Abstract
Several applications of chalcones and their derivatives encouraged researchers to increase their synthesis as an alternative for the treatment of pathogenic bacterial and fungal infections. In the present study, chalcone derivatives were synthesized through cross aldol condensation reaction between 4-(N,N-dimethylamino)benzaldehyde and multiarm aromatic ketones. The multiarm aromatic ketones were synthesized through nucleophilic substitution reaction between 4-hydroxy acetophenone and benzyl bromides. The benzyl bromides, multiarm aromatic ketones, and corresponding chalcone derivatives were evaluated for their activities against eleven clinical pathogenic Gram-positive, Gram-negative bacteria, and three pathogenic fungi by the disk diffusion method. The minimum inhibitory concentration was determined by the microbroth dilution technique. The results of the present study demonstrated that benzyl bromide derivatives have strong antibacterial and antifungal properties as compared to synthetic chalcone derivatives and ketones. Benzyl bromides (1a and 1c) showed high ester activity against Gram-positive bacteria and fungi but moderate activity against Gram-negative bacteria. Therefore, these compounds may be considered as good antibacterial and antifungal drug discovery. However, substituted ketones (2a–b) as well as chalcone derivatives (3a–c) showed no activity against all the tested strains except for ketone (2c), which showed moderate activity against Candida albicans.
Introduction
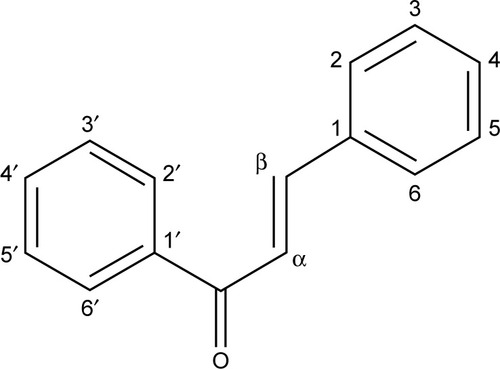
Chalcones are open-chain flavonoids with two aromatic rings joined by a three-carbon α,β-unsaturated carbonyl system (). They are found abundantly in fruits and vegetables.Citation1 Chalcones are used as precursors in the biosynthesis of flavonoids and isoflavonoids. Some of them were found to have remarkable biological activities and can act as antibacterial,Citation2 antifungal,Citation3 antiinflammatory,Citation4 antimalarial,Citation5 and anticancer agents.Citation6 The presence of α,β-unsaturated carbonyl moiety as the functional group makes the chalcones biologically active.Citation7 Chalcones are used as intermediates in the synthesis of various heterocyclic compounds such as pyrazoles, which are used as anticancer, antiinflammatory, antitumor, antibacterial, antipyretic, antidiabetic, and anticonvulsant agents and as monoamine oxidase inhibitors.Citation8–Citation11
Chalcones are found as dimmers, oligomers, and conjugates of different kinds. Additionally, various groups of hydroxyl, methoxy, and alkenyl functionalities can be attached to the framework of chalcone and contribute to its structural diversity.Citation12,Citation13 They are found as yellow pigments in flowers, roots, and leaves of species of genera Angelica, Artocarpus, and Dorstenia and widely distributed in fruits, vegetables, spices, tea, and beer.Citation14 Increase in resistance to antimicrobial agents has been a major concern for public health over the last few decades. Thus, the need for new drugs/compounds for the new disease and resistant strains is an essential part of medical care.
