Abstract
Background
G-protein-coupled bile acid receptor 1, also known as TGR5 is known to be involved in glucose homeostasis. In animal models, treatment with a TGR5 agonist induces incretin secretion to reduce hyperglycemia. Betulinic acid, a triterpenoid present in the leaves of white birch, has been introduced as a selective TGR5 agonist. However, direct activation of TGR5 by betulinic acid has not yet been reported.
Methods
Transfection of TGR5 into cultured Chinese hamster ovary (CHO-K1) cells was performed to establish the presence of TGR5. Additionally, TGR5-specific small interfering RNA was employed to silence TGR5 in cells (NCI-H716 cells) that secreted incretins. Uptake of glucose by CHO-K1 cells was evaluated using a fluorescent indicator. Amounts of cyclic adenosine monophosphate and glucagon-like peptide were quantified using enzyme-linked immunosorbent assay kits.
Results
Betulinic acid dose-dependently increases glucose uptake by CHO-K1 cells transfected with TGR5 only, which can be considered an alternative method instead of radioligand binding assay. Additionally, signals coupled to TGR5 activation are also increased by betulinic acid in cells transfected with TGR5. In NCI-H716 cells, which endogenously express TGR5, betulinic acid induces glucagon-like peptide secretion via increasing calcium levels. However, the actions of betulinic acid were markedly reduced in NCI-H716 cells that received TGR5-silencing treatment. Therefore, the present study demonstrates the activation of TGR5 by betulinic acid for the first time.
Conclusion
Similar to the positive control lithocholic acid, which is the established agonist of TGR5, betulinic acid has been characterized as a useful agonist of TGR5 and can be used to activate TGR5 in the future.
Introduction
It has been established that G-protein-coupled bile acid (BA) receptor 1 (also known as TGR5) agonists show potential for treating diabetic disorders.Citation1,Citation2 Essentially, BA induces TGR5 receptor internalization, activation of extracellular signal-regulated kinase, and intracellular cyclic adenosine monophosphate (cAMP) production in cells.Citation1,Citation2 In animal models, treatment with TGR5 agonist(s) produces glucagon-like peptide (GLP-1) secretion, which in turn induces insulin secretion to lower blood glucose and/or increase the basal energy expenditure.Citation3 Therefore, TGR5 has been recognized as a target for developing new antidiabetic agents.Citation4 In the gut, BAs are also modified by the gut flora to produce secondary BAs, deoxycholic acid, and lithocholic acid (LCA). BAs can be divided into hydrophobic and hydrophilic subgroups. LCA is hydrophobic and could activate TGR5Citation5 but was highly toxic to liver.Citation6
Betulinic acid, a triterpenoid, as shown in , that is present in the leaves of white birch, has been introduced as a selective TGR5 agonist with moderate potency to produce antihyperglycemic actions.Citation7,Citation8 Due to its potential as a TGR5 agonist, derivatives of betulinic acid have widely been studied.Citation9,Citation10 Recently, the antidiabetic action of betulinic acid has been reviewed with two other triterpenic acids, oleanolic acid, and ursolic acid, in detail.Citation11 However, the activation of TGR5 was not shown in that report, probably due to poor evidence. Therefore, data showing the direct effect of betulinic acid on TGR5 are likely to be helpful.
In the present study, we used cells transfected with TGR5 to identify the effect of betulinic acid. Additionally, TGR5-silenced cells were applied to confirm the deletion of betulinic acid-induced actions. Therefore, the TGR5-mediated actions of betulinic acid can be observed directly.
Materials and methods
Materials
Betulinic acid (Tokyo Chemical Institute, Tokyo, Japan) and LCA (Sigma-Aldrich Chemical Co., St Louis, MO, USA) were dissolved in dimethyl sulfoxide. Additionally, myristoylated PKI 14-22 amide (Tocris, Avonmouth, Bristol, UK), an inhibitor of protein kinase A, was dissolved in an aqueous solution. Protein was assayed using a bicinchoninic acid assay kit (Thermo Sci., Rockford, IL, USA).
Cell cultures
The commercial human NCI-H716 cells (BCRC No CCL-251) obtained from the Culture Collection and Research Center of the Food Industry Institute (Hsin-Chiu, Taiwan) were maintained in medium supplemented with 10% (v/v) fetal bovine serum and 2 mM l-glutamine at 5% CO2. Additionally, Chinese hamster ovary (CHO-K1) cells (BCRC No CCL-61) were maintained in growth medium composed of F-12K supplemented with 10% fetal bovine serum. Cells were subcultured once every 3 days by trypsinization (GIBCO-BRL Life Technologies, Gaithersburg, MD, USA), and the medium was changed every 2–3 days.
