Abstract
Background
The use of glucose as the only osmotic agent in peritoneal dialysis (PD) solutions (PDSs) is believed to exert local (peritoneal) and systemic detrimental actions, particularly in diabetic PD patients. To improve peritoneal biocompatibility, we have developed more biocompatible PDSs containing xylitol and carnitine along with significantly less amounts of glucose and have tested them in cultured Human Vein Endothelial Cells (HUVECs) obtained from the umbilical cords of healthy (C) and gestational diabetic (GD) mothers.
Methods
Primary C- and GD-HUVECs were treated for 72 hours with our PDSs (xylitol 0.7% and 1.5%, whereas carnitine and glucose were fixed at 0.02% and 0.5%, respectively) and two glucose-based PDSs (glucose 1.36% or 2.27%). We examined their effects on endothelial cell proliferation (cell count), viability (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide assay), intracellular nitro-oxidative stress (peroxynitrite levels), Vascular Cell Adhesion Molecule-1 and Intercellular Adhesion Molecule-1 membrane exposure (flow cytometry), and HUVEC-monocyte interactions (U937 adhesion assay).
Results
Compared to glucose-based PDSs, our in vitro studies demonstrated that the tested PDSs did not change the proliferative potential both in C- and GD-HUVECs. Moreover, our PDSs significantly improved endothelial cell viability, compared to glucose-based PDSs and basal condition. Notably, glucose-based PDSs significantly increased the intracellular peroxynitrite levels, Vascular Cell Adhesion Molecule-1 and Intercellular Adhesion Molecule-1 membrane exposure, and endothelial cell–monocyte interactions in both C- and GD-HUVECs, as compared with our experimental PDSs.
Conclusion
Present results show that in control and diabetic human endothelial cell models, xylitol–carnitine-based PDSs do not cause cytotoxicity, nitro-oxidative stress, and inflammation as caused by hypertonic glucose-based PDSs. Since xylitol and carnitine are also known to favorably affect glucose homeostasis, these findings suggest that our PDSs may represent a desirable hypertonic solution even for diabetic patients in PD.
Introduction
Peritoneal dialysis (PD) is a well-established mode of renal replacement therapy for patients suffering from end-stage renal disease, and has been used by approximately 11% of dialysis patients worldwide.Citation1 It is primarily a home-based treatment, which can be performed manually (continuous ambulatory PD [CAPD]) or employing a mechanical device (automated PD).
PD is based on the exchange of solutes and fluid between the peritoneal capillary blood and a solution (dialysate) introduced into the peritoneal cavity through an implanted catheter. PD solution (PDS) contains electrolytes, a buffer (lactate or bicarbonate), and an osmotic agent needed to remove excess water from the patient’s body (peritoneal ultrafiltration). Glucose is the osmotic agent almost universally used in PD due to its acceptable safety profile, efficacy, delivery of energy source, and low cost. Regulatory wise, the active osmotic ingredient present in PDSs is regarded as a drug and it must go through the traditional drug development process in order to achieve market authorization by the US Food and Drug Administration and by the European Medicines Agency.
