Abstract
Theophylline is a natural compound present in tea. Because of its property to relax smooth muscle it is used in pharmacology for the treatment of airway diseases (ie, chronic obstructive pulmonary disease, asthma). However, this effect on smooth muscle is dose dependent and it is related to the development of side effects. Recently, an increasing body of evidence suggests that theophylline, at low concentrations, also has anti-inflammatory effects related to the activation of histone deacetylases. In this study, we evaluated the effects of theophylline alone and in combination with corticosteroids on human bronchial epithelial cells under inflammatory stimuli. Theophylline administrated alone was not able to reduce growth-stimulating signaling via extracellular signal-regulated kinases activation and matrix metalloproteases release, whereas it strongly counteracts this biochemical behavior when administered in the presence of corticosteroids. These data provide scientific evidence for supporting the rationale for the pharmacological use of theophylline and corticosteroid combined drug.
Introduction
Asthma is characterized by activated mast cells, increased numbers of eosinophils, increased numbers of T helper 2 and T helper 17 lymphocytes, and increase in sensory neurogenic release.Citation1 Allergens, some pathogens, and some diseases have been implicated in the exacerbation of asthma in both animal models and humans.Citation2–Citation7 Theophylline is a natural compound present in tea. Because of its property to relax smooth muscle, oral theophylline has been administered to improve the bronchoconstriction in patients with severe asthma or chronic obstructive pulmonary disease (COPD). Theophylline has dose-dependent effects, and the therapeutic index of “high dose” theophylline is narrow. Therefore, its use at “high dose” (plasma levels 10–20 mg/L) as a bronchodilator, frequently, is related with the development of side effects.Citation8
Recently, however, preclinical studies have demonstrated that theophylline at “low dose” (plasma levels 1–5 mg/L) has anti-inflammatory effects not related to the adenosine receptor antagonism or phosphodiesterase (PDE) inhibition, which requires high dose.Citation9 In fact, at “low dose”, theophylline inhibits phosphoinositide 3-kinase (PI3K);Citation10 PI3K generates lipid second messengers involved in airways inflammation.Citation11 Moreover, To et alCitation12 documented that low concentrations of theophylline restore corticosteroid sensitivity by the enhancement of histone deacetylase (HDAC)-2 activity through inhibition of the d-isoform of PI3K and that this is independent from PDE inhibition and adenosine antagonism. In agreement with these data, Sun et al,Citation13 in an experimental study documented that a treatment with theophylline at low dose might improve the anti-inflammatory effects of steroids by increasing HDAC-2 activity.
In patients with airways diseases, particularly with COPD, tumor necrosis factor-alpha (TNF-alpha) and interleukin (IL)-1 blood levels correlate with COPD severity (the higher the levels of these inflammatory stimuli the more severe is COPD expression).Citation14
Montaño et alCitation15 documented increased plasma levels of matrix metalloproteinases (MMP-1, MMP-7, and MMP-9) in patients with COPD, compared to healthy subjects. In agreement, more recently, Ostridge et alCitation16 evaluating inflammatory cytokines in bronchoalveolar lavage from 24 COPD and eight control subjects documented higher levels of MMPs in lungs of subjects with COPD, suggesting that MMPs play a pivotal role in the development of COPD. Taken together, these studies documented that MMPs, ILs, and TNF-alpha may all be involved in the inflammatory pathways of COPD.
Moreover, inflammatory and structural cells, including neutrophils, macrophages, and epithelial cells, which are activated in the airways of patients with COPD release the oxidative stress that activates some transcription factors, for example, nuclear factor-κB (NF-κB) which switches on multiple inflammatory genes, resulting in amplification of the inflammatory response.Citation17 NF-κB is regulated by complex signal transduction pathways mediated by mitogen-activated protein kinases (MAPKs). The ERK1/2 subgroup of MAPKs is activated by an MAPK named Raf (most commonly Raf-1), whose activation in turn requires the guanosine-5′-triphosphate-bound form of Ras family proteins.Citation18 Once activated, Raf-1 phosphorylates the MAPKs MEK1 and MEK2, that finally stimulate ERK1 and ERK2. Airborne pollutants and cigarette smoke can induce the bronchial epithelium to acquire a proinflammatory phenotype, characterized by an increased production of autacoids, cytokines, and chemokines.Citation19 Oxidant-induced phenotypic changes may thus significantly contribute to the key pathogenic role played by bronchial epithelial cells in inflammatory airway disorders (eg, asthma and COPD).
