV injection, the cumulative food intake was recorded at 1, 2, 4, 8, 12, and 24 hours. The optimal dose of urocortin 3 counteracting acyl ghrelin-induced hyperphagia was chosen from this step.
Roles of ICV CRF receptor 2 in central urocortin 3 counteracting acyl ghrelin-induced hyperphagia in freely fed rats
In the third experiment, a vehicle (3.3 μL of saline) or astressin2-B (20 nmol) was ICV injected immediately before a vehicle (3.3 μL of saline), or urocortin 3 (2.0 nmol) in rats receiving either ICV-infused O-n-octanoylated ghrelin (0.1 nmol) or des-Gln14-ghrelin (0.1 nmol) stimulation on food intake. The total volume of ICV injection was 10 μL for each rat. Immediately after the ICV injection, the cumulative food intake was recorded at 1, 2, 4, 8, 12, and 24 hours. The dosage of urocortin 3 was selected based on the above studies. The ratio of astressin2-B/urocortin 3 was modified from those described in the previous studies.Citation18,Citation19
Gastric emptying of charcoal, non-nutrient, semiliquid meal
After being deprived of food for 16 hours, with free access to water, the non-nutrient semiliquid gastric emptying was measured in conscious rats (food-deprived). The total volume of ICV injection was 10 μL for each rat. Fifteen minutes after the ICV injection, the conscious rats were gavaged via a catheter (PE-205, ID: 1.67 mm, OD: 2.42 mm, Clay Adams, Becton, Dickinson and Company, Franklin Lakes, NJ, USA) with test meal containing Na2 51 CrO4 (0.5 μCi/mL) and 10% charcoal in physiological saline (3 mL/kg) on the day of the experiment (around 9 am). The test meal was continuously stirred before intubation. Additional air (0.5 mL) was added to flush the residual charcoal suspension remaining in the catheter. Thirty minutes later, the rats were decapitated.Citation17 The details are described in the previous study.Citation20 Semiliquid gastric emptying was calculated as (total radioactivity in the small intestine/total radioactivity in the stomach and small intestine) ×100%, 30 minutes after gavage.Citation20
Influences of two splice variants of acyl ghrelin and urocortin 3 on gastric emptying in food-deprived rats
In the first experiment, a vehicle (10 μL of saline), O-n-octanoylated ghrelin (0.1 nmol), des-Gln14-ghrelin (0.1, 0.3, or 1.0 nmol), or urocortin 3 (0.1, 0.3, or 1.0 nmol) were ICV injected. The optimal dosage of O-n-octanoylated ghrelin on gastric emptying was chosen according to our previous study.Citation17 The optimal dose of des-Gln14-ghrelin on gastric emptying was selected from this step.
Effects of ICV infusion of urocortin 3 on central splice variants of acyl ghrelin-induced acceleration of gastric emptying in food-deprived rats
In the second experiment, a vehicle (5 μL of saline) or urocortin 3 (0.1, 0.3, or 1.0 nmol) was ICV injected immediately before O-n-octanoylated ghrelin (0.1 nmol) or des-Gln14-ghrelin (0.1 nmol). The dose of urocortin 3 counteracting acyl ghrelin-induced gastroprokinetic responses was chosen from this step.
Roles of ICV CRF receptor 2 in central urocortin 3 counteracting acyl ghrelin-induced acceleration of gastric emptying in food-deprived rats
In the third experiment, a vehicle (3.3 μL of saline) or astressin2-B (3 nmol) was ICV injected immediately before a vehicle (3.3 μL of saline) or urocortin 3 (0.3 nmol) in rats receiving either ICV O-n-octanoylated ghrelin (0.1 nmol) or des-Gln14-ghrelin (0.1 nmol) stimulation on gastric emptying. The optimal dosage of urocortin 3 was selected based on the above studies, whereas the ratio of astressin2-B/urocortin 3 was modified from that described in the previous studies.Citation18,Citation19
Statistical analyses
All results were expressed as mean ± standard error of the mean. Food intake or gastric emptying was compared between O-n-octanoylated ghrelin and des-Gln14-ghrelin-treated rats using a two-way analysis of variance. One-way analysis of variance followed by a Student–Newman–Keuls post hoc test was used to analyze difference among groups, when analyzing one-variable experiments with more than two groups. Differences were considered statistically significant if P<0.05.