Transfection of TGR5 in CHO-K1 cells
As described in a previous report,Citation12 CHO-K1 cells were transiently transfected with human G-protein-coupled BA receptor 1 and an expression vector (pCMV6-Entry; OriGene, Rockville, MD, USA). We used the TurboFect transfection reagent (Thermo Fisher Scientific, Pittsburgh, PA, USA) to transfect the cells, which were seeded at 5×104 cells per well in six-well plates. Twenty-four hours later, the success of transfection was confirmed using the Western blotting analysis. Then, the TGR5-CHO-K1 cells were incubated with betulinic acid or LCA at the indicated concentrations.
Uptake of 2-NBDG into CHO-K1 cells
Similar to our previous report,Citation13 glucose uptake into cells was investigated using 2-(N-[7-nitrobenz-2-oxa-1,3-diazol-4-yl] amino)-2-deoxyglucose (2-NBDG) as a fluorescent indicator. Each assay used 1×106 cells/mL. The medium was removed, and the cells were washed gently with phosphate-buffered solution (PBS). Cells were detached from the dish by trypsinization, suspended in 0.2 mM 2-NBDG and the testing agent at the indicated concentration in PBS, and then incubated in a 37°C water bath for 60 minutes in the dark. The cells were centrifuged (4°C, 5,000× g, 10 minutes), and the supernatant was discarded. The pellet was washed three times with cold PBS and cooled on ice. The pellet was suspended in 1 mL of PBS. The fluorescence intensity in cell suspension was evaluated using a fluorescence spectrofluorometer (Hitachi F-2000, Tokyo, Japan), with excitation and emission wavelengths of 488 and 520 nm, respectively. The intensity of fluorescence showed the uptake of 2-NBDG in the cells that were incubated with betulinic acid or LCA at the indicated concentrations for suitable time.
Small interfering RNA in NCI-H716 cells
Based on a previous method,Citation12 we purchased a validated small interference RNA (siRNA) targeted against TGR5 from a commercial source (Dharmacon RNA Technology, Lafayette, CO, USA). The validated siRNAs were as follows: ON-TARGETplus SMARTpool siTAS1R3 (human NM_152228 sequence), ON-TARGETplus SMARTpool siGNAT3 (human NM_001102386 sequence), and ON-TARGETplus SMARTpool siGPBAR1 (TGR5) (human NM_170699 sequence). ON-TARGETplus SMARTpool non-targeting siRNA pool was used as a negative control to distinguish sequence-specific silencing from nonspecific effects. TurboFect transfection reagent (Thermo Fisher Scientific) was used to transfer siRNA. Success of silencing was evaluated using the Western blotting analysis after the transfection. The NCI-H716 cells were transfected with siRNA and differentiated for another 24 hours before the assay.
Western blotting analysis
Cells were harvested 24 hours after transfection and were lysed with ice-cold lysis buffer containing 300 mmol/L NaCl, 20 mmol/L Tris-HCl (pH 7.8), 2 mmol/L ethylenediaminetetraacetic acid, 2 mmol/L dithiothreitol, 2% Nonidet P-40, 0.2% sodium lauryl sulfate, and a cocktail of protease inhibitors (Sigma-Aldrich). Then, 30 μg of cell lysate was separated by 10% sodium dodecyl sulfate–polyacryl-amide gel electrophoresis. The blots were then transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). After blocking with 10% skim milk for 1 hour, the blots were developed using a primary antibody against TGR5 (Abcam, Rockville, MD, USA). The blots were subsequently hybridized using horseradish peroxidase-conjugated goat antirabbit or antimouse immunoglobulin G (Calbiochem, San Diego, CA, USA) and developed using a chemiluminescence kit (PerkinElmer, Waltham, MA, USA). The optical densities of the bands for TGR5 (32 kDa) and β-actin (43 kDa) were determined using GEL-PRO ANALYSER software 4.0 (Media Cybernetics, Silver Spring, MD, USA), and quantified as the ratio to β-actin.