Several studies have shown that the introduction of different functional groups can improve the biological activity of chalcones. Regarding the antifungal activities of chalcones, the emergence of resistant strains of Candida species due to the intensive use of antifungal drugs increased the interest to develop new therapeutics.Citation15 The antifungal activity of chalcone is due to its effect on the cell wall, which depends largely on its potential to interact with sulfhydryl groups.Citation16
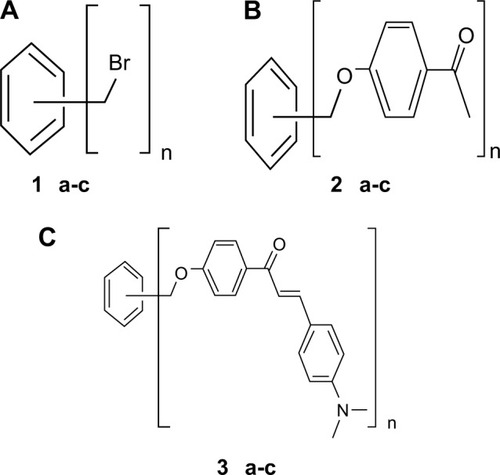
The present study examined the antibacterial and antifungal activities of prepared benzyl bromides (1a–c), substituted ketones (2a–c), and substituted chalcone derivatives (3a–c) against selected clinical pathogenic strains of bacteria and fungi. Chalcone derivatives (3a–c) were synthesized through cross aldol condensation reaction between aromatic ketones (2a–c) and 4-(N,N-dimethylamino)benzaldehyde in basic medium (). The procedure of synthesis, structures of various compounds, elemental analyses, nuclear magnetic resonance (1H- and 13C-nuclear magnetic resonance), infrared, and mass spectral data of all compounds were previously published by Al-Smadi and Al-Momani.Citation17
Materials and methods
Synthesized compounds
All synthesized compounds including benzyl bromides (1a–c), ketones (2a–c) and chalcone derivatives (3a–c) were obtained from Al-Smadi and Al-Momani.Citation17 The multiarm chalcone derivatives (3a–c) were synthesized (prepared) in basic medium by cross aldol condensation reaction between ketones (2a–c) and 4-(N,N-dimethylamino) benzaldehyde.Citation17
Test microorganisms
As shown in , a total of 14 reference clinical bacterial and fungal strains were obtained from King Abdullah University Hospital (KAUH) diagnostic laboratories. The pathogenic bacterial strains were Staphylococcus aureus, Pseudomonas aeruginosa, Enterococcus faecalis, Escherichia coli, Shigella dysenteriae, Salmonella typhi, Streptococcus pyogenes, Citrobacter freundii, Enterobacter aerogenes, Klebsiella pneumoniae, and Acinetobacter species. The pathogenic fungal strains were Candida albicans, Candida krusei ATCC14243, and Aspergillus niger ATCC16404. Antibacterial and antifungal activities were evaluated by the disk diffusion and the microbroth dilution methods as recommended by the Clinical and Laboratory Standards Institute (CLSI)Citation18–Citation20 and the European Committee on Antimicrobial Susceptibility Testing (EUCAST).Citation21,Citation22 As bacterial samples were obtained from the pathology laboratory stored samples, and were not identified to a certain patient, the Institutional Review Board of Jordan University of Science and Technology does not require ethics approval to be obtained.
Table 1 Pathogenic bacteria and fungi used for the antimicrobial assays
Antibacterial activity of the synthetic compounds using disk diffusion
Preparation of synthetic compounds for microbiological assay
A stock solution of 10 mg of each synthetic compound dissolved in 1 mL of dimethyl sulfoxide (DMSO) as solvent was prepared. The antimicrobial activity of the synthesized compounds was evaluated by the disk diffusion method and the microbroth dilution method, which determines minimum inhibitory concentration (MIC).Citation23
Determination of antibacterial activity by disk diffusion method
Antibacterial activity of the prepared synthetic compounds against the Gram-negative and the Gram-positive bacterial strains were examined by disk diffusion assay. Bacterial cultures were obtained from KAUH diagnostic laboratories. Isolated pure colonies from fresh grown bacteria were transferred from the plates into sterile normal saline solution and vortexed to form bacterial homogenous suspensions. The turbidity was then adjusted to 0.5 McFarland standard units, and the suspensions were poured over Mueller–Hinton agar (MHA) plates. Sterile filter paper disks with a diameter of 6 mm were placed over these plates. The sterile disks were impregnated with 20 µL of the tested compounds (10 mg/mL dissolved in DMSO). Positive control (Amoxicillin) and negative control (sterile distilled water) were used. Finally, the plates were incubated at 37°C for 24 hours. The inhibition zones were measured in millimeter.Citation24
Antifungal disk diffusion method
The fungal strains were cultured on Sabouraud dextrose agar and incubated at 35°C for 24 hours and for 5 days on potato dextrose agar slant for the mold fungi. Using a sterile loop, pure colonies of the Candida species were transferred into a tube containing sterile normal saline. For the mold, 1 mL of sterile distilled water supplemented with 0.1% Tween 20 was used to cover and resuspend the colonies. Using a hemocytometer, the suspension was adjusted to 2–5×106 conidia/mL. The suspension was further diluted 1:10 to obtain final working inoculums 2–5×105 conidia/mL. The inoculums were poured over MHA supplemented with 2% of glucose. The sterile 6 mm disks that were impregnated with 20 µL test compound (with a concentration of 10 mg/mL) were placed over the plate.
