Abstract
Background
This work evaluated the hypothesis that 3,4-methylenedioxybenzoyl-2-thienylhydrazone (LASSBio-294), an agonist of adenosine A2A receptor, could be beneficial for preventing cardiac dysfunction due to hypertension associated with myocardial infarction (MI).
Methods
Male spontaneously hypertensive rats (SHR) were randomly divided into four groups (six animals per group): sham-operation (SHR-Sham), and myocardial infarction rats (SHR-MI) were treated orally either with vehicle or LASSBio-294 (10 and 20 mg.kg−1.d−1) for 4 weeks. Echocardiography and in vivo hemodynamic parameters measured left ventricle (LV) structure and function. Exercise tolerance was evaluated using a treadmill test. Cardiac remodeling was accessed by LV collagen deposition and tumor necrosis factor α expression.
Results
Early mitral inflow velocity was significantly reduced in the SHR-MI group, and there was significant recovery in a dose-dependent manner after treatment with LASSBio-294. Exercise intolerance observed in the SHR-MI group was prevented by 10 mg.kg−1.d−1 of LASS-Bio-294, and exercise tolerance exceeded that of the SHR-Sham group at 20 mg.kg−1.d−1. LV end-diastolic pressure increased after MI, and this was prevented by 10 and 20 mg.kg−1.d−1 of LASSBio-294. Sarcoplasmic reticulum Ca2+ ATPase levels were restored in a dose-dependent manner after treatment with LASSBio-294. Fibrosis and inflammatory processes were also counteracted by LASSBio-294, with reductions in LV collagen deposition and tumor necrosis factor α expression.
Conclusion
In summary, oral administration of LASSBio-294 after MI in a dose-dependent manner prevented the development of cardiac dysfunction, demonstrating this compound’s potential as an alternative treatment for heart failure in the setting of ischemic heart disease with superimposed chronic hypertension.
Introduction
Chronic heart failure (HF) is increasing in incidence and prevalence worldwide. This cardiovascular disease is often preceded by myocardial infarction (MI) and hypertension.Citation1,Citation2 HF presents with a complex constellation of symptoms, including dyspnea and exercise intolerance.Citation3–Citation5 The primary underlying defect in HF is an inability of the left ventricle (LV) to maintain an adequate forward stroke volume to meet the metabolic demands of the body, either because of impaired LV contractile function or insufficient LV filling.Citation6
The dynamic cellular and molecular processes that lead to permanent alterations in the geometry and function of the ventricles after an MI or due to chronic hypertension remain an active area of research. Current interventions used to prevent and treat LV remodeling include beta-blockers, angiotensin-converting enzyme inhibitors, aldosterone antagonists, and cardiac rehabilitation. The overall efficacy of these treatments in terms of cardiovascular morbidity and mortality is modest.Citation7 Therefore, continued exploration of novel pharmacological targets for the treatment of cardiac ischemic and nonischemic HF is reasonable.
Among the potential therapeutic targets are adenosine receptors (ARs), which upon activation by adenosine, appear to ameliorate the deleterious consequences of HF. AR activation inhibits fibroblast proliferation and collagen synthesis,Citation8,Citation9 thus limiting cardiac tissue remodeling and progression to failure. Moreover, activated ARs have favorable regulatory effects on several phenomena associated with ischemic and pressure-overload insults, including cytokine release,Citation10,Citation11 cell death processes,Citation12 and oxidative stress.Citation13,Citation14
Recently, we showed that 3,4-methylenedioxybenzoyl-2-thienylhydrazone (LASSBio-294), an A2AR ligand (IC50 9.5 µM obtained in a binding assay performed by CEREP S.A., Celle-Lévescault, France), prevented the development of diastolic dysfunctionCitation15 and attenuated exercise intoleranceCitation16 in normotensive rats with MI.
However, the beneficial effect of LASSBio-294 in rats with MI superimposed on preexisting hypertension is still unknown. Indeed, spontaneously hypertensive rat (SHR) strains have provided a useful model of the transition from stable, compensated hypertrophy to decompensated HF in the context of an ischemic insult.Citation17–Citation19 Accordingly, we hypothesized that chronic activation of A2AR by LASSBio-294 after MI would mitigate the progression to HF, as defined by myocardial extracellular matrix remodeling, LV dysfunction, and exercise intolerance, in male SHRs.
Methods
Animals
Male SHRs (12 weeks old) were maintained at 22°C±2°C with a relative humidity of 60%–70%, 12/12-hour light/dark cycles. Food and water were available ad libitum. All procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (National Institute of Health) and the study was approved by the Ethics and Research Committee of the Federal University of Rio de Janeiro (No DFBCICB049A), Rio de Janeiro Brazil.