PD therapy may provide a clinical outcome comparable to hemodialysis (HD)Citation2 and an even better quality of life.Citation3,Citation4 In addition, several studies underpin the important link between maintained residual renal function (RRF) in PD and survival benefit,Citation5–Citation7 whereas HD is linked with a loss of RRF compared with that observed in PD.Citation8
However, a major Achilles’ heel of the treatment is still represented by the poor local and systemic biocompatibility of current standard PDSs. Though several factors have been alleged,Citation9 glucose along with toxic glucose degradation products (GDPs) generated during heat sterilization of glucose-based solutionsCitation10 is by far thought as the main culprit for the bioincompatibility of PDS.Citation11 Glucose has been associated with functional and morphological damage to the peritoneal membraneCitation12 and to vascular cells such as the endothelial cells.Citation13,Citation14 Glucose and GDPs also induce apoptosis of peritoneal mesothelial cells and endothelial cells and, in particular, GDPs show a stronger reactivity than glucose in the formation of advanced glycation end-products, a known cause for microvascular complications and arteriosclerosis.Citation15 Moreover, excessive glucose absorption (up to 200 g/day) may cause or aggravate metabolic disturbances frequently encountered in end-stage renal disease, such as dyslipidemia, insulin resistance, hyperinsulinemia, inflammation, and altered adipokine levels.Citation16 Although PD has been traditionally considered a more physiological technique than HD, these results raise some doubts with respect to inflammation and endothelial damage.Citation17
Based on these evidences, is not surprising that strategies designed to reduce/eliminate glucose-associated toxicity form one of the modern goals of PD.Citation11,Citation18
A novel glucose-sparing approach may be represented by the use of osmo-metabolic agents in the PDS that are not only able to reduce intraperitoneal glucose load without compromising ultrafiltration, but also to independently mitigate underlying metabolic disorders.Citation19 Osmo-metabolic agents may be used singly, or in combination in order to maximize their therapeutic effects. In this context, our recent studies support the use of l-carnitine, which is involved in the mitochondrial oxidation of long-chain fatty acids,Citation20 as a suitable osmotic agent in PD.Citation21 The presence of l-carnitine in the solution was safe and well tolerated,Citation21,Citation22 and proved to be more biocompatible than glucose in several experimental models.Citation21,Citation23 In addition, a PDS containing l-carnitine significantly increased insulin sensitivity in a 4-month randomized controlled study in nondiabetic CAPD patients.Citation22
Furthermore, a study conducted some years ago highlights the potential beneficial effect of xylitol,Citation24 which is involved in the pentose phosphate shunt and has low glycemic properties.Citation25 Effect of xylitol used for at least 5 months as an osmotic agent fully replacing glucose in the PD fluid of six type 1 diabetic patients on CAPD proved to be safe, maintained peritoneal ultrafiltration, and significantly improved the glycemic control.Citation24
Aside from the different types of agents that may replace glucose, when developing a new PDS, one should also examine its impact on the peritoneal membrane, which consists of three layers: the capillary endothelium, the interstitium, and the mesothelium.Citation26 In PD therapy, the capillary endothelium is the major barrier of the peritoneum to the transport of water and solutes. In addition, it progressively emerged that the microvascular endothelium is not only a permeability barrier and a thromboresistant surface, but also the location of relevant synthetic and metabolic activities.Citation27
In the present study, we investigated the biocompatibility of a new experimental PDS containing l-carnitine, xylitol, and low amount of glucose, instead of glucose alone, on Human Vein Endothelial Cells (HUVECs) obtained from umbilical cords of healthy mothers and of mothers suffering from gestational diabetes (GD). GD is associated with increased oxidative stress, inflammation, and overexpression of inflammatory cytokines,Citation14,Citation28 which are the common abnormalities in patients on PD.Citation29–Citation31
Methods
Antibodies and materials
Experimental PDSs () were formulated in order to achieve an osmolarity (calculated) comparable to that of the commercially available low-GDPs glucose-based PDSs (Physioneal 40, glucose 1.36% or 2.27%; Baxter Healthcare, Mc Gaw Park, IL, USA), steam-sterilized, and provided in sterile disposable 2 L bags (HBiofluids Srl, Tovo S. Agata, Italy). In particular, xylitol and carnitine concentrations were selected according to the current approved dosages for parenteral administration as described by the US Food and Drug Administration.Citation32 Dulbecco’s Modified Eagle’s Medium (DMEM)-Low Glucose, M199 endothelial growth medium, penicillin–streptomycin, glutamine, phosphate-buffered saline, and 0.05% trypsin/0.02% ethylenediaminetetraacetic acid (EDTA) were purchased from Mascia Brunelli (Milan, Italy). Fetal bovine serum was purchased from Gibco by Life Technologies (Monza, MB, Italy), and tissue-culture disposables were from Eppendorf (Hamburg, Germany). Phycoerythrin (PE)-labeled anti-Vascular Cell Adhesion Molecule-1 (anti-VCAM-1) and fluorescein isothiocyanate (FITC)-labeled anti-Intercellular Adhesion Molecule-1 (anti-ICAM-1) antibodies were from BioLegend (San Diego, CA, USA). Endothelial cell growth factor, bovine serum albumin, dimethyl sulfoxide, and 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) were from Sigma Aldrich (St Louis, MO, USA). HKGreen-4A probe was synthesized and kindly provided by Prof Dan Yang’s lab.Citation33
Table 1 Composition of tested peritoneal dialysis solutions
Cell cultures and experimental procedures
Umbilical cords were obtained from randomly selected healthy mothers (Control, C) and from mothers with GD delivering at the hospitals of Chieti and Pescara. All procedures were in agreement with the University G. d’Annunzio Chieti-Pescara Ethical Comittee (Reference Number: 1879/09COET) and with the Declaration of Helsinki principles. After obtaining approval of the protocol from the University G. d’Annunzio Chieti-Pescara Ethical Comittee, signed informed consent was obtained from each participating subject.