Measurement of intracellular calcium
Changes in intracellular calcium concentrations were detected using the fluorescent probe fura-2. In brief, NCI-H716 cells were placed in a buffered physiological saline solution (PSS), to which 5 mmol/L fura-2 was added. Cells were then incubated for 1 hour in humidified 5% CO2 and 95% air at 37°C. Then, cells were washed and incubated for an additional 30 minutes in PSS. The cells were inserted into a preheated (37°C) cuvette containing 2 mL PSS, and the testing agent at the indicated concentration for the mentioned time. In addition, in some experiments, TGR5-deleted NCI-H716 cells were incubated with the testing agent in the same manner. Fluorescence was recorded continuously using a fluorescence spectrofluorometer (F-2000; Hitachi, Tokyo, Japan). Values of [Ca2+]i were determined, as described in our previous report.Citation14 Background autofluorescence was measured in unloaded cells and was subtracted from all measurements.
GLP-1 secretion from NCI-H716 cells
After being cultured for 48 hours, NCI-H716 cells (5×105 cells per well) were placed in buffered PSS containing betulinic acid or positive control (LCA) at the indicated concentration to incubate under 37°C for 1 hour. The supernatants were collected and immediately assayed using a GLP-1 active enzyme-linked immunosorbent assay kit (EZGLP1T-36K, EMD Millipore Co., Billerica, MA, USA). Experiments were performed in duplicate from the indicated samples.
Measurement of intracellular cAMP levels
The TGR5-CHO-K1 cells were plated at 5×104 cells/well in 96-well plates and incubated with betulinic acid or LCA at the indicated concentrations for 72 hours before cAMP measurements. Intracellular cAMP accumulation was measured using a cAMP enzyme-linked immunosorbent assay kit (ADI-900-066, Enzo Life Sciences, Farmingdale, NY, USA). Experiments were performed in duplicate from the indicated samples.
Statistical analysis
Results are presented as the mean±standard error of the mean from the sample number(n) of each group. One-way analysis of variance was followed by post hoc Tukey’s test and t-test using SPSS for Windows, version 17 (SPSS Inc., Chicago, IL, USA). Two-tailed P≤0.05 was considered significant.
Results
Betulinic acid increases glucose uptake in TGR5-transfected CHO cells
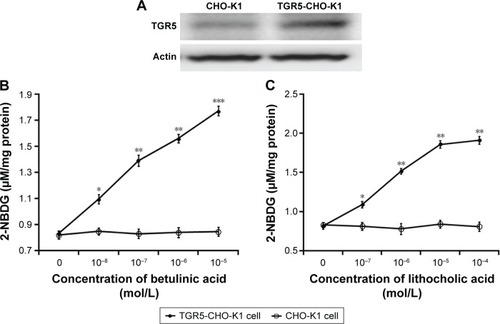
The successful transfection of TGR receptor into CHO-K1 cells was confirmed using Western blots, as shown in . Then, we investigated the functional response of TGR5-transfected CHO-K1 cells using glucose uptake as an indicator. Similar to the action of LCA, betulinic acid increases glucose uptake in a dose-dependent manner. However, glucose uptake was not induced by betulinic acid () or LCA () in CHO-K1 cells transfected with empty vector. We also evaluated the possibility of cytotoxicity induced by betulinic acid in CHO-K1 cells transfected with TGR5 receptor or with empty vector. Betulinic acid at the highest dose (0.1 mM) did not influence the viability of TGR5-transfected CHO-K1 cells or CHO-K1 cells transfected with empty vector only in the preliminary experiments. Similar results of the MTT assay were observed in LCA-treated TGR5-transfected CHO-K1 cells or CHO-K1 cells transfected with empty vector only (data not shown).
Figure 2 Role of TGR5 in glucose uptake in Chinese hamster ovary (CHO-K1) cells.
Abbreviations: CHO-K1, Chinese hamster ovary cells; 2-NBDG, 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl]amino)-2-deoxyglucose; SE, standard error.
Betulinic acid increases signals in TGR5-transfected CHO-K1 cells
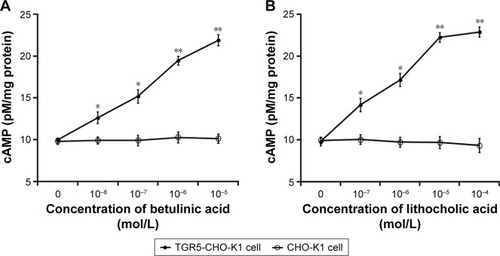
It has been established that cAMP is the main signal coupled to TGR5 receptor activation.Citation15 Therefore, we determined the change of cAMP levels in CHO-K1 cells-expressed TGR5. Betulinic acid induced a marked increase in cAMP levels in cells transfected with TGR5 receptor, but not in cells transfected with empty vector (). The 50% effective dose value of betulinic acid was ~0.5 µM. The effect of betulinic acid was produced in a dose-dependent fashion over the same range as that used to increase the glucose uptake. Similarly, LCA (the well-known agonist of TGR5) induced a dose-dependent increase in cAMP levels in CHO-K1 cells transfected with TGR5 receptor (). LCA is known to increase intracellular cAMP levels,Citation3 and it was used as a positive control in this study.