Results
Effects of ICV injection of O-n-octanoylated ghrelin, des-Gln14-ghrelin, and urocortin 3 on spontaneous food intake in freely fed rats
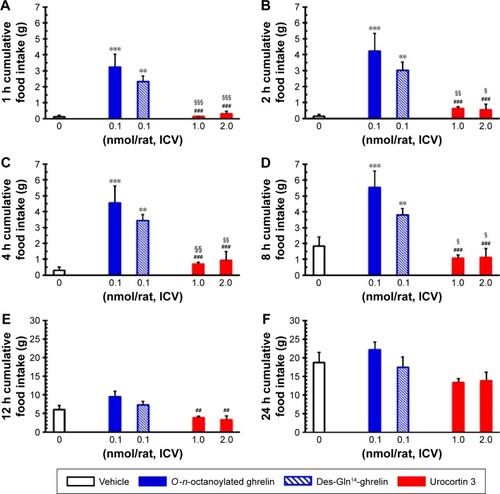
In freely fed conscious rats, ICV injection of O-n-octanoylated ghrelin and des-Gln14-ghrelin at the dosage of 0.1 nmol/rat significantly increased the cumulative food intake at 1, 2, 4, and 8 hours (), whereas ICV infusion of urocortin 3 at dosage of 1.0 and 2.0 nmol/rat did not affect any cumulative food intake compared to vehicle control group ().
Figure 1 The influence of ICV injection of O-n-octanoylated ghrelin, des-Gln14-ghrelin, and urocortin 3 on cumulative food intake in freely fed satiated rats.
Abbreviations: ICV, intracerebroventricular; SEM, standard error of the mean.
Effects of ICV injection of urocortin 3 on central splice variants of acyl ghrelin-induced hyperphagia in freely fed rats
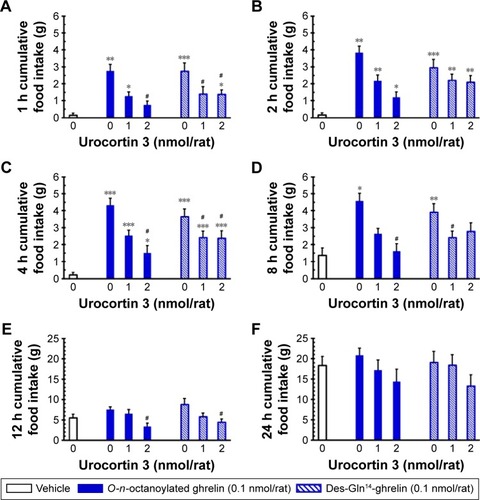
In freely fed conscious rats, ICV injection of urocortin 3 (2.0 nmol/rat) significantly attenuated central O-n-octanoylated ghrelin-induced orexigenic effects in 1-, 4-, 8-, and 12-hour cumulative food intake (). Similarly, des- Gln14-ghrelin also effectively increased the cumulative food intake in 1–8 hours. ICV-infused urocortin 3 at 1.0 nmol/rat or 2.0 nmol/rat also significantly prevented the stimulation in 1-, 4-, and 8-hour () or 1-, 4-, and 12-hour () cumulative food intake induced by ICV-injected des-Gln14-ghrelin in freely fed rats, respectively.
Figure 2 The influence of ICV injection of urocortin 3 on central splice variants of acyl ghrelin-induced hyperphagia in freely fed satiated rats.
Abbreviations: ICV, intracerebroventricular; SEM, standard error of the mean.