Standard antifungal drug Nystatin was used as positive control and sterile distilled water as negative control and incubated at 35°C for 48 hours. The zone of inhibition was measured in millimeter.Citation23
MICs by microbroth dilution method
The MIC was determined by measuring the absorbance of microtiter plates at 570 nm for bacteria and 530 nm for fungi. While for A. niger, MIC was determined visually. The optical density from each well was compared with optical density from the positive control wells, the lowest concentration with optical density <0.1 signifies inhibition and considered as MIC.
Antibacterial susceptibility testing by microbroth dilution method
The pure bacterial culture of each microorganism was adjusted to 0.5 McFarland standards in Mueller–Hinton broth (MHB). The bacterial inoculums were vortexed and diluted in MHB. The bacterial suspension was used within 30 minutes after turbidity adjustment to avoid duplication of the bacterial cells. Eight different concentrations were prepared from each stock with the medium (0.125, 0.25, 0.5, 1, 2, 4, 6, and 8 mg/mL). A total of 50 µL of each compound concentration was added to sterile 96-well microtiter plates. After that, 50 µL of diluted bacterial inoculums were added to each well including the negative control lane, and 100 µL of broth was added to positive control lane. (Each well reached the final desired concentration of 5×105 CFU/mL.) The plates were then incubated at 37°C for 18–24 hours. Then, they were examined to check where growth had taken place, and the inhibition of growth was determined by measuring the absorbance at 570 nm using an enzyme-linked immunosorbent assay (ELISA) reader.Citation24
Antifungal susceptibility testing by microbroth dilution method
The MICs for C. albicans and C. krusei were determined using the reference procedure of the Antifungal Susceptibility Testing of CLSI M27-A3Citation19 and EUCAST for the testing of fermentative yeasts.Citation22 MICs for A. niger (mold) were determined in accordance with EUCASTCitation22 and CLSI M38-A.Citation20 Briefly, testing was performed in sterile 96-well microtiter plates with Roswell Park Memorial Institute (RPMI) 1640 medium with L-glutamine, without sodium bicarbonate (NaHCO3, RPMI 1640; Gibco, Carlsbad, CA, USA) supplemented with 2% glucose, buffered to pH 7.0 with 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) medium.
Preparation of fungal (yeasts and molds) inoculums
Regarding preparation of yeast inoculums, the fungal strains were subcultured on Sabouraud’s dextrose agar slant and incubated for 24–48 hours at 35°C to obtain a freshly grown pure culture. Homogenous suspension was adjusted to 0.5 McFarland standards. Then, the inoculum size was further adjusted to 0.5×105 or 2.5×105. In addition, regarding the preparation of mold inoculums, the mold suspension of conidia was obtained from 5 days culture Sabor and dextrose agar slant incubated at 35°C. Colonies were covered with 5 mL of sterile distilled water supplemented with Tween 20. The conidia were collected with a sterile cotton swab, transferred to a sterile tube, and vortexed to homogenize the suspension. The suspension was standardized by counting the conidia in a hemocytometer to 2–5×106 conidia/mL. The suspension was diluted 1:10 with RPMI to obtain final inoculums of 2–5×105 conidia/mL. A total of 50 µL of each compound concentration and 50 µL of fungal suspension were added to each well for the negative control lane, but 100 µL of broth was added to the positive control lane (each well reached the final desired concentration of 2–5×105 CFU/mL). The plate was sealed with aluminum foil and incubated at 35°C for 2,448 hours in humid atmosphere. The MIC was determined using an ELISA reader at 530 nm for the yeast species and visually for mold species after 48 hours of incubation as the lowest concentration of drug that resulted in 50% inhibition of growth compared to that drug-free growth control.Citation18–Citation20