Figure 3 Changes in cAMP levels in CHO-K1 cells.
Abbreviations: cAMP, cyclic adenosine monophosphate; CHO-K1, Chinese hamster ovary cells; SE, standard error.
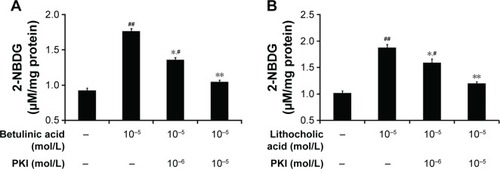
Additionally, glucose uptake increased by betulinic acid was markedly reduced by blockade of protein kinase A using protein kinase A inhibitor (PKI) in a dose-related manner (). Similar results were observed in LCA-treated TGR5-transfected CHO-K1 cells as shown in .
Figure 4 Effect of protein kinase A inhibition on the changes in glucose uptake in CHO-K1 cells.
Abbreviations: CHO-K1, Chinese hamster ovary cells; 2-NBDG, 2-(N-[7-nitrobenz-2-oxa-1,3-diazol-4-yl]amino)-2-deoxyglucose; SE, standard error.
Betulinic acid increases GLP-1 secretion in NCI-H716 cells
Glucose from the digestion of carbohydrates in foods is the well-known stimulator of GLP-1 secretion in the intestine.Citation16 NCI-H716 cells are widely used in the study of GLP-1 secretion,Citation17 and the presence of TGR5 receptor in NCI-H716 cells has been characterized.Citation10 Therefore, we used NCI-H716 cells to investigate the effect of betulinic acid on GLP-1 secretion in vitro. Additionally, as shown in , we applied siRNA to silence TGR5 receptor in NCI-H716 cells.
Figure 5 Role of TGR5 in GLP-1 secretion from NCI-H716 cells.
Abbreviations: GLP-1, glucagon-like peptide-1; SE, standard error; siRNA, small interfering RNA.
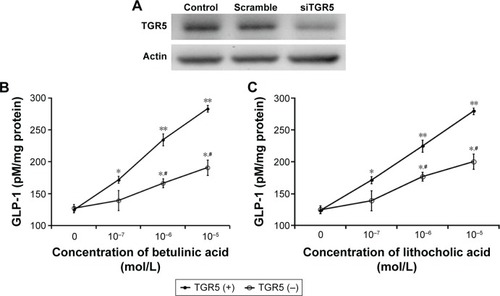
Betulinic acid increases GLP-1 secretion in a dose-dependent manner after incubation with NCI-H716 cells (). Similar changes were also observed in LCA-treated NCI-H716 cells ().
The effectiveness of betulinic acid was markedly attenuated once TGR5 receptor was silenced by siRNA (). Secretion of GLP-1 by betulinic acid was reduced with the absence of TGR5 receptor in NCI-H716 cells. Similar results were also observed in LCA-treated NCI-H716 cells (). This shows that TGR5 receptor mediates the secretion of GLP-1.
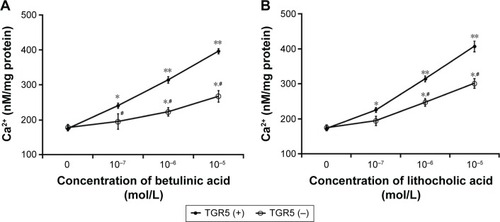
Betulinic acid increases calcium levels in NCI-H716 cells
We used a nonradioactive assay to determine intracellular calcium levels in NCI-H716 cells because GLP-1 secretion from cells is associated with calcium levels.Citation18
Betulinic acid also dose-dependently increases calcium levels in NCI-H716 cells (). Additionally, the same changes were obtained in LCA-treated NCI-H716 cells. However, the effect of betulinic acid was markedly reduced after TGR5 receptor was silenced by siRNA (). Calcium level increased by betulinic acid was attenuated after the silencing of TGR5 receptor in NCI-H716 cells. Similar results were also produced in LCA-treated NCI-H716 cells ().