Effects of ICV injection of astressin2-B on central urocortin 3 counteracting acyl ghrelin-induced hyperphagia in freely fed rats
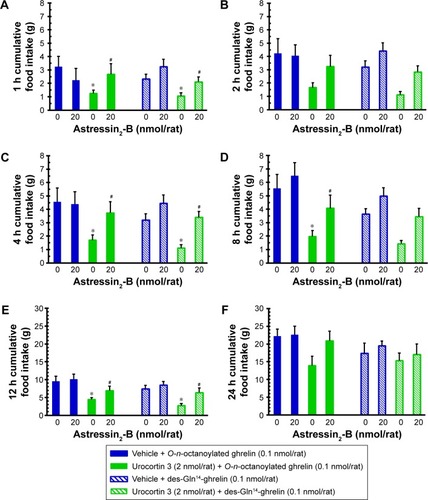
In freely fed conscious rats, ICV injection of astressin2-B (20 nmol/rat), the selective CRF receptor 2 antagonist had no significant effect on central O-n-octanoylated ghrelin and des-Gln14-ghrelin-induced stimulation of cumulative food intake up to 24 hours (). Interestingly, infusion of astressin2-B (20 nmol/rat) significantly prevented the counteracting responses from ICV-infused urocortin 3 (2.0 nmol/rat) on central O-n-octanoylated ghrelin-induced stimulation in 1, 4, 8, and 12 hours cumulative food intake (). Similarly, ICV infusion of astressin2-B (20 nmol/rat) also significantly opposed the counteracting responses from ICV-infused urocortin 3 (2.0 nmol/rat) on central des-Gln14-ghrelin-induced stimulation in 1-, 4-, and 12-hour cumulative food intake in conscious freely fed rats ().
Figure 3 The influence of ICV injection of corticotropin-releasing factor receptor 2 antagonist, astressin2-B, on central urocortin 3 counteracting acyl ghrelin-induced hyperphagia in freely fed satiated rats.
Abbreviations: ICV, intracerebroventricular; SEM, standard error of the mean.
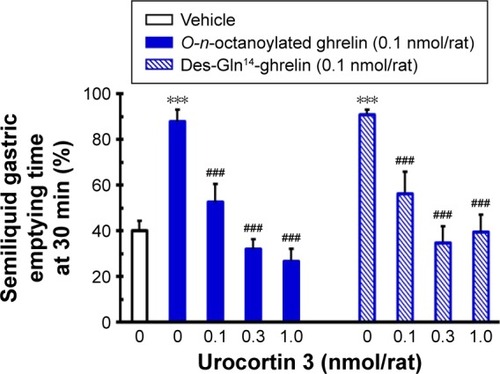
Effects of ICV injection of O-n-octanoylated ghrelin, des-Gln14-ghrelin, and urocortin 3 on gastric emptying
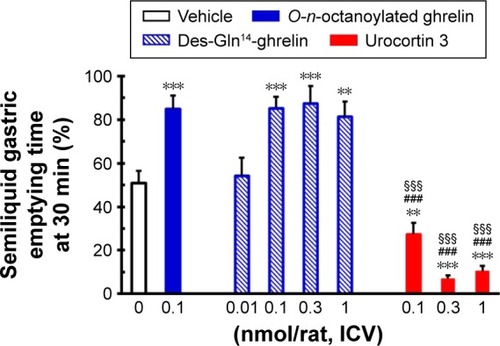
In food-deprived conscious rats, ICV injection of O-n- octanoylated ghrelin (0.1 nmol/rat) and des-Gln14-ghrelin (0.01, 0.1, 0.3, and 1.0 nmol/rat) significantly enhanced gastric emptying, whereas ICV-infused urocortin 3 (0.1, 0.3, and 1.0 nmol/rat) effectively suppressed gastric emptying (). The optimal dosage of des-Gln14-ghrelin at 0.1 nmol/rat was selected for the subsequent gastric emptying experiments.
Figure 4 The influence of ICV injection of O-n-octanoylated ghrelin, des-Gln14-ghrelin, and urocortin 3 on gastric emptying in conscious fasted rats.
Abbreviations: ICV, intracerebroventricular; SEM, standard error of the mean.
Effects of ICV-infused urocortin 3 on central splice variants of acyl ghrelin-induced acceleration of gastric emptying
In food-deprived conscious rats, ICV injection of urocortin 3 (0.1, 0.3, and 1.0 nmol/rat) significantly attenuated the enhanced gastric emptying resulting from ICV-infused O-n-octanoylated ghrelin and des-Gln14-ghrelin (). The optimal dosage of urocortin 3 (0.3 nmol/rat) to counteract acyl ghrelin-induced gastroprokinetic effect was chosen for the subsequent gastric emptying experiments.