Coronary artery ligation
To induce MI, under isoflurane anesthesia (3% v/v), rats were subjected to ligation of the anterior descending coronary artery, as previously described by Costa et al.Citation15 The same procedure was employed for a control group (Sham) without suturing the coronary artery. MI was confirmed 4–6 hours after surgery by the presence of anterior wall motion abnormalities and thinning of anterior and posterior LV walls, using M-mode echocardiographic images, obtained in the two-dimensional short-axis view. The SHRs were randomized into the following four groups (six animals per group): without MI treated with vehicle, dimethyl sulfoxide (SHR-Sham), MI treated with dimethyl sulfoxide (SHR-MI), MI treated with 10 mg.kg−1.d−1 LASSBio-294 (SHR-MI + LASSBio-294 [10 mg.kg−1.d−1]), and MI treated with 20 mg.kg−1.d−1 LASSBio-294 (SHR-MI + LASSBio-294 [20 mg.kg−1.d−1]). LASSBio-294 was administered orally by gastric gavage at doses of 10 or 20 mg.kg−1.d−1 for 4 weeks starting on the day of the surgical procedure. LASSBio-294 was synthesized at Laboratório de Avaliação de Substâncias Bioativas of the Federal University of Rio de Janeiro (Rio de Janeiro, RJ, Brazil).
Experimental protocol
The day after the surgical procedure, MI was confirmed by echocardiogram. Only animals that underwent surgery and had absence of motility of the anterior wall of the LV in M-mode were included in the study. After echocardiographic data collection, they were subsequently treated with dimethyl sulfoxide or LASSBio-294 according to their respective groups, which was administered daily for 4 weeks. On the last 2 days, the animals underwent: i) an additional echocardiographic evaluation (26th day) and ii) an exercise fatigue tolerance test (27th day). On the final day, hemodynamic parameters were obtained before euthanasia. After cervical decapitation under anesthesia, the lung and heart were removed for weighing, and the hearts were then prepared for histological evaluation and immunohistochemical analyses.
Exercise fatigue tolerance test
Rats performed a graded treadmill test on a customized rodent treadmill (EP–131, Insight, São Paulo, Brazil). In the fourth week, a treadmill test was conducted as follows: after acclimatization for 3 minutes in the cages, the animals were induced to run for 3 minutes at 8 m.min−1, 3 minutes at 12 m.min−1, and until volitional fatigue at 18 m.min−1. Exercise intolerance was determined by measuring the running distance until volitional fatigue, which was confirmed by loss of the animal’s righting reflex.Citation20
Echocardiographic measures of systolic and diastolic function
LV function was assessed with an echocardiograph equipped with a 10 MHz mechanical transducer (Esaote model, CarisPlus, Firenze, Italy). The animals were anesthetized with 3% (v/v) isoflurane and maintained with 1% (v/v). LV M-mode images were obtained in the two-dimensional short-axis view, close to the papillary muscles. Anterior and posterior wall thickness (AWT and PWT, respectively) and internal diameter during LV end-diastolic and end-systolic dimensions were measured. The LV ejection fraction (EF) and fractional shortening, indices of global systolic function, were calculated as: EF = (LVDV – LVSV)/LVDV, where LVDV is LV end-diastolic volume and LVSV is LV end-systolic volume; and fractional shortening = (LVDD – LVSD)/LVDD) ×100, where LVDD is LV end-diastolic and LVSD end-systolic dimensions. Mitral inflow measurements of early filling velocities (Emax) and late filling velocities (Amax) were used to calculate the E/A ratio to evaluate diastolic function.Citation21 All measurements were obtained according to the American Society of Echocardiography guidelines.
Cardiac mass and hemodynamic measurements
After 4 weeks, the animals were anesthetized with pentobarbital sodium (50 mg.kg−1) and prepared for terminal arterial blood and LV pressure measurements. A catheter (PE-50) was introduced into the right carotid artery and connected to a pressure transducer (MLT884, ADInstruments, Inc.; Colorado Springs, CO, USA) that measured systolic (SBP), diastolic (DBP) and mean (MBP) blood pressures. Subsequently, the catheter was introduced into the LV to record intracavitary pressure. The LV systolic pressure, LV end-diastolic pressure (LVEDP), and LV contraction and relaxation rates were assessed by maximal positive and negative dP/dt, respectively. Data were recorded on a polygraph (Powerlab, ADInstruments, Inc., Sydney, NSW, Australia) using LabChart software (Version 7.0, ADInstruments, Inc., Sydney, NSW, Australia). Then the animals were euthanized, the heart and lung weighed, and the tissues prepared for histological and immunohistochemical analyses.