Primary HUVECs were obtained as described previously and used between the third and fifth passages in vitro.Citation14 In this study, nine different HUVEC batches were employed and each experiment was performed on cells coming at least from three different batches. In all experiments, primary C- and GD-HUVECs were grown to confluency and exposed for 72 hours in 50:50 medium and PDSs (each of the four PDSs is reported in ).
Cell count
After treatment with the four different PDSs () for 72 hours, C- and GD-HUVECs were detached by using Trypsin-EDTA (10 min at 37°C), resuspended in culture medium, and then counted with Burker’s chamber.
MTT assay
The effect of the glucose-based and the experimental PDSs () on HUVEC viability was assessed by the MTT method. Briefly, C- and GD-HUVECs were plated in 96-well tissue culture plates (2,000 cells/cm2) stimulated as described above and MTT solution (0.5 mg/mL) was added to each well and incubated for 3 hours. Then, 200 μL of dimethyl sulfoxide was added to the cells for crystal solubilization. The spectrometric absorbance at 540 nm was read using a microplate reader (SpectraMAX 190; Molecular Devices, Sunnyvale, CA, USA).
Intracellular peroxynitrite levels
The intracellular levels of peroxynitrite (ONOO−) were detected in C- and GD-HUVECs stimulated with PDSs as described above by using the HKGreen-4A probe (10 μM, 30 min at 37°C), which was synthesized by Prof Dan Yang’s lab.Citation33
All data were analyzed using FACS Diva (BD Biosciences) and FlowJo™ Version 8.8.6 software (Tree-Star, Ashland, OR, USA) and expressed as percentage of positive cells.Citation34
Adhesion molecules membrane exposure
For fluorescence cytometry, C- and GD-HUVECs were stimulated as described in the experimental protocol and flow cytometry analysis performed as previously reported.Citation35 Briefly, nonpermeabilized cells were detached by EDTA 5 mM solution, washed, and resuspended in bovine serum albumin (0.5%). Cells were pelleted by centrifugation at 800 rpm for 15 min and then incubated with the primary antibodies anti-VCAM-1 PE-conjugate (1:100, PE; Biolegend) and anti-ICAM-1 FITC-conjugate (1:100, FITC), both for 30 min at room temperature. The incubation with primary antibody was followed by incubation with the specific FITC-labeled secondary antibody. All samples were analyzed on an FACS Canto II flow cytometer (BD Biosciences) using CellQuest™ software 3.2.1.f1 (BD Biosciences).Citation36 Quality control included a regular check-up with Cytometer Setup and Tracking beads (BD Biosciences). Debris was excluded from the analysis by gating on morphological parameters; 10,000 nondebris events in the morphological gate were recorded for each sample. All antibodies were titrated under assay conditions and optimal photomultiplier gains were established for each channel. Data were analyzed using FlowJo Version 8.8.6 software (TreeStar) and expressed as mean fluorescence intensity (MFI) ratio. The MFI ratio was calculated by dividing the MFI of positive events by the MFI of negative events.
Monocyte adhesion assays
We evaluated U937 monocyte adhesion to C- and GD-HUVECs using a cell adhesion assay in the normal growth condition (basal) and after incubation for 72 hours with the different PDSs (). Cells were grown to confluence in six-well tissue culture plates and U937 cell adhesion was evaluated as previously described.Citation35 Briefly, 1×106 U937 cells/mL were added to each HUVEC monolayer under rotating conditions (63 rev/min) at room temperature. After 20 min, nonadhering cells were removed and the monolayers were fixed with 1% paraformaldehyde. As experimental control, some monolayers were treated for 16 hours with tumor necrosis factor alpha 1 ng/mL and at 1 hour before the assay with mouse anti-human monoclonal antibody against VCAM-1 and ICAM-1. The number of adherent cells was assessed by counting eight different high-power fields (3.5 mm2). Photos of randomly chosen high-power fields were taken at half-radius distance from the center of the well in one of three comparative experiments of similar design, showing U937 monocytoid cell adhesion to C- and GD-HUVECs.