Figure 5 The influence of ICV infusion of urocortin 3 on central splice variants of acyl ghrelin-induced acceleration of gastric emptying in conscious, fasted rats.
Abbreviations: ICV, intracerebroventricular; SEM, standard error of the mean.
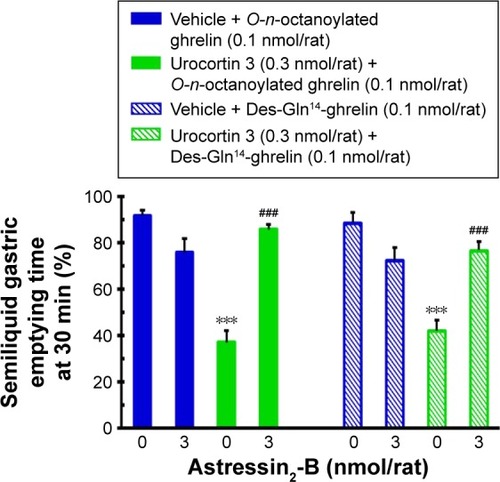
Effects of ICV-infused astressin2-B on central urocortin 3 counteracting acyl ghrelin-induced gastroprokinetic responses
In food-deprived conscious rats, ICV injection of the selective CRF receptor 2 antagonist, astressin2-B (3.0 nmol/rat) had a slight but not significant decrease of the gastric emptying time. ICV-infused urocortin 3 (0.3 nmol/rat) significantly blocked the central O-n-octanoylated ghrelin and des-Gln14- ghrelin-induced acceleration of gastric emptying and this counteracting response was significantly reversed by the infusion of astressin2-B ().
Figure 6 The influence of ICV injection of corticotropin-releasing factor receptor 2 antagonist, astressin2-B, on central urocortin 3 counteracting acyl ghrelin-induced acceleration of gastric emptying in conscious, fasted rats.
Abbreviations: ICV, intracerebroventricular; SEM, standard error of the mean.
Discussion
Ghrelin and growth hormone secretagogue receptor 1a signaling pathways in the hypothalamus play important roles in the central nervous control of feeding behavior and energy partition.Citation21 Des-Gln14-ghrelin constitutes one-fifth of ghrelin immunoreactivity in the circulation.Citation22 The orexigenic effects elicited by central O-n-octanoylated ghrelin and des-Gln14-ghrelin,Citation15,Citation16 as well as the anorexigenic effects induced by central urocortin 3 in rodents,Citation23 have been reported previously. Despite the fact that there are several studies reporting controversial effects of urocortin 1 on ghrelin levels, either the suppressing effect in ratsCitation24 (central administration) and humansCitation25 (peripheral administration) or the enhancing effect in ratsCitation26 (peripheral administration), our investigation is the first to explore the peptide–peptide interactions between central urocortin 3 and splice variants of acyl ghrelin on food intake, as well as to extend the knowledge regarding the role of CRF receptor 2 in mediating these actions. We clearly demonstrated that ICV-infused urocortin 3 attenuated central acyl ghrelin-elicited hyperphagic responses, whereas the administration of the selective CRF receptor 2 antagonist, astressin2-B, effectively prevented the counteractions in feeding.
CRF family peptides are known to be engaged with coping, resilience, and sensitivity with regard to anxiety and stress, while cachectic cancer patients often display higher anxiety scores.Citation3 Additionally, urocortin 3 in the brain also mediates the anorexigenic and weight loss activities of other peptides, such as parathyroid hormone-related protein.Citation27 In agreement with a previous finding that intrahypothalamic urocortin 3 reducing food intake by prolonging the post-meal interval,Citation28 we not only demonstrated that ICV-injected urocortin 3 antagonized the ghrelin-induced food intake but also elucidated that the feeding suppression was mediated through central CRF receptor 2. This is consistent with the finding that CRF receptor 2-mediated stress-induced anorexia.Citation29 According to our current findings, it is reasonable to hypothesize that hypothalamic urocortin-3/CRF receptor 2 pathway may be responsible for hypophagia caused by chronic diseases. Together, these results suggest that the central urocortin 3/CRF receptor 2 pathway is an important mechanism in modulating stress, food intake, and energy metabolism.