Cardiac fibrosis
The LV interstitial collagen volume was examined with Picrosirius red staining. The collagen fraction was determined by averaging six fields/heart of the fibrotic areas within the infarct border zone. The amount of collagen in the tissue was determined as a percentage of the total area by optical microscopy.Citation15
Immunohistochemistry
Immunohistochemical analysis of heart sections (4-µm thick) for tumor necrosis factor α (TNF-α) and sarcoplasmic reticulum (SR) Ca2+-ATPase (SERCA2) was performed by standard procedures. Formalin-fixed, paraffin-embedded LV sections were deparaffinized and exposed to 3% (v/v) hydrogen peroxide, then subjected to antigen retrieval via immersion in citric acid (pH 6.0, 0.01 mol/L) at 96°C, followed by slow cooling to 60°C. Subsequently, the sections were incubated with primary anti-TNF-α antibody (AB6671-Abcam, Cambridge, UK) or anti-SERCA2 antibody (1:100) (AB2817, Abcam) overnight at 4°C, rinsed with phosphate-buffered saline, and incubated with biotinylated secondary IgG (Vector Laboratories, Burlingame, CA, USA) at 4°C. Antibody binding was detected by incubation with the peroxidase substrate solution, diaminobenzidine. The tissue sections were counterstained with hematoxylin, dehydrated, mounted, and observed under light microscopy with a 40× objective. The expression was quantified using Image-Pro Plus 6 software (ImageJ), measuring images obtained from the infarct border zone.
Statistical analysis
The data were expressed as the mean ± standard errors of the mean. Differences among groups were considered statistically significant when the P-value was <0.05 using one-way analysis of variance followed by a post hoc Dunnett’s test.
Results
Effects of LASSBio-294 on exercise intolerance in infarcted SHRs
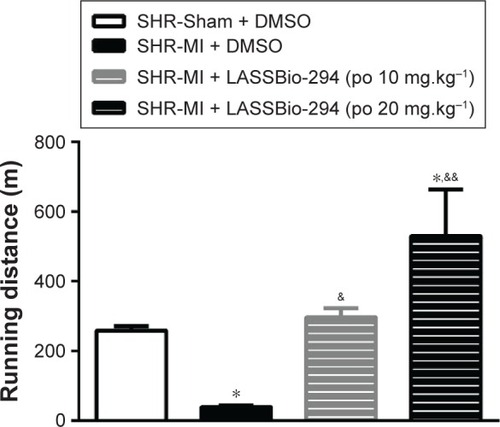
Exercise capacity data, as determined by the graded treadmill test, are shown in . The SHR-MI group had a significantly shorter mean running distance (39.01±4.39 m) than the SHR-Sham group (257.9±13.19 m; P<0.05). This exercise intolerance was prevented by treatment with LASSBio-294 at a dose of 10 mg.kg−1.d−1, with the SHR-MI + LASSBio-294 (10 mg.kg−1.d−1) group running a mean distance of 296±26.47 m (P<0.05 vs SHR-MI), which was similar to the distance observed for the noninfarcted group. The mean running distance of SHR-MI + LASSBio-294 (20 mg.kg−1.d−1) animals was 529.8±133.3 m, far exceeding that observed for both the SHR-MI (P<0.01) and SHR-Sham (P<0.05) groups.
Figure 1 Exercise tolerance was improved with LASSBio-294 treatment. Measurements performed 4 weeks after surgery. SHR-Sham treated with DMSO and SHR-MI treated with DMSO or LASSBio-294 orally, 10 and 20 mg.kg−1. Data are expressed as mean ± SEM.
Abbreviations: DMSO, dimethyl sulfoxide; MI, myocardial infarction; SHR, spontaneously hypertensive rats; LASSBio-294, 3,4-methylenedioxybenzoyl-2-thieny lhy-drazone; po, by mouth.
Echocardiographic changes induced in infarcted SHR
Echocardiographic evaluation and assessment of LV structural parameters revealed a significant decrease in diastolic and systolic AWT of the SHR-MI group compared with the SHR-Sham group. Changes in AWT were prevented in the SHR-MI+LASSBio-294 (10 mg.kg−1.d−1) and SHR-MI+LASSBio-294 (20 mg.kg−1.d−1) groups, relative to those observed in the untreated SHR-MI. No significant differences in diastolic and systolic PWT were observed between the groups. Diastolic LV internal chamber diameter was elevated in the SHR-MI group, and treatment with 20 mg.kg−1.d−1 of LASSBio-294 prevented this effect (). Both doses of LASSBio-294 reversed the MI-induced EF decrease, but only the higher dose (20 mg.kg−1.d−1) prevented the decline in fractional shortening ().