Results
Antibacterial and antifungal susceptibility testing
The benzyl bromides (1a–c), ketones (2a–c), and chalcone derivatives (3a–c) were tested against eleven clinical pathogenic strains of bacteria and three pathogenic fungal strains. The methods include the disk diffusion method and broth microdilution method for MIC determination in accordance with CLSI and EUCAST.Citation18–Citation22
As shown in and , the antibacterial and antifungal activities of the nine synthetic derivatives (benzyl bromide derivatives [1a–c], ketones [2a–c], and chalcone derivatives [3a–c]) were tested against clinical pathogenic bacterial and fungal strains. The benzyl bromide derivatives were the most effective compounds against the following Gram-positive bacteria: S. aureus, S. pyogenes, and E. faecalis with a range of inhibition zones (10–17 mm). Benzyl bromide derivative (1a) showed moderate effect against the Gram-negative bacteria E. coli, C. freundii, K. pneumoniae, and S. typhi with an inhibition zone of 7 mm. Benzyl bromide derivatives (1b) and (1c) showed moderate antibacterial activity against the tested Gram-positive bacteria S. aureus and E. faecalis with a range of inhibition zones (8–9 mm). Benzyl bromide derivative (1b) was highly active against S. pyogenes, with an inhibition zone of 15 mm. In addition, the benzyl bromide derivatives showed no activity against the tested Gram-negative bacteria as shown in . Benzyl bromide derivatives (1a–c) were highly effective against both C. albicans and C. krusei and showed inhibition zones ranging from 9 to 35 mm. Furthermore, benzyl bromide derivative (1a) was the only highly effective compound against A. niger with an inhibition zone of 14 mm. Ketone (2c) had a moderate effect against C. albicans with an inhibition zone of 7 mm as shown in . On the other hand, substituted chalcone derivatives (3a–c) as well as ketones (2a–b) showed no activity against all the tested pathogenic bacterial and fungal strains except for ketone (2c), which showed moderate activity against C. albicans with an inhibition zone of 7 mm.
Table 2 The antibacterial activity of the prepared synthetic compounds, the inhibition zone is measured in millimeter
Table 3 The antifungal activity of the nine prepared synthetic compounds, the inhibition zone is measured in millimeter
MIC determination
The antimicrobial potential of the synthesized compounds was evaluated (assessed) by determining the MIC values by the microdilution method. The MICs were determined by measuring the absorbance of microtiter plates at 570 nm for bacteria and 530 nm for fungi (yeasts). For A. niger, MICs were determined visually. The optical density from each well was compared with optical density from the positive control wells, the lowest concentration with optical density <0.1 signifies inhibition and considered as our MIC value ( and ). Benzyl bromides (1a) and (1c) were the most effective synthetic compounds having the least MIC value with widest spectrum of antibacterial activity as compared to the other tested compounds. The MIC value for benzyl bromide (1a) was 1 mg/mL against S. aureus and 2 mg/mL against S. pyogenes, E. faecalis, K. pneumoniae, S. typhi, and E. coli. The MIC value for benzyl bromide (1c) was 0.5 mg/mL against S. pyogenes, 2 mg/mL against E. faecalis, and 4 mg/mL for S. aureus, K. pneumoniae, and S. typhi. In addition, benzyl bromide (1b) showed poor and nondetectable antimicrobial activity against both Gram-negative and Gram-positive bacteria. Regarding MIC for pathogenic fungi, benzyl bromide (1a) had the lowest MIC with 0.25 mg/mL as the most effective compound against C. albicans. The lowest MIC value obtained for benzyl bromide (1c) was against C. krusei (0.5 mg/mL).