Treatment for the tricky anorexia–cachexia syndrome has been an underestimated and unmet clinical need. In cachectic cancer patients, higher-than-normal ghrelin levels were observed,Citation30 suggesting that there are unknown, underlying mechanisms of the chronic wasting status, such as ghrelin resistance or insufficient increase of ghrelin. Due to anatomical vicinity, functional relationships between acyl ghrelin and CRF receptors located near the paraventricular nuclei area have been examined, including the interactions on anxiety,Citation31 ingestive behavior,Citation15 and secretion of luteinizing hormone.Citation32 In our present study, we have clearly shown that the opposing effect of ICV-induced urocortin 3 against central acyl ghrelin-elicited hyperphagia is through CRF receptor 2 in satiated rats ( and ). Based on our results, we may provide some clinical implications for the potential management of poor appetite in cachexia. In addition to enhancing signaling of the ghrelin/growth hormone secretagogue receptor 1a system in the brain,Citation9,Citation10 a blockade of the central CRF receptor 2 can be considered as one of the strategies in alleviating hypophagia in certain chronic wasting disorders as well as those conditions with insufficient compensatory elevation of central acyl ghrelin leading to ineffective compensatory hyperphagia. However, as the precise role of CRF receptors in the modulation of feeding under chronic wasting diseases remains less investigated, further studies are required to validate the efficacy of CRF receptor 2 antagonists in various anorectic disorders.
Gastric emptying plays a pivotal role in the control of gastric tension and intestinal exposure of nutrients, and subsequently regulates hunger, satiation, and satiety.Citation33 The strong association between gastric emptying and body weight implies that gastric motility may be involved in the long-term modulation of body weight balance. Previous studies also confirmed that upper gut motilityCitation34 and liquid gastric emptyingCitation35 were either reduced or delayed in anorectic conditions, such as in cancer subjects. However, disturbed gastric emptying is often underrecognized and not easily measured in chronic wasting conditions. With our findings that des-Gln14-ghrelin was as potent as O-n-octanoylated ghrelin in the acceleration of gastric emptying in fasted rats (), it is reasonable to speculate that insufficient ghrelin levels and inadequate ghrelin response may also participate in the dysfunction of gut motility in hypophagic patients with chronic diseases. Further investigation in this area may provide important clues for future clinical application of ghrelin and its derivatives.Citation36
On the other hand, ICV injection of urocortin 3 has been shown to inhibit nutrient, solid, gastric emptying in mice.Citation23 Similarly, we demonstrated that ICV-infused urocortin 3 effectively delayed non-nutrient, semiliquid gastric emptying in food-deprived rats (). Therefore, urocortin 3 may regulate the motility of the whole gastrointestinal tract via neurological control from the central nervous system. Our results also confirmed that CRF receptor 2 is responsible for the opposing effect of urocortin 3 on acyl ghrelin-induced gastroprokinetic response, as shown by the fact that the selective CRF receptor 2 antagonist, astressin2-B, reversed the counteracting reactions (). Hence, targeting CRF receptor 2 and/or growth hormone secretagogue receptor 1a in the brain may be promising in ameliorating gastrointestinal ileus in various chronic wasting diseases.
Conclusion
In conclusion, ICV-infused urocortin 3 counteracts central acyl ghrelin-elicited hyperphagia via CRF receptor 2 in satiated rats. Central administration of O-n-octanoylated ghrelin and des-Gln14-ghrelin are equally potent in enhancing nonnutrient gastric emptying in fasted rats, whereas ICV-infused urocortin 3 delays gastric emptying and opposes ICV acyl ghrelin-induced gastroprokinetic effects via CRF receptor 2. Therefore, enhancing the ghrelin/growth hormone secretagogue receptor 1a system and blocking CRF receptor 2 in the hypothalamus may provide a new therapeutic avenue in ameliorating anorexia and gastric ileus during human negative energy balance conditions, thus fulfilling the currently unmet medical needs of the real world.