Table 1 LASSBio-294 improves systolic and diastolic structural changes in echocardiographic evaluation
Table 2 Echocardiographic evaluation of systolic and diastolic function of infarcted SHR after treatment with LASSBio-294
Diastolic function obtained through the analyses of early transmitral filling velocity to late transmitral filling velocity ratio (E/A) showed that the SHR-MI group developed a significant impairment of the filling pattern compared with the SHR-Sham group. On the other hand, this index was significantly increased with LASSBio-294 treatment in a dose-dependent manner ().
LASSBio-294 treatment improves hemodynamic changes in infarcted SHRs
SBP or DBP did not differ significantly between the SHR-Sham and SHR-MI groups. However, as shown in , LASSBio-294 treatment did reduce SBP significantly, at both the 10 mg.kg−1.d−1 (P<0.05 vs SHR-MI and SHR-Sham) and 20 mg.kg−1.d−1 (P<0.01 vs SHR-MI and SHR-Sham) doses. DBP was also reduced in a dose-dependent manner by the treatment ().
Figure 2 Effect of LASSBio-294 on the blood pressure in infarcted SHRs (A) SBP, systolic blood pressure; (B) DBP, diastolic blood pressure; (C) MBP, mean blood pressure; (D) heart rate; (E) double product. SHR-Sham treated with DMSO and SHR-MI treated with DMSO or LASSBio-294 orally, 10 and 20 mg.kg−1. Data are expressed as mean ± SEM.
Abbreviations: DBP, diastolic blood pressure; DMSO, dimethyl sulfoxide; MBP, mean blood pressure; SBP, systolic blood pressure; SHR, spontaneously hypertensive rats; LASSBio-294, 3,4-methylenedioxybenzoyl-2-thienylhydrazone; po, by mouth; MI, myocardial infarction.
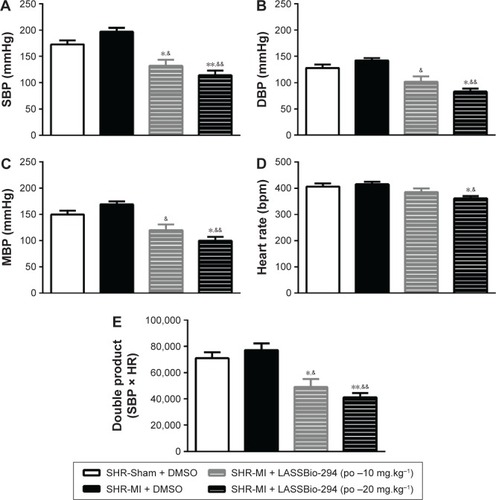
MBP in the SHR-MI + LASSBio-294 at dose 10 mg.kg−1.d−1 or 20 mg.kg−1.d−1 groups was reduced (P<0.05 and P<0.01, respectively) in a dose-dependent manner compared with the SHR-MI group (). The MBP of the SHR-MI + LASSBio-294 (20 mg.kg−1.d−1) group was even significantly lower than that of the SHR-Sham group (P<0.05).
Only the 20 mg.kg−1.d−1 dose of LASSBio-294 reduced the heart rate significantly in SHR-MI (P<0.05 vs SHR-MI and SHR-Sham) (). Nevertheless, the double rate product was reduced in both the SHR-MI + LASSBio-294 (10 mg.kg−1.d−1) (P<0.05) and SHR-MI + LASSBio-294 (20 mg.kg−1.d−1) (P<0.01) groups when compared with the SHR-MI and SHR-Sham groups ().
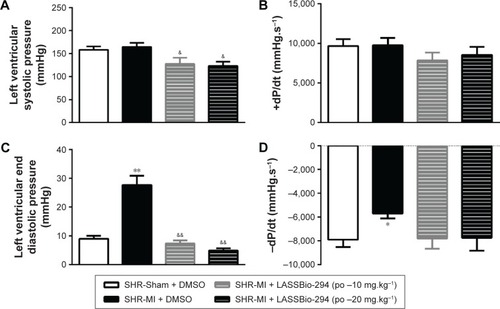
LV systolic pressure was reduced in both the SHR-MI + LASSBio-294 (10 mg.kg−1.d−1 and 20 mg.kg−1.d−1) groups compared with that in the SHR-MI group (P<0.05) (). However, there were no significant group differences in +dP/dt (). LVEDP was increased in the SHR-MI group (27.69±3.23 mmHg) compared with the SHR-Sham group (8.98±1.10 mmHg; P<0.01) and reduced in the SHR-MI + LASSBio-294 (10 mg.kg−1.d−1) (7.37±1.05 mmHg) and SHR-MI + LASSBio-294 (20 mg.kg−1.d−1) (4.91±0.76 mmHg) groups, compared with that in the SHR-MI group (both P<0.01) (). The rate of ventricular relaxation, represented by −dP/dt, was reduced in the SHR-MI group compared with that in the SHR-Sham group (P<0.05). Treatment with 10 mg.kg−1.d−1 or 20 mg.kg−1.d−1 of LASSBio-294 resulted in ventricular relaxation rates similar to that observed in the SHR-Sham group ().