Statistical analysis
All experiments were repeated at least three times, and the results are presented as mean ± standard deviation (SD). Statistical analysis was performed by the unpaired Student’s t-test to compare basal C- and GD-HUVECs, or by one-way analysis of variance followed by the post hoc Bonferroni’s multiple comparison test whenever the analysis of variance indicated the presence of a statistical significance, to compare the effects of the different PDSs. Significance was defined as a P-value <0.05.
Results
Effect of PDSs on C- and GD-HUVECs viability
Treatment with glucose-based PDSs (glucose 1.36% and glucose 2.27%) and with the two experimental PDSs (xylitol 0.7% and xylitol 1.5%) for 72 hours did not alter the proliferative potential either in C- or in GD-HUVECs (data not shown). Notably, under the same experimental conditions, the experimental PDSs significantly improved C-HUVECs viability as compared to both glucose-based PDSs (0.80±0.05 and 0.88±0.03 vs 0.54±0.06 and 0.55±0.07 Abs540 nm, P<0.05 for xylitol 0.7% and xylitol 1.5% vs glucose 1.36% and glucose 2.27%, respectively) and basal condition (0.80±0.05 and 0.88±0.03 vs 0.51±0.05 Abs540 nm, P<0.05 for xylitol 0.7% and xylitol 1.5% vs basal, respectively), while for GD-HUVECs, a positive trend that did not reach significance was found. In contrast, glucose-based PDSs did not improve cell viability both in C- and GD-HUVECs (data not shown).
Effect of PDSs on C- and GD-HUVECs nitro-oxidative stress
In order to determine the potential protective effect of our experimental PDSs on nitro-oxidative stress, we evaluated the intracellular peroxynitrite levels in our cellular models.
Treatment with experimental PDSs significantly decreased peroxynitrite levels in GD-HUVECs, as compared to glucose-based PDSs and basal condition (). In C-HUVEC cultures, it was observed that xylitol-PDSs reduced nitro-oxidative stress and this reached statistical significance at the concentration of 1.5% ().
Figure 1 Effect of PDSs on intracellular peroxynitrite levels in C- and GD-HUVECs.
Abbreviations: C, control; GD, gestational diabetes; HUVECs, Human Vein Endothelial Cells; PDS, peritoneal dialysis solution; SD, standard deviation.
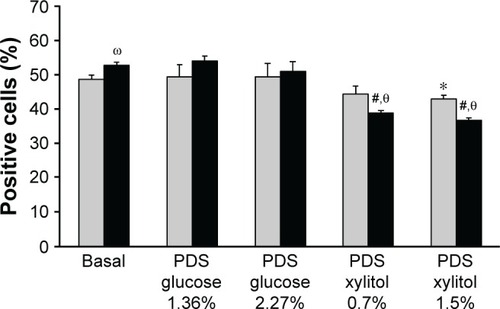
In addition, as previously demonstrated, unstimulated GD-HUVECs showed a significantly higher nitro-oxidative stress than C-HUVECs.Citation14
Effect of PDSs on adhesion molecule membrane exposure in C- and GD-HUVECs
We next evaluated whether adhesion molecule membrane exposure might be modified upon treatment with the different PDSs.
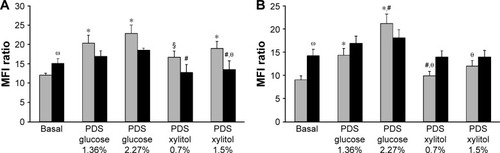
As shown in , following incubation with glucose-based PDSs, ICAM-1 () and VCAM-1 () membrane exposure increased in our cellular models, as compared to basal condition. Interestingly, our experimental PDSs decreased both ICAM-1 and VCAM-1 exposure compared to glucose-based PDSs, both in C- and GD-HUVECs (). Furthermore, as previously demonstrated, at baseline, GD-HUVECs displayed higher ICAM-1 and VCAM-1 exposure levels than C-HUVECs.Citation14
Figure 2 Effect of PDSs on adhesion molecules exposure in C- and GD-HUVECs.