Authors contributions
CY, C-HT, M-LD, and S-DL participated in the conception, design, and acquisition of data of the research. C-WC and C-YC wrote and revised the paper. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
This project was funded by grants from Taiwan Ministry of Science and Technology (NSC 97-2314-B-010-024-MY2 to C-YC) and in part supported by a grant from the Cheng Hsin General Hospital (103F003C13 to S-DL and C-YC). We would like to thank Miss Yu-chi Lee for her secretarial assistance in editing this manuscript, as well as the Clinical Research Core Laboratory of Taipei Veterans General Hospital for providing experimental space and facilities.
Disclosure
The authors report no conflicts of interest in this work.
References
- Plata-SalamánCRCentral nervous system mechanisms contributing to the cachexia-anorexia syndromeNutrition200016101009101211054608
- ChenCYTsaiCYLeePCLeeSDLong-term etanercept therapy favors weight gain and ameliorates cachexia in rheumatoid arthritis patients: roles of gut hormones and leptinCurr Pharm Des201319101956196423305268
- ChenCYNeuroplasticity of central corticotropin-releasing factor and serotonergic systems in anxiety-related behaviorNeuropeptides201448418718824909502
- TsengLYInuiAChenCYCorticotropin-releasing hormone receptor 1 antagonist with or without corticotropin-releasing hormone receptor 2 remain promising in relieving human acute psychological stress and related intestinal hyperpermeabilityJ Biochem Mol Biol2015123031
- TanakaCAsakawaAUshikaiMComparison of the anorexigenic activity of CRF family peptidesBiochem Biophys Res Commun2009390388789119850009
- JamiesonPMLiCKukuraCVaughanJValeWUrocortin 3 modulates the neuroendocrine stress response and is regulated in rat amygdala and hypothalamus by stress and glucocorticoidsEndocrinology2006147104578458816809443
- AtakaKNagaishiKAsakawaAInuiAFujimiyaMAlteration of antral and proximal colonic motility induced by chronic psychological stress involves central urocortin 3 and vasopressin in ratsAm J Physiol Gastrointest Liver Physiol20123034G519G52822651925
- KupermanYIsslerORegevLPerifornical urocortin-3 mediates the link between stress-induced anxiety and energy homeostasisProc Natl Acad Sci U S A2010107188393839820404164
- LundholmKGunneboLKörnerUEffects by daily long term provision of ghrelin to unselected weight-losing cancer patients: a randomized double-blind studyCancer201011682044205220186829
- FujitsukaNAsakawaAUezonoYPotentiation of ghrelin signaling attenuates cancer anorexia-cachexia and prolongs survivalTransl Psychiatry20111e2322832525
- AliSChenJAGarciaJMClinical development of ghrelin axis-derived molecules for cancer cachexia treatmentCurr Opin Support Palliat Care20137436837524145681
- KhanMSKaiyaHTachibanaTCentral injection of urocortin-3 but not corticotrophin-releasing hormone influences the ghrelin/GHS-R1a system of the proventriculus and brain in chicksDomest Anim Endocrinol201447273424484650
- HornerKMByrneNMCleghornGJNäslundEKingNAThe effects of weight loss strategies on gastric emptying and appetite controlObes Rev2011121193595121729233
- ChenCYFujimiyaMLavianoAChangFYLinHCLeeSDModulation of ingestive behavior and gastrointestinal motility by ghrelin in diabetic animals and humansJ Chin Med Assoc201073522522920685586
- ChenCYTsaiCYLeeWJIntracerebroventricular O-n-octanoylated ghrelin and its splice variant-induced feeding is blocked by insulin, independent of obestatin or CRF receptor, in satiated ratsNutrition2012287–881282022465905
- TingCHChiCPLiCPChenCYDifferential modulation of endogenous cannabinoid CB1 and CB2 receptors in spontaneous and splice variants of ghrelin-induced food intake in conscious ratsNutrition201531123023525466669