Figure 3 Effect of LASSBio-294 on left ventricular pressure in infarcted SHRs. (A) LVSP, Left ventricular systolic pressure; (B) +dP/dt, derived left ventricular systolic pressure; (C) LVEDP, left ventricular end diastolic pressure; (D) −dP/dt, derived left ventricular diastolic pressure; SHR-Sham treated with DMSO and SHR-MI treated with DMSO or LASSBio-294 orally, 10 and 20 mg⋅kg−1. Data are expressed as mean ± SEM.
Notes: **P<0.01 compared to SHR-Sham; *P<0.05 compared to SHR-Sham; &&P<0.01 compared to SHR-MI; &P<0.05 compared to SHR-MI, n=6.
Effect of LASSBio-294 on cardiac hypertrophy and pulmonary edema in infarcted SHRs
Absolute heart weight and the heart-to-body weight ratio were significantly higher in the SHR-MI group than in the SHR-Sham group. Treatment with 10 mg.kg−1.d−1 or 20 mg.kg−1.d−1 LASSBio-294 reduced both heart weight and heart-to-body weight ratio, compared with the SHR-MI group. Similar results were observed with lung weight and lung-to-body weight ratio, which were increased in the SHR-MI group compared with the SHR-Sham group, but reduced in SHR-MI rats treated with LASSBio-294 at either dose ().
Table 3 Cardiac hypertrophy and pulmonary edema after myocardial infarction
Effect of LASSBio-294 on cardiac fibrosis and expression of TNF-α and SERCA2 in cardiac muscle in infarcted SHR
The presence of collagen in the left ventricle tissue was observed in sections stained with picrusirus (). Representative images of TNF-α () and SERCA () expression which were analyzed by immunohistochemistry in tissues from different groups.
Figure 4 Effect of LASSBio-294 on cardiac fibrosis and protein expression in infarcted SHRs.
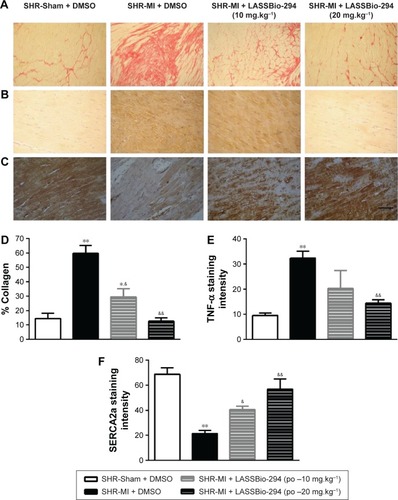
As expected, the SHR-MI group exhibited an increase in collagen percentage compared with that in the SHR-Sham group (; P<0.01). Treatment of SHR-MI with 10 mg.kg−1.d−1 LASSBio-294 reduced the collagen percentage by nearly half (P<0.05 vs SHR-MI), whereas treatment with 20 mg.kg−1.d−1 LASSBio-294 led to a full recovery, approximating the collagen percentage observed in the SHR-Sham group.
We demonstrated increased TNF-α expression in the SHR-MI group (P<0.01) compared with that observed in the SHR-Sham group (). TNF-α expression was reduced significantly in the SHR-MI + LASSBio-294 (20 mg.kg−1.d−1) group only (P<0.01 vs SHR-MI).
The SERCA2 expression was reduced in the SHR-MI group compared with the SHR-Sham group (21.46%±2.35% vs 68.75%±5.12%; P<0.01). Treatment led to a partial (in SHR-MI + LASSBio-294 [10 mg.kg−1.d−1]) or complete (in SHR-MI + LASSBio-294 [20 mg.kg−1.d−1]) recovery of SERCA2 expression (P<0.05, P<0.01, respectively), relative to the levels observed in animals without MI ().