Table 4 The MIC values of benzyl bromides against pathogenic bacterial strains
Table 5 The MIC values of benzyl bromides against pathogenic fungal strains
Discussion
In the present study, the antibacterial and antifungal activities of nine synthesized compounds including the benzyl bromides (1a–c), ketones (2a–c), and chalcone derivatives (3a–c) were evaluated and tested against different pathogenic bacterial strains and fungal strains. The obtained results showed that benzyl bromides have strong antibacterial and antifungal properties as compared to the corresponding ketones and chalcone derivatives. The antimicrobial activity measurements of the nine tested synthetic compounds were performed by the disk diffusion method. All synthetic derivatives proved themselves to be more active against Gram-positive bacteria than Gram-negative. Benzyl bromide (1a) was the most active compound (highly effective) against Gram-positive cocci including S. aureus, S. pyogenes, and E. faecalis. Additionally, benzyl bromide (1a) has a moderate antimicrobial effect against the Gram-negative bacteria: E. coli, C. freundii, K. pneumoniae, and S. typhi. Furthermore, benzyl bromides (1b) and (1c) showed a moderate to high antibacterial activity against the tested Gram-positive bacteria S. aureus, E. faecalis, and S. pyogenes. The mechanism of bactericidal action is thought to be due to disruption of intermolecular interactions. This can cause dissociation of cellular membrane lipid bilayers, which compromises cellular permeability controls and induces leakage of cellular contents. The chalcones structure–activity relationship potency against C. albicans depends to a large extent on their ability to interact with thiol and hydroxyl chalcones.Citation15 Furthermore, the location of the hydroxyl group is strongly involved, the ortho-position and not the para- or the meta-being the most favorable, because of a mechanism by which the o-hydroxyl groups are involved in intermolecular hydrogen bond formation facilitating the addition of thiols.Citation25 Strong antifungal effects were obtained for the compounds of the series against dermatophytes, a group of fungi that infect the keratinized areas of the body. Regarding the influence of the substituents on the B ring, an interesting structure–activity correlation could be observed: electron-donating groups tended to weaken the antifungal activity while electron-withdrawing groups in the para-position increased the potency so that a nitro substituent enhanced the activities and an –N(CH3)2 had almost the lowest activity.Citation26 Therefore, it would be interesting to try a withdrawing group such as NO2 or CN or a halogen in the para rather than –N(CH3)2.
The antifungal activity measurements of the nine tested synthetic compounds were performed by the disk diffusion method. Benzyl bromides (1a), (1b), and (1c) were all active (highly effective) against both pathogenic fungi: C. albicans and C. krusei. Additionally, benzyl bromide (1a) was found to be distinguished among the nine tested compounds and showed to be highly active against the pathogenic fungus A. niger. Regarding ketones, ketone (2c) was found to have moderate antifungal effect against C. albicans. The chalcone derivatives (3a–c) as well as ketones (2a–b) showed no antibacterial or antifungal activity against all the tested pathogenic bacterial and fungal strains.
Furthermore, in order to evaluate the antimicrobial activity of the tested compounds in the present study; the MIC which is the lowest concentration of the tested compound that produced no visible bacterial growth (no turbidity) when compared with the control was determined by the microdilution method. Benzyl bromides (1a) and (1c) gave the least (MIC) values against Gram-positive cocci strains: S. aureus, S. pyogenes, E. faecalis, K. pneumoniae, S. typhi, and E. coli and proved themselves to be the most active of the nine compounds. Benzyl bromide (1b) showed poor and nondetectable antibacterial activity against both Gram-positive and Gram-negative bacteria. Regarding (MIC) pathogenic fungi, benzyl bromide (1a) was found to be the most active (effective) compound against C. albicans. The results obtained showed that the tested chalcone derivatives that contain aromatic amine substituents have no antibacterial or antifungal activity against all the tested clinical pathogenic species of bacteria and fungi. Many chalcones are known to exhibit reasonable antimicrobial and antifungal activity due to the presence of α,β-unsaturated carbonyl groups that can enhance the activity.Citation3,Citation4 In case of chalcone derivatives (3a–c), there are more than one α,β-unsaturated carbonyl group (chalcone) substituted on one benzene ring, which leads to electronic and steric interactions between the substituents. In addition to the large size of the substituents, the presence of an electron donating group (N,N-dimethyl amine) on the para-position of the chalcone that increases the direct resonance conjugation between the amino group and the chalcone might be the reason for the low antimicrobial and antifungal activity of the chalcone derivatives (3a–c). The presence of small substituents containing an electron withdrawing group (bromo group) that is surrounded by high electron density might be the reason for the high antibacterial and antifungal activity of the benzyl bromides (1a–c). Therefore, the inactivity of chalcone derivatives (3a–c) observed in the present study can be due to the presence of variations in compound structure and mode of substitution. Future research may need to be done to investigate antifungal and other biological activities such as antioxidant, antiinflammatory, and anticancer activity of the chalcone derivatives (3a–c).