Culpitt et alCitation20 in an in vitro study documented that the release of TNF-alpha and MMP-9 from macrophages taken from healthy subjects and normal smokers is inhibited by corticosteroids, whereas they are relatively ineffective in macrophages from patients with COPD. The reasons for resistance to corticosteroids in patients with COPD might be the marked reduction in activity of HDAC-2, which is recruited to activated inflammatory genes by glucocorticoid receptors to switch off inflammatory genes.Citation21 The decreased activity of HDAC-2 is related to both increased secretion of cytokines (eg, TNF-alpha) and reduced response to corticosteroids.Citation17 However, a recent finding highlights that the theophylline by itself has the capability to restore corticosteroid sensitivity.Citation22
With this knowledge, in this study, we evaluated the effects of theophylline alone and theophylline in combination with corticosteroids on primary cultures of human bronchial epithelial cells (HBECs) under inflammatory stimuli.
Methods
Reagents
Anti-phospho-ERK1/2, Anti-phospho-NF-κB, and monoclonal antibodies were purchased from New England Biolabs (Beverly, MA, USA); an anti-(total)-ERK1/2 polyclonal antibody was commercially provided by Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). All reagents and drugs needed for cell culture, protein extraction, and Western blotting were purchased from Sigma (St Louis, MO, USA). The drugs were obtained from Sigma and then dissolved in agreement with the datasheet of each drug, in saline (0.9%) or in light-protected dimethyl sulfoxide (DMSO) as a stock solution (stored at −20°C). Stock solution was then further diluted in cell culture medium to create working concentrations. The maximum final concentration of DMSO was <0.1%.
Recombinant TNF-alpha was obtained from PeproTech, Inc. (Rocky Hill, NJ, USA). Enzyme-linked immunosorbent assay (ELISA) for MMP-2 and MMP-9 monoclonal antibody (Biotrak Human ELISA System, Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, UK) was performed according to manufacturer’s protocol.
Primary cultures of HBECs
Adjacent normal lung tissue from a surgical specimen of lung tumor was obtained with the written informed consent of the patients. The study was approved by the Research Ethics Committee (EUDRACT number 2010019530-27), and was carried out in accordance with the Declaration of Helsinki and Guidelines for Good Clinical Practice. To avoid contamination of tumor tissue, the normal tissue was obtained far from the tumor lesion. Therefore, HBECs were obtained from fresh surgical specimens derived from patients who underwent either lobectomy or pneumectomy, in agreement with our previous papers.Citation23–Citation27
The cells were then harvested and cultured in bronchial epithelial growth medium (BEGM; Clonetics, Sand Diego, CA, USA) in the presence of antibiotics (100 U/mL of penicillin and 100 µg/mL of streptomycin; Sigma) and fungizone (1 µg/mL; Gibco BRL, Gaithersburg, MD, USA). Cells were maintained at 37°C in a humidified incubator, with 5% CO2. The cell line generated by primary epithelial culture medium was referred to as HBECs.
Experimental protocol
For assays, cells at passage 3 or 4 (4 days for each passage) were seeded into 24-well cell culture plates (1 mL BEGM/well containing 5×104 cells/mL). When cells reached 70%–80% confluence, they were then treated for 24 h with TNF-alpha (50 ng/mL) or IL-1 (1 ng/mL) in the presence or absence of an overnight treatment with methylprednisone (10−5 and 10−10 M), hydrocortisone (10−5 and 10−10 M), and theophylline (10−5 M). The medium was not changed after the treatment. The solvent employed to dissolve these drugs was used as control. After this period, the medium was collected for ELISA determination and cells were processed for protein extraction and immunoblotting.