Figure 6 Role of TGR5 in calcium influx into NCI-H716 cells.
Abbreviations: siRNA, small interfering RNA; SE, standard error.
Discussion
In this study, we demonstrated that the natural product betulinic acid is an agonist of TGR5 receptor. Using CHO-K1 cells transfected with human TGR5 receptor, we found for the first time that betulinic acid increases glucose uptake through activation of TGR5 receptor. A similar result was also produced using LCA, a well-known agonist of TGR5 receptor and the positive control in this study.
In CHO-K1 cells transfected with TGR5 receptor, betulinic acid increases glucose uptake in a dose-dependent manner. However, similar changes were not observed in CHO-K1 cells transfected with empty vector only. That the same results were observed in LCA-treated cells provide additional support for the betulinic acid-induced glucose uptake through TGR5 receptor. Moreover, betulinic acid dose-dependently increases the cAMP level in CHO-K1 cells transfected with TGR5 receptor. Previous report has identified cAMP as the subcellular signal coupled to TGR5 receptor activation,Citation6 further supporting the mediation of TGR5 receptor in betulinic acid-induced action. Finally, we applied an inhibitor of protein kinase A to block the betulinic acid-induced glucose uptake in CHO-K1 cells transfected with TGR5 receptor. It is thus plausible that TGR5 receptors are activated by betulinic acid to enhance glucose uptake through a cAMP-dependent pathway. Notably, this view has not been reported previously.
NCI-H716 cells have been introduced as a useful in vitro tool in the study of GLP-1 secretion from the human intestine,Citation17 and in which GLP-1 secretion has been reported to be linked to increased levels of intracellular calcium.Citation18 We found that betulinic acid dose-dependently increased GLP-1 secretion in parallel with the increase in calcium levels in NCI-H716 cells. Similar changes were induced by LCA, the well-known agonist of TGR5 receptor that was used as a positive control in this study. Interestingly, actions of betulinic acid were markedly reduced once TGR5 receptor was silenced by siRNA in NCI-H716 cells. To produce the stable silencing, the siRNA used for TGR5 receptor included three targets: TAS1R3 and α-gustducin in addition to TGR5 receptor.Citation12 However, it has been demonstrated that neither siRNA targeting TAS1R3 nor siRNA targeting α-gustducin in NCI-H716 cells influenced the GLP-1 secretion that was induced by a TGR5 receptor agonist.Citation12 TGR5 receptor is known to be a Gαs-coupled receptor.Citation19 TGR5 receptor activation stimulates GLP-1 secretion via Gαs, which increases the intracellular calcium level in NCI-H716 cells.Citation20 Our data are consistent with this view, supporting the idea that the action of betulinic acid in NCI-H716 cells to increase GLP-1 secretion occurs mainly through TGR5 receptor activation. Some reports have indicated that betulinic acid is able to induce TGR5 receptor activation.Citation9,Citation10 However, direct stimulation of TGR5 receptor by betulinic acid using radioligand binding assay or similar approaches has still not been reported. Using an alternative method, the present study demonstrated for the first time that betulinic acid-induced action is mediated by TGR5 receptor activation. However, chemical ligand(s) are known to produce nonspecific action, particularly at other receptor sites such as farnesoid X receptor. It is the limitation of this report, and more studies are required to clarify the specificity of betulinic acid in advance.
GLP-1 plays an essential role in the maintenance of glucose homeostasis by its multiple actions, which include stimulation of glucose-dependent insulin secretion, proinsulin gene expression, and β-cell proliferative and antiapoptotic pathways.Citation21 Moreover, GLP-1 regulates energy homeostasis via its neural actions, which include inhibiting glucagon release, gastric emptying, food intake, and appetite.Citation22,Citation23 Betulinic acid appears to regulate energy homeostasis via GLP-1 secretion. In addition, betulinic acid has been characterized as an agonist of human TGR5 receptor, which is expressed in the intestine, brown adipose tissue, skeletal muscle, and selected regions of the central nervous system. TGR5 receptor activation is known to regulate glucose and/or energy homeostasis, immune function, and liver function.Citation24 Therefore, betulinic acid can produce multiple actions in vivo.
Conclusion
In the present study, we demonstrated that betulinic acid is an agonist of human TGR5 receptor. Betulinic acid increases glucose uptake and stimulates GLP-1 secretion in cells via TGR5 receptor activation. Therefore, betulinic acid is a potential agent for activating TGR5 receptor, which has multiple actions in vivo.