Many studies have shown that chalcone derivatives exhibit several significant biological (antibacterial and antifungal) activities with the aim to discover the therapeutic values of them. A series of chalcones containing thiophene aromatic ring instead of benzene were synthesized and showed significant antimicrobial activity.Citation27,Citation28 Heterocyclic chalcone derivatives were also prepared and showed moderate activity against methicillin-sensitive S. aureus and methicillin-resistant Staphylococcus and no activity against vancomycin-resistant S. aureus as reported by Tran et al.Citation29 The antifungal activity of some chalcones against a dermatophytic fungus was investigated and showed that the presence of electron-donating substituents makes chalcone a weak antifungal agent, while electron-withdrawing substituents in the para position increase the antifungal activity.Citation26 Other studies reported that chalcones substituted with thiazole ring showed good antimicrobial activity.Citation30 Chalcones with a hybrid of rhodanine-3-acetic acid were reported to possess a potent antimicrobial activity.Citation31 Furthermore, a series of quinolinyl chalcones were tested for their in vitro antibacterial activities against different strains of Gram-negative and Gram-positive bacteria and showed significant antimicrobial activity.Citation32 Pyrazolyl-substituted chalcone derivatives exhibited strong antimicrobial activities.Citation33
Conclusion
The present study has clinical application as it will establish antimicrobial and antifungal behavior of some synthetic derivatives, which will be basic for future research on developing alternative antibiotics focusing on the development of better antibiotics against infectious organisms. The obtained results indicate that the benzyl bromides (1a–c) may have a considerable potential for therapeutic application as a novel drug candidate against bacterial and fungal infections. Depending on the obtained results, some of the studied compounds have proved themselves to be promising candidates for additional efficacy evaluation.
Disclosure
The authors report no conflicts of interest in this work.
References
- Di CarloGMascoloNIzzoAACapassoFFlavonoids: old and new aspects of a class of natural therapeutic drugsLife Sci199965433735310421421
- BandgarBPGawandeSSBodadeRGGawandeNMKhobragadeCNSynthesis and biological evaluation of a novel series of pyrazole chalcones as anti-inflammatory, antioxidant and antimicrobial agentsBioorg Med Chem200917248168817319896853
- GuptaDJainDKChalcone derivatives as potential antifungal agents: synthesis, and antifungal activityJ Adv Pharm Technol Res20156311411726317075
- BandgarBPPatilSAGaccheRNSynthesis and biological evaluation of nitrogen-containing chalcones as possible anti-inflammatory and antioxidant agentsBioorg Med Chem Lett201020273073320005707
- YadavNDixitSKBhattacharyaAAntimalarial activity of newly synthesized chalcone derivatives in vitroChem Biol Drug Des201280234034722429524
- BandgarBPGawandeSSSynthesis and biological screening of a combinatorial library of beta-chlorovinyl chalcones as anticancer, anti-inflammatory and antimicrobial agentsBioorg Med Chem20101852060206520138527
- SuwitoHJuminaMustofaKristantiAPuspaningsihNNChalcones: synthesis, structure diversity and pharmacological aspectsJ Chem Pharmaceut Res20146510761088
- HsiehHKLeeTHWangJPWangJJLinCNSynthesis and anti-inflammatory effect of chalcones and related compoundsPharm Res199815139469487544
- RamalingamKThyvelikakathGXBerlinKDSynthesis and biological activity of some derivatives of thiochroman-4-one and tetrahydrothiapyran-4-oneJ Med Chem1977206847850406397
- UlusoyNSynthesis and antituberculosis activity of cycloalkylidene-hydrazide and 4-aza-1-thiaspiro[4.5]decan-3-one derivatives of imidazo[2,1-b]thiazoleArzneimittelforschung200252756557112189781
- ChimentiFCarradoriSSecciDSynthesis and inhibitory activity against human monoamine oxidase of N1-thiocarbamoyl-3,5-di(hetero)aryl-4,5-dihydro-(1H)-pyrazole derivativesEur J Med Chem201045280080419926363