Abbreviations: C, control; GD, gestational diabetes; HUVECs, Human Vein Endothelial Cells; ICAM-1, Intercellular Adhesion Molecule-1; MFI, mean fluorescence intensity; PDS, peritoneal dialysis solution; SD, standard deviation; VCAM-1, Vascular Cell Adhesion Molecule-1.
Effect of PDSs on U937 monocyte adhesion to C- and GD-HUVECs
By using the in vitro protocol of monocyte adhesion to the endothelium, which is close to the in vivo physiopathological state, we tested the effect of the four different PDSs on both C- and GD-HUVECs.
Stimulation with glucose-based PDSs caused an increase in the adhesion of monocytes to C- and GD-HUVECs, as compared to unstimulated cells (). Of note, and as expected, this proinflammatory effect was absent when both C- and GD-HUVECs were exposed to our experimental PDSs, thus confirming the absence of proinflammatory vascular effects.
Figure 3 Effect of PDSs on monocyte adhesion to C- and GD-HUVECs.
Abbreviations: C, control; GD, gestational diabetes; HUVECs, Human Vein Endothelial Cells; PDS, peritoneal dialysis solution; SD, standard deviation.
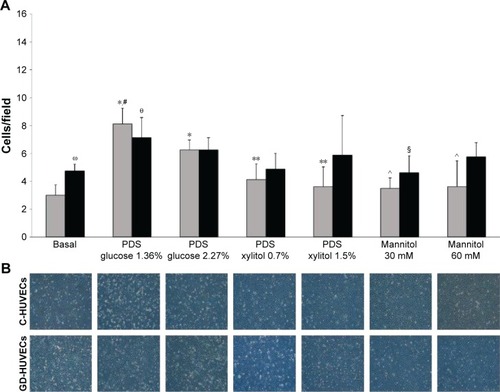
Interestingly, as compared to glucose-based PDSs, stimulation with xylitol-based PDSs induced a decreased trend in the adhesion of monocytes to GD-HUVECs, which reached statistical significance for C-HUVECs (). Moreover, the hyperosmolar control (mannitol at doses of 30 and 60 mM) did not induce any effect on monocyte adhesion to C- and GD-HUVECs, indicating that the proinflammatory vascular effects of glucose-based PDSs were independent of their increased osmolarity. In addition, as previously demonstrated, at basal condition, monocyte adhesion to GD-HUVECs was significantly higher than that of C-HUVECs.Citation14
Treating cells with anti-VCAM-1 or anti-ICAM-1 antibodies at saturating concentrations resulted in blocking U937 adhesion to both C- and GD-HUVECs, thus suggesting that hyperexpression of these molecules on the cell surface was among the main mechanisms for increased U937 adhesion to HUVECs (data not shown).
Discussion
In recent years, several studies have highlighted the detrimental effect of PDSs containing glucose on the longevity of PD patients.Citation11,Citation37,Citation38 Hence, a major challenge of PD therapy is the development of glucose-sparing strategies that are able to provide an efficacious ultrafiltration profile without jeopardizing patient health.
Today, glucose sparing can be primarily offered by the use of PDSs containing the glucose polymer icodextrin or aminoacids as osmotic agents replacing glucose. These formulations, either alone or in combination, have been shown to be effective and PD patients may benefit from their use.Citation18 However, both icodextrin and aminoacids can only replace 30%–50% of daily glucose absorption,Citation11 and their use is limited to a single daily peritoneal exchange.Citation39,Citation40 Furthermore, two recent randomized, controlled studies (IMPENDIA and EDEN) showed that a low-glucose regimen based on dextrose-based solutes, icodextrin and aminoacids, though improving metabolic indices in diabetic PD patients, was associated with an enhanced risk of extracellular fluid volume expansion, causing an increase in serious adverse events and deaths.Citation41
Thus, based on such findings, it is clear that the search for new solutions that manage to minimize the negative effects of PD represents an important objective. In the present study, we tested the biocompatibility of new experimental PDSs containing more than one osmo-metabolic agent, xylitol, glucose, and l-carnitine. Most of the osmotic strength of our PDSs is achieved by the presence of xylitol and carnitine, osmo-metabolic ingredients extremely stable from the chemical standpoint, even when steam-sterilized in an acidic environment (http://pubchem.ncbi.nlm.nih.gov).