Discussion
This study showed that chronic treatment with oral LASSBio-294 after MI improved exercise tolerance and attenuated the development of LV dysfunction and remodeling. In a previous work, we had demonstrated that LASSBio-294 prevented the development of HF in normotensive animals when administered intraperitoneally. This is the first time that oral administration of this substance has been shown to be efficacious in preventing the progression of LV dysfunction after MI in a preclinical model of preexisting hypertension.Citation15,Citation16
Exercise intolerance of untreated, infarcted rats was confirmed in our model by the profound reduction in the running distance traveled on the treadmill. Intolerance to physical exercise is one of the main symptoms of chronic diastolic and systolic dysfunction and is a quality of life determinant of patients with HF.Citation22 LASSBio-294 enhanced the running capacity of SHR-MI groups above that of their respective SHR-Sham group. The results of this study suggest that, by improving hemodynamic parameters, LASSBio-294 may contribute to exercise tolerance. However, we cannot exclude direct effects of the substance on skeletal muscle contractility. We have previously demonstrated that LASSBio-294 prevented MI-induced decrease of skeletal muscle function analyzed by the contractile response in vitro and exercise intolerance associated with SERCA2 expression decrease in the soleus muscle.Citation16 Our results are also in agreement with Gonzalez-Serratos et alCitation23 who demonstrated in single fiber frog skeletal muscles that LASSBio-294 reduces development of fatigue and accelerates the recovery of maximal tetanic tension after fatigue is developed. Although we did not directly investigate the effects of LASSBio-294 in SHR-MI rat skeletal muscle, it is reasonable to suggest that LASSBio-294 may act in both cardiac and skeletal muscles to improve exercise tolerance in infarcted SHRs.
LASSBio-294 resulted in maintenance of LV AWT in the injured myocardium and prevented the development of LV systolic and diastolic dysfunction after MI. The normalization of EF in LASSBio-294-treated rats could be attributed to the compound’s positive inotropic activity.Citation15,Citation24 All four AR subtypes, A1, A2A, A2B, and A3, have been detected in the heart,Citation12,Citation13 and stimulation of A2ARs is associated with increased cardiac contractility, SERCA2 expression, and reuptake of Ca2+ by the SRCitation25 effects which would be expected to be beneficial to cardiac function and hemodynamics. Moreover, the improvements in EF, as well as maintenance of SERCA2 expression, could have led to improvements in myocardial relaxation, which were characterized by the increase in E/A ratio, without a concomitant increase in LVEDP after MI. Indeed, either MI or hypertension in isolation can increase LVEDP, particularly when both conditions are present.Citation26 The treatment-associated reductions in LVEDP, increases in E/A, and improvements in systolic function brought the functional profile of the treated SHR-MI groups toward that seen in the SHR-Sham group.
Treatment with LASSBio-294 had an antihypertensive effect. In addition, LASSBio-294 led to a normalized heart rate and double product in SHR-MI in a dose-dependent manner. Indeed, both hypertension and MI promote development of vascular dysfunction. An attenuated vasodilator response to endothelium-dependent vasodilator agents associated with preexisting hypertension and ischemia has been described by Wiemer et al;Citation27 an effect could be related to a marked reduction of NO production. A probable mechanism by which the drug could lead to an antihypertensive effect could be attributed to agonism of A2AR. Stimulation of the A2AR activates the adenylate cyclase system, resulting in increased production of cyclic adenosine monophosphate (cAMP) and protein kinase A, which stimulate K+ channels, leading to adenosine triphosphate-dependent hyperpolarization of vascular smooth muscle cells.Citation28
Furthermore, infusion of the A2AR agonist ATL-146e, which has anti-inflammatory and vasodilatory effects, for 2–5 hours was reported to reduce infarct area,Citation29 associated with a substantial reduction of inflammation and neutrophil infiltration.Citation30 AMP579, an agonist of A1R and A2AR, provided a cardioprotective effect that was inhibited by a selective A2AR antagonist.Citation31 It is possible that LASSBio-294 could have prevented the onset of ventricular hypertrophy by activating A2ARs. In previous studies, we demonstrated that agonists of A2aRs prevented the development of pulmonary vascular remodeling and reduced cardiac hypertrophy in a model of pulmonary hypertension.Citation32,Citation33 Those results were in accordance with the reduction of pulmonary edema in SHR-MI and furthermore, reinforced by the reduction of pulmonary edema induced by stimulation of A2aA/A2B receptors described by Lu et al.Citation34
Other antiremodeling actions of LASSBio-294 in SHR-MI are exemplified by the reduction in cardiac collagen deposition and consequent myocardial fibrosis, which in LASSBio-294-treated rats may be related to the activation of A2ARs.Citation14,Citation31,Citation35,Citation36 This finding suggests that cAMP-dependent signaling by A2AR activation may limit cardiac remodeling and progression to HF by promoting fibroblast-induced inhibition of collagen synthesis.Citation37 According to Cabiati et al,Citation38 MI increased TNF-α gene expression in pigeon’s hearts but did not alter A2aR gene expression. Although A2aR and TNF-α messenger RNA levels apparently have not shown substantial correlation, we consider that LASSBio-294 reduced TNF-α expression by activating A2aR, based on the fact that A2aR activation inhibits cytokine secretion from activated macrophages and prevents TNF-α synthesis.Citation39 Taken together, the cardioprotective effect and reduction of post-myocardial injury induced by LASSBio-294 could be the result of inhibition of pro-inflammatory responses via stimulation of A2AR during ischemia.