- Al-SmadiMAl-MomaniFSynthesis, characterization and antimicrobial activity of new 1,2,3-selenadiazolesMolecules200813112740274918985951
- ZhangEHWangRFGuoSZLiuBAn update on antitumor activity of naturally occurring chalconesEvid Based Complement Alternat Med2013201381562123690855
- NowakowskaZA review of anti-infective and anti-inflammatory chalconesEur J Med Chem200742212513717112640
- BatovskaDParushevSSlavovaAStudy on the substituents’ effects of a series of synthetic chalcones against the yeast Candida albicansEur J Med Chem2007421879217007965
- BoeckPLealPCYunesRAAntifungal activity and studies on mode of action of novel xanthoxyline-derived chalconesArch Pharm (Weinheim)20053382–3879515799012
- Al-SmadiMMohammadSSynthesis, characterization, and reactions of selected multichalcone derivativesJ Heterocyclic Chem2009462201206
- Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST)Method for the determination of Broth dilution MICs of antifungal agents for fermentative yeastsClin Microbiol Infect Diseases2008144398405
- CLSIReference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard-Third Edition; CLSI Document M27-A3Wayne, PAClinical and Laboratory Standards Institute2008
- CLSIReference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard CLSI Document M38-A2Wayne, PAClinical and Laboratory Standards Institute2008
- ChryssanthouECuenca-EstrellaMComparison of the EUCAST-AFST broth dilution method with the CLSI reference broth dilution method (M38-A) for susceptibility testing of posaconazole and voriconazole against Aspergillus sppClin Microbiol Infect200612990190416882296
- Cuenca-EstrellaMMooreCBBarchiesiFMulticenter evaluation of the reproducibility of the proposed antifungal susceptibility testing method for fermentative yeasts of the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antimicrobial Susceptibility Testing (AFST-EUCAST)Clin Microbiol Infect20039646747412848721
- EsmadiFTKhabourOFAlbarqawiAIAbabnehMAl-TalibMSynthesis and characterization of some transition metal complexes of thiocarbohydrazone Schiff basesJordan J Chem2013813143
- WiegandIHilpertKHancockREAgar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substancesNat Protoc20083216317518274517
- Dinkova-KostovaATMassiahMABozakREHicksRJTalalayPPotency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groupsProc Natl Acad Sci U S A20019863404340911248091
- LopezSNCastelliMVZacchinoSAIn vitro antifungal evaluation and structure-activity relationships of a new series of chalcone derivatives and synthetic analogues, with inhibitory properties against polymers of the fungal cell wallBioorg Med Chem2001981999201311504637
- BagSGhoshSTulsanRDesign, synthesis and biological activity of multifunctional alpha,beta-unsaturated carbonyl scaffolds for Alzheimer’s diseaseBioorg Med Chem Lett20132392614261823540646
- YinBTYanCYPengXMSynthesis and biological evaluation of alphatriazolyl chalcones as a new type of potential antimicrobial agents and their interaction with calf thymus DNA and human serum albuminEur J Med Chem20147114815924291568
- TranTDNguyenTTDoTHHuynhTNTranCDThaiKMSynthesis and antibacterial activity of some heterocyclic chalcone analogues alone and in combination with antibioticsMolecules20121766684669622728362
- LiarasKGeronikakiAGlamoclijaJCiricASokovicMThiazole-based chalcones as potent antimicrobial agents. Synthesis and biological evaluationBioorg Med Chem201119103135314021524583
- ChenZHZhengCJSunLPPiaoHRSynthesis of new chalcone derivatives containing a rhodanine-3-acetic acid moiety with potential anti-bacterial activityEur J Med Chem201045125739574320889240
- ChikhaliaKHPatelMJVashiDBDesign, synthesis and evaluation of novel quinolylchalcones as antibacterial agentsARKIVOC200813189197
- SiddiquiZNMusthafaTNAhmadAKhanAUThermal solvent-free synthesis of novel pyrazolyl chalcones and pyrazolines as potential antimicrobial agentsBioorg Med Chem Lett201121102860286521507638