In addition, the concept to introduce more than one osmo-metabolic agent in our PDSs is somehow derived from the well-known approach of polypharmacy or combination therapy, whereby the aim is to achieve a favorable synergetic action.Citation42,Citation43 Note that our experimental PDSs have a lower pH and a higher lactate concentration, conditions thought to affect biocompatibility of PD fluids,Citation9 than the tested, commercially available, normal pH, low-GDP PDS, which is regarded as a “biocompatible” solution.Citation44 Our PDSs were also steam-sterilized in a single-chambered bag containing a lactate-buffered glucose solution at pH 5.5, a procedure known to generate more than fourfold acetaldehyde, a reliable indicator of GDPs, than in the two-chambered commercial bag tested in our study.Citation45 The use of these xylitol–carnitine-based PDSs in our in vitro study proved not to change the proliferative potential in both C- and GD-HUVECs, compared to glucose-based PDSs. In addition, our PDSs significantly improved endothelial cell viability compared to basal condition.
Our results also show that glucose-based PDSs significantly increased VCAM-1 and ICAM-1 membrane exposure as compared to basal conditions in both C- and GD-HUVECs. Such proinflammatory vascular effect may have pathophysiologic consequences in the pathogenesis of atherosclerosis. In fact, increased expression of ICAM-1 and VCAM-1 on endothelial cell surface may promote adhesion of monocytes, which is a crucial event in vascular inflammation and the early atherosclerotic process.Citation46 Moreover, upon being exposed on the endothelial cells, VCAM-1 and ICAM-1 can be released into the circulation; increased plasma levels of adhesion molecules,Citation47 as found in PD patients,Citation48 have been associated with cardiovascular events and RRF.Citation49,Citation50 Indeed, we found that glucose-based PDSs caused a significant increase in monocyte interaction with both C- and GD-HUVECs compared to basal condition. Notably, when endothelial cells were exposed to experimental PDSs, all the above unfavorable vascular effects were absent.
Thus, PD therapy seems to induce a significant proinflammatory effect on endothelial cells, which has been attributed to the high glucose concentrations and/or GDPs present in PD standard solutions.Citation17 Although our experimental PDSs did contain some glucose (), this was not enough to trigger a comparable proinflammatory effect or nitro-oxidative stress as that seen for glucose-based PDSs in both C- and GD-HUVECs. This indicates that a small amount of glucose may be maintained in the PDS, in order to take advantage of its ultrafiltration ability and to provide energy source to patients who are often malnourished.
Conclusion
Our results show that in control and diabetic human endothelial cell models, xylitol–carnitine-based PDSs do not cause cytotoxicity and inflammation that are caused by the neutral pH, low-GDP hypertonic glucose-based PDSs. Since xylitol significantly inhibits hepatic glucose production,Citation51 is a poor insulin secretagogue, and possesses a low glycemic index, whereas carnitine improves muscle glucose disposal,Citation20 these findings suggest that osmo-metabolic-based PDSs may represent a desirable hypertonic solution even for diabetic patients in PD.
Acknowledgments
We gratefully acknowledge Daniela Heuberger for the excellent technical assistance. This work was in part supported by funds from Programma Operativo Nazionale (01_00937) – MIUR “Modelli sperimentali biotecnologici integrati per lo sviluppo e la selezione di molecole di interesse per la salute dell’uomo”.
Disclosure
AA is an employee of CoreQuest. The authors report no other conflicts of interest in this work.