Conclusion
Oral administration of the A2AR agonist, LASSBio-294 improved exercise tolerance and reduced hypertension and MI-related increase in blood pressure in a dose-dependent manner. It also prevented the development of diastolic and systolic dysfunction in SHRs after experimentally induced MI. Moreover, A2AR activation with this compound mitigated several consequences in SHR-MI animals, including changes in filling pressure, hypertrophy, fibrosis, and inflammation, apparently at least in part through decreased TNF-α expression. Previously, we observed that LASSBio-294 prevented cardiac remodeling in normotensive myocardial infarcted rats when administered intraperitoneally. The originality of the present work is in the fact that we could demonstrate that the oral administration of a novel A2a agonist rescued cardiovascular system from even more drastic damages induced by hypertension in association with MI, a condition that is often observed in clinics. The present data could be ratified and reinforced by using an A2A antagonist in order to observe if it counteracted the effects induced by LASSBio-294. However, we believe that the present findings have sufficient scientific evidences to support the notion that LASSBio-294 is a promising compound for the treatment of ischemia and hypertension-induced HF.
Acknowledgments
This work was supported by Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Instituto Nacional de Ciência e Tecnologia de Fármacos e Medica-mentos (INCT-INOFAR), and Centro Nacional de Biologia Estrutural e Bioimagem (CENABIO).
Disclosure
The authors report no conflicts of interest in this work.
References
- RapsomanikiETimmisAGeorgeJBlood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million peopleLancet20143839932 1899 191124881994
- MuschTIGhaulMRTranchitellaVZelisRSkeletal muscle glycogen depletion during submaximal exercise in rats with chronic heart failureBasic Res Cardiol1990856 606 6182076096
- MunkvikMLundePKAronsenJMBirkelandJASjaastadISejerstedOMAttenuated fatigue in slow twitch skeletal muscle during isotonic exercise in rats with chronic heart failurePLoS One201167 e2269521799933
- CiampiQVillariBRole of echocardiography in diagnosis and risk stratification in heart failure with left ventricular systolic dysfunctionCardiovasc Ultrasound20075 3417910744
- LibbyPBonowROMannDLZipesDPBraunwald’s Heart Disease: A Textbook of Cardiovascular Medicine, 2-Volume SetElsevier Health Sciences2007
- MandinovLEberliFRSeilerCHessOMDiastolic heart failureCardiovascular Res2000454 813 825
- TamargoJLopez-SendonJNovel therapeutic targets for the treatment of heart failureNat Rev Drug Discov2011107 536 55521701502
- Albrecht-KupperBELeineweberKNellPGPartial adenosine A1 receptor agonists for cardiovascular therapiesPurinergic signal20128Suppl 1 91 99
- CronsteinBNKramerSBRosensteinEDKorchakHMWeissmannGHirschhornROccupancy of adenosine receptors raises cyclic AMP alone and in synergy with occupancy of chemoattractant receptors and inhibits membrane depolarizationBiochem J19882523 709 7152844154
- CiarkaAvan de BornePPathakAMyocardial infarction, heart failure and sympathetic nervous system activity: new pharmacological approaches that affect neurohumoral activationExpert Opin Investig Drugs2008179 1315 1330
- JordanJEZhaoZQSatoHTaftSVinten-JohansenJAdenosine A2 receptor activation attenuates reperfusion injury by inhibiting neutrophil accumulation, superoxide generation and coronary endothelial adherenceJ Pharmacol Exp Ther19972801 301 3098996210
- HeadrickJPPeartJNReicheltMEHaselerLJAdenosine and its receptors in the heart: regulation, retaliation and adaptationBiochim Biophys Acta201118085 1413 142821094127
- LaylandJCarrickDLeeMOldroydKBerryCAdenosine: physiology, pharmacology, and clinical applicationsJACC Cardiovasc Interv201476 581 59124835328
- PeartJNHeadrickJPAdenosinergic cardioprotection: multiple receptors, multiple pathwaysPharmacol Ther20071142 208 22117408751
- CostaDGda SilvaJSKummerleAELASSBio-294, a compound with inotropic and lusitropic activity, decreases cardiac remodeling and improves Ca(2)(+) influx into sarcoplasmic reticulum after myocardial infarctionAm J Hypertens20102311 1220 122720671720