- ChenCYDoongMLChienEJIntracerebroventricular ghrelin enhances non-nutrient semiliquid gastric emptying in fasted conscious ratsGastroenterol J Taiwan2008253242248
- KiharaNFujimuraMYamamotoIItohEInuiAFujimiyaMEffects of central and peripheral urocortin on fed and fasted gastroduodenal motor activity in conscious ratsAm J Physiol Gastrointest Liver Physiol20012803G406G41911171623
- SmedhUMoranTHPeptides that regulate food intake: separable mechanisms for dorsal hindbrain CART peptide to inhibit gastric emptying and food intakeAm J Physiol Regul Integr Comp Physiol20032846R1418R142612468444
- DoongMLLuCCKauMMInhibition of gastric emptying and intestinal transit by amphetamine through a mechanism involving an increased secretion of CCK in male ratsBr J Pharmacol19981246112311309720782
- YadaTKohnoDMaejimaYNeurohormones, rikkunshito and hypothalamic neurons interactively control appetite and anorexiaCurr Pharm Des201218314854486422632865
- HosodaHKojimaMMatsuoMHKangawaKPurification and characterization of rat des-Gln14-Ghrelin, a second endogenous ligand for the growth hormone secretagogue receptorJ Biol Chem200027529219952200010801861
- UshikaiMAsakawaASakoguchiTTanakaCInuiACentrally administered urocortin 3 inhibits food intake and gastric emptying in miceEndocrine201139211311721061090
- YakabiKNoguchiMOhnoSUrocortin 1 reduces food intake and ghrelin secretion via CRF(2) receptorsAm J Physiol Endocrinol Metab20113011E72E8221540451
- DavisMEPembertonCJYandleTGUrocortin-1 infusion in normal humansJ Clin Endocrinol Metab20048931402140915001641
- WangLStengelAGoebel-StengelMShaikhAYuanPQTachéYIntravenous injection of urocortin 1 induces a CRF2 mediated increase in circulating ghrelin and glucose levels through distinct mechanisms in ratsPeptides20133916417023183626
- AsakawaAFujimiyaMNiijimaAParathyroid hormone-related protein has an anorexigenic activity via activation of hypothalamic urocortins 2 and 3Psychoneuroendocrinology20103581178118620188481
- FeketeEMInoueKZhaoYDelayed satiety-like actions and altered feeding microstructure by a selective type2 corticotropin-releasing factor agonist in rats: intra-hypothalamic urocortin 3 administration reduces food intake by prolonging the post-meal intervalNeuropsychopharmacology20073251052106817019404
- OhataHShibasakiTInvolvement of CRF2 receptor in the brain regions in restraint-induced anorexiaNeuroreport2011221049449821666520
- TakahashiMTerashinmaMTakaganeAOyamaKFujiwaraHWakabayashiGGhrelin and leptin levels in cachectic patients with cancer of the digestive organsInt J Clin Oncol200914431532019705241
- AsakawaAInuiAKagaTA role of ghrelin in neuroendocrine and behavioral responses to stress in miceNeuroendocrinology200174314314711528215
- VulliémozNRXiaoEXia-ZhangERivierJFerinMAstressin B, a nonselective corticotropin-releasing hormone receptor antagonist, prevents the inhibitory effect of ghrelin on luteinizing hormone pulse frequency in the ovariectomized rhesus monkeyEndocrinology2008149386987418063681
- JanssenPVanden BerghePVerschuerenSLehmannADepoortereITackJReview article: the role of gastric motility in the control of food intakeAliment Pharmacol Ther201133888089421342212
- NelsonKAWalshTDSheehanFGO’DonovanPBFalkGWAssessment of upper gastrointestinal motility in the cancer-associated dyspepsia syndromeJ Palliat Care19939127318492233
- ChenCYLuCLChangFLDelayed liquid gastric emptying in patients with hepatocellular carcinomaAm J Gastroenterol200095113230323711095347
- ChenCYTsaiCYFrom endocrine to rheumatism: Do gut hormones play roles in rheumatoid arthritis?Rheumatology (Oxford)201453220521223882111