References
- JainAKBlakePCordyPGargAXGlobal trends in rates of peritoneal dialysisJ Am Soc Nephrol201223353354422302194
- MehrotraRChiuYWKalantar-ZadehKBargmanJVoneshESimilar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal diseaseArch Intern Med2011171211011820876398
- BoatengEAEastLThe impact of dialysis modality on quality of life: a systematic reviewJ Ren Care201137419020022035363
- ZhangAHChengLTZhuNSunLHWangTComparison of quality of life and causes of hospitalization between hemodialysis and peritoneal dialysis patients in ChinaHealth Qual Life Outcomes200754917678543
- MaiorcaRBrunoriGZubaniRPredictive value of dialysis adequacy and nutritional indices for mortality and morbidity in CAPD and HD patients. A longitudinal studyNephrol Dial Transplant19951012229523058808229
- BargmanJMThorpeKEChurchillDNCPDS GroupRelative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA studyJ Am Soc Nephrol200112102158216211562415
- TermorshuizenFKorevaarJCDekkerFWvan ManenJGBoeschotenEWKredietRTNECOSAD Study GroupThe relative importance of residual renal function compared with peritoneal clearance for patient survival and quality of life: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2Am J Kidney Dis20034161293130212776283
- MoistLMPortFKOrzolSMPredictors of loss of residual renal function among new dialysis patientsJ Am Soc Nephrol200011355656410703680
- JorresATopleyNGahlGMBiocompatibility of peritoneal dialysis fluidsInt J Artif Organs199215279831555880
- Nilsson-ThorellCBMuscaluNAndrenAHKjellstrandPTWieslanderAPHeat sterilization of fluids for peritoneal dialysis gives rise to aldehydesPerit Dial Int19931332082138369351
- HolmesCJGlucotoxicity in peritoneal dialysis – solutions for the solution!Adv Chronic Kidney Dis200714326927817603981
- Diaz-BuxoJAGotloibLAgents that modulate peritoneal membrane structure and functionPerit Dial Int2007271163017179504
- PandolfiADe FilippisEAChronic hyperglicemia and nitric oxide bioavailability play a pivotal role in pro-atherogenic vascular modificationsGenes Nutr20072219520818850175
- Di FulvioPPandolfiAFormosoGFeatures of endothelial dysfunction in umbilical cord vessels of women with gestational diabetesNutr Metab Cardiovasc Dis201424121337134525438716
- KimYLChoJHChoiJYKimCDParkSHSystemic and local impact of glucose and glucose degradation products in peritoneal dialysis solutionJ Ren Nutr201323321822223510669
- BurkartJMetabolic consequences of peritoneal dialysisSemin Dial200417649850415660581
- CaballoCPalomoMCasesANFkappaB in the development of endothelial activation and damage in uremia: an in vitro approachPLoS One201278e4337422937042
- HolmesCMujaisSGlucose sparing in peritoneal dialysis: implications and metricsKidney Int Suppl2006103S104S10917080098
- Di PietroNGiardinelliASirolliVNitric oxide synthetic pathway and cGMP levels are altered in red blood cells from end-stage renal disease patientsMol Cell Biochem20164171–215516727206740
- ArduiniABonominiMSavicaVAmatoAZammitVCarnitine in metabolic disease: potential for pharmacological interventionPharmacol Ther2008120214915618793670
- BonominiMPandolfiADi LiberatoLl-carnitine is an osmotic agent suitable for peritoneal dialysisKidney Int201180664565421525850
- BonominiMDi LiberatoLDel RossoGEffect of an l-carnitine-containing peritoneal dialysate on insulin sensitivity in patients treated with CAPD: a 4-month, prospective, multicenter randomized trialAm J Kidney Dis201362592993823725973
- GaggiottiEArduiniABonominiMPrevention of peritoneal sclerosis: a new proposal to substitute glucose with carnitine dialysis solution (biocompatibility testing in vitro and in rabbits)Int J Artif Organs200528217718715770606
- BazzatoGColiULandiniSXylitol as osmotic agent in CAPD: an alternative to glucose for uremic diabetic patients?Trans Am Soc Artif Intern Organs1982282802866761936
- LiveseyGHealth potential of polyols as sugar replacers, with emphasis on low glycaemic propertiesNutr Res Rev200316216319119087388
- Di PaoloNSacchiGAtlas of peritoneal histologyPerit Dial Int200020Suppl 3S5S96