- da SilvaJSPereiraSLMaia RdoCN-acylhydrazone improves exercise intolerance in rats submitted to myocardial infarction by the recovery of calcium homeostasis in skeletal muscleLife Sci2014941 30 3624269214
- PfefferJPfefferMFletcherPBraunwaldEAlterations of cardiac performance in rats with established spontaneous hypertensionAm J Cardiol1979445 994 998158974
- PfefferJMPfefferMAFishbeinMCFrohlichEDCardiac function and morphology with aging in the spontaneously hypertensive ratAm J Physiol19792374 H461 H468495731
- PfefferMAPfefferJMFrohlichEDPumping ability of the hypertrophying left ventricle of the spontaneously hypertensive ratCirc Res1976385 423 429131652
- LiuQAndersonCBroydeAGlucagon-like peptide-1 and the exenatide analogue AC3174 improve cardiac function, cardiac remodeling, and survival in rats with chronic heart failureCardiovasc Diabetol20109 7621080957
- LangRMBierigMDevereuxRBRecommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of CardiologyJ Am Soc Echocardiogr20051812 1440 146316376782
- KitzmanDWGrobanLExercise intoleranceHeart Fail Clin200841 99 11518313628
- Gonzalez-SerratosHChangRPereiraEFA novel thienylhydrazone, (2-thienylidene)3,4-methylenedioxybenzoylhydrazine, increases inotropism and decreases fatigue of skeletal muscleJ Pharmacol Exp Ther20012992 558 56611602667
- SudoRTZapata-SudoGBarreiroEJThe new compound, LASSBio 294, increases the contractility of intact and saponin-skinned cardiac muscle from Wistar ratsBr J Pharmacol20011343 603 61311588115
- ChanTOFunakoshiHSongJCardiac-restricted overexpression of the A(2A)-adenosine receptor in FVB mice transiently increases contractile performance and rescues the heart failure phenotype in mice overexpressing the A(1)-adenosine receptorClin Transl Sci200812 126 13320354569
- XiaQGReineckeADorenkampMDaemenMJSimonRUngerTEffects of endothelin ETA receptor blocker LU 135252 on cardiac remodeling and survival in a hypertensive rat model of chronic heart failureActa pharmacol Sin20062711 1417 142217049116
- WiemerGItterGMalinskiTLinzWDecreased nitric oxide availability in normotensive and hypertensive rats with failing hearts after myocardial infarctionHypertension2001386 1367 137111751719
- GemignaniASAbbottBGThe emerging role of the selective A2A agonist in pharmacologic stress testingJ Nucl Cardiol2010173 494 49720221857
- GloverDKRiouLMRuizMReduction of infarct size and postischemic inflammation from ATL-146e, a highly selective adenosine A2A receptor agonist, in reperfused canine myocardiumAm J Physiol Heart Circ Physiol20052884 H1851 H185815591104
- PatelRAGloverDKBroisatAReduction in myocardial infarct size at 48 hours after brief intravenous infusion of ATL-146e, a highly selective adenosine A2A receptor agonistAm J Physiol Heart Circ Physiol20092972 H637 H64219502555
- SmitsGJMcVeyMCoxBFPerroneMHClarkKLCardioprotective effects of the novel adenosine A1/A2 receptor agonist AMP 579 in a porcine model of myocardial infarctionJ Pharmacol Exp Ther19982862 611 6189694911
- AlencarAKPereiraSLda SilvaFEN-acylhydrazone derivative ameliorates monocrotaline-induced pulmonary hypertension through the modulation of adenosine AA2R activityInt J Cardiol20141732 154 16224630383
- AlencarAKPereiraSLMontagnoliTLBeneficial effects of a novel agonist of the adenosine A2A receptor on monocrotaline-induced pulmonary hypertension in ratsBr J Pharmacol20131695 953 96223530610
- LuQHarringtonEONewtonJAdenosine protected against pulmonary edema through transporter- and receptor A2-mediated endothelial barrier enhancementAm J Physiol Lung Cell Mol Physiol20102986 L755 L76720228181
- GeraetsDKienzleMClinical use of adenosineIowa Med1992821 25 281735634
- NeubauerSThe failing heart – an engine out of fuelN Engl J Med200735611 1140 115117360992
- VillarrealFEppersonSARamirez-SanchezIYamazakiKGBruntonLLRegulation of cardiac fibroblast collagen synthesis by adenosine: roles for Epac and PI3KAm J Physiol Cell Physiol20092965 C1178 C118419279233
- CabiatiMMartinoAMattiiLAdenosine receptor expression in an experimental animal model of myocardial infarction with preserved left ventricular ejection fractionHeart Vessels2014294 513 51923843027
- FengWSongYChenCLuZZZhangYStimulation of adenosine A(2B) receptors induces interleukin-6 secretion in cardiac fibroblasts via the PKC-delta-P38 signalling pathwayBr J Pharmacol20101598 1598 160720050850