Acknowledgments
The authors thank Ya-Pin Lin and Yi-Zhi Chen for their assistance in experiments. We also thank American Journal Expert for editing. This study was supported in part by a grant from the Ministry of Science and Technology (MOST 104-2320-B-384-004-MY3) in Taiwan, the Republic of China.
Disclosure
The authors report no conflicts of interest in this work.
References
- MaruyamaTMiyamotoYNakamuraTIdentification of membrane-type receptor for bile acids (M-BAR)Biochem Biophys Res Commun2002298571471912419312
- KawamataYFujiiRHosoyaMA G protein-coupled receptor responsive to bile acidsJ Biol Chem2003278119435944012524422
- ThomasCGioielloANoriegaLTGR5-mediated bile acid sensing controls glucose homeostasisCell Metab200910316717719723493
- CoppleBLLiTPharmacology of bile acid receptors: evolution of bile acids from simple detergents to complex signaling moleculesPharmacol Res201610492126706784
- De Aguiar VallimTQTarlingEJEdwardsPAPleiotropic roles of bile acids in metabolismCell Metab201317565766923602448
- FickertPFuchsbichlerAMarschallHULithocholic acid feeding induces segmental bile duct obstruction and destructive cholangitis in miceAm J Pathol2006168241042216436656
- TakikawaHHHirookaMSasakiMThe first synthesis of (±)-brevione B, an allelopathic agent isolated from Penicillium spTetrahedron Lett2003442852355238
- BlazevskiJPetkovicFMomcilovicMBetulinic acid regulates generation of neuroinflammatory mediators responsible for tissue destruction in multiple sclerosis in vitroActa Pharmacol Sin201334342443123377550
- GenetCStrehleASchmidtCStructure-activity relationship study of betulinic acid, a novel and selective TGR5 agonist, and its synthetic derivatives: potential impact in diabetesJ Med Chem201053117819019911773
- WangXYZhangSYLiJLiuHNXieXNanFJHighly lipophilic 3-epi-betulinic acid derivatives as potent and selective TGR5 agonists with improved cellular efficacyActa Pharmacol Sin201435111463147225283506
- SilvaFSGOliveiraPJDuarteMFOleanolic, ursolic and betulinic acids as food supplements or pharmaceutical agents for type 2 diabetes – promise or illusion?J Agric Food Chem201664152991300827012451
- KimKParkMLeeYMRhyuMRKimHYGinsenoside metabolite compound K stimulates glucagon-like peptide-1 secretion in NCI-H716 cells via bile acid receptor activationArch Pharm Res20143791193120024590628
- HuangCHChenMFChungHHChengJTAntihyperglycemic effect of syringaldehyde in streptozotocin-induced diabetic ratsJ Nat Prod20127581465146822880723
- ChengKCLiYXAsakawaACharacterization of preptin-induced insulin secretion in pancreatic β-cellsJ Endocrinol20122151434922787110
- VettorazziJFRibeiroRABorckPCThe bile acid TUDCA increases glucose-induced insulin secretion via the cAMP/PKA pathway in pancreatic beta cellsMetabolism2016653546326892516
- SteinertREBeglingerCNutrient sensing in the gut: interactions between chemosensory cells, visceral afferents and the secretion of satiation peptidesPhysiol Behav20111051627021376067
- KuhreREWewer AlbrechtsenNJDeaconCFPeptide production and secretion in GLUTag, NCI-H716, and STC-1 cells: a comparison to native L-cellsJ Mol Endocrinol201656320121126819328
- Le NevéBDanielHSelected tetrapeptides lead to a GLP-1 release from the human enteroendocrine cell line NCI-H716Regul Pept20111671142021070823
- ReimannFTolhurstGGribbleFMG-protein coupled receptors in intestinal chemosensationCell Metab201215442143122482725
- BehrensMMeyerhofWGustatory and extragustatory functions of mammalian taste receptorsPhysiol Behav2011105141321324331
- KuhreREHolstJJKappeCThe regulation of function, growth and survival of GLP-1-producing L-cellsClin Sci (Lond)20161302799126637406
- DruckerDJThe biology of incretin hormonesCell Metab20063315316516517403
- LimGEBrubakerPLGlucagon-like peptide 1 secretion by the L-cell: the view from withinDiabetes200655Suppl 2S70S77
- PolsTWNoriegaLGNomuraMAuwerxJSchoonjansKThe bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammationJ Hepatol20115461263127221145931