- ThorgeirssonGRobertsonALJrThe vascular endothelium-pathobiologic significanceAm J Pathol1978933803848362947
- OzuguzUIsikSBerkerDGestational diabetes and subclinical inflammation: evaluation of first year postpartum outcomesDiabetes Res Clin Pract201194342643321917349
- WangAYConsequences of chronic inflammation in peritoneal dialysisSemin Nephrol201131215917121439430
- StenvinkelPKettelerMJohnsonRJIL-10, IL-6, and TNF-alpha: central factors in the altered cytokine network of uremia – the good, the bad, and the uglyKidney Int20056741216123315780075
- LocatelliFCanaudBEckardtKUStenvinkelPWannerCZoccaliCOxidative stress in end-stage renal disease: an emerging threat to patient outcomeNephrol Dial Transplant20031871272128012808161
- SchneiderASSchettlerAMarkowskiAAssessment of xylitol serum levels during the course of parenteral nutrition including xylitol in intensive care patients: a case control studyClin Nutr201433348348823916161
- PengTWongNKChenXMolecular imaging of peroxynitrite with HKGreen-4 in live cells and tissuesJ Am Chem Soc201413633117281173425058034
- LanutiPRottaGAlmiciCEndothelial progenitor cells, defined by the simultaneous surface expression of VEGFR2 and CD133, are not detectable in healthy peripheral and cord bloodCytometry A201689325927026305912
- Di TomoPDi SilvestreSCordoneVGCentella asiatica and lipoic acid, or a combination thereof, inhibit monocyte adhesion to endothelial cells from umbilical cords of gestational diabetic womenNutr Metab Cardiovasc Dis201525765966626026207
- LanutiPMarchisioMCantilenaSA flow cytometry procedure for simultaneous characterization of cell DNA content and expression of intracellular protein kinase C-zetaJ Immunol Methods20063151–2374816945385
- DaviesSJPhillipsLGriffithsAMRussellLHNaishPFRussellGIWhat really happens to people on long-term peritoneal dialysis?Kidney Int1998546220722179853287
- ChaudharyKKhannaRBiocompatible peritoneal dialysis solutions: do we have one?Clin J Am Soc Nephrol20105472373220093342
- JonesMHagenTBoyleCATreatment of malnutrition with 1.1% amino acid peritoneal dialysis solution: results of a multicenter outpatient studyAm J Kidney Dis19983257617699820445
- JohnsonDWAgarJCollinsJRecommendations for the use of icodextrin in peritoneal dialysis patientsNephrology (Carlton)2003811715012742
- LiPKCulletonBFArizaARandomized, controlled trial of glucose-sparing peritoneal dialysis in diabetic patientsJ Am Soc Nephrol201324111889190023949801
- American Diabetes Association(7) Approaches to glycemic treatmentDiabetes Care201538Suppl 1S41S4825537707
- TaddeiSCombination therapy in hypertension: what are the best options according to clinical pharmacology principles and controlled clinical trial evidence?Am J Cardiovasc Drugs201515318519425850749
- SeoEYAnSHChoJHEffect of biocompatible peritoneal dialysis solution on residual renal function: a systematic review of randomized controlled trialsPerit Dial Int201434772473125185015
- CookerLALuneburgPFaictDChooCHolmesCJReduced glucose degradation products in bicarbonate/lactate-buffered peritoneal dialysis solutions produced in two-chambered bagsPerit Dial Int19971743733789284465
- RossRAtherosclerosis – an inflammatory diseaseN Engl J Med199934021151269887164
- GearingAJNewmanWCirculating adhesion molecules in diseaseImmunol Today199314105065127506035
- BonominiMRealeMSantarelliPStuardSSettefratiNAlbertazziASerum levels of soluble adhesion molecules in chronic renal failure and dialysis patientsNephron19987943994079689154
- WangAYLamCWWangMCirculating soluble vascular cell adhesion molecule 1: relationships with residual renal function, cardiac hypertrophy, and outcome of peritoneal dialysis patientsAm J Kidney Dis200545471572915806475
- ChoiHYLeeJEHanSHAssociation of inflammation and protein-energy wasting with endothelial dysfunction in peritoneal dialysis patientsNephrol Dial Transplant20102541266127119926717
- KishorePKehlenbrinkSHuMXylitol prevents NEFA-induced insulin resistance in ratsDiabetologia20125561808181222460760