Protein extraction and immunoblot analysis
Following treatment with TNF-alpha or IL-1, cells were lysed for Western blotting in radioimmunoprecipitation assay buffer as previously reported.Citation28–Citation32
Briefly, protein extracts were then separated on 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes (Amersham Pharmacia Biotech). Immunoblotting was performed using an anti-phospho-ERK1/2 monoclonal antibody. Membranes were reprobed with a polyclonal antibody against total (phosphorylated and unphosphorylated) protein. Antibody binding was visualized by enhanced chemiluminescence-Plus (Amersham Pharmacia Biotech); intensities of experimental bands were analyzed by computer-assisted densitometry (ImageJ software for Windows) and expressed as arbitrary units (control levels set at 100). These experiments were performed in triplicate.
Enzyme-linked immunosorbent assay
A commercially available sandwich ELISA kit for MMP-2 and MMP-9 determination based on monoclonal antibody (Human Biotrak ELISA System, Amersham Pharmacia Biotech) was used to determine MMP levels, in agreement, with our previous papers.Citation33–Citation35 These experiments were performed in triplicate.
Statistical analysis
All data are expressed as mean ± standard error of the mean. Statistical evaluation of the results was performed by analysis of variance (ANOVA). Differences identified by ANOVA were pinpointed by paired Student’s t-test. The threshold of statistical significance was set at P<0.05.
Results
ERKs phosphorylation and NF-κB modulation under proinflammatory stimuli and steroid drugs
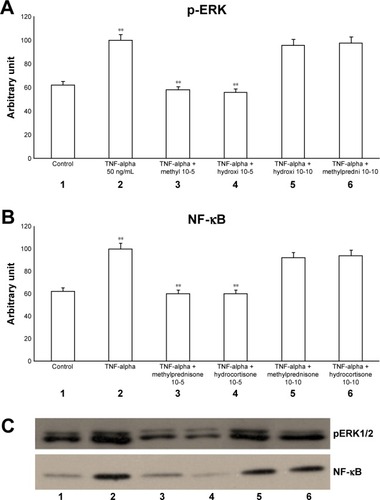
The exposure of HBECs for 24 h to TNF-alpha (50 ng/mL) induced a significant increase in the ERK1/2 phosphorylation (pERKs; P<0.01; , lane 2). In agreement with pERK, an increase in the activation of NF-κB (P<0.01) was recorded (, lane 2). The effect of TNF-alpha on both pERKs and NF-κB was significantly inhibited (P<0.01) by a 24-h treatment with methylprednisone and hydrocortisone at high dosage (10−5) (, lanes 3, 4). In contrast, corticosteroids at low dosage (10−10) did not modify the effects of TNF-alpha on both pERKs and NF-κB (, lanes 5, 6).
Figure 1 Effects of TNF-alpha (50 ng/mL) in the presence or absence of either methylprednisone (10−5 M) or hydrocortisone (10−10 M) in a 24-h treatment on (A) p-ERK and (B) NF-κB evaluated by (C) Western blot analysis in primary HBECs under the same treatments described in the graphs (lanes 1–6).
Abbreviations: HBECs, human bronchial epithelial cells; NF-κB, nuclear factor-κB; p-ERK, phosphorylated ERK 1/2; TNF, tumor necrosis factor.
The exposure of HBECs for 24 h to IL-1 (1 ng/mL) significantly increased (P<0.01) both pERK1/2 and NF-κB (, lane 2). These effects were significantly inhibited (P<0.01) by a 24-h treatment with methylprednisone and hydrocortisone at high concentrations (10−5) (, lanes 3, 4). In contrast, a 24-h treatment with methylprednisone and hydrocortisone at low concentrations (10−10) did not modify the effects of IL-1 on pERK and NF-κB (, lanes 5, 6). The treatment with theophylline (10−5) did not modify the effects of both TNF-alpha and IL-1 on p-ERK and NF-κB (, lane 2; 4A–C, lane 2), but potentiated the inhibitory effects of methylprednisone and hydrocortisone on both TNF-alpha and IL-1 (, lanes 5, 6; , lanes 5, 6). Both TNF-alpha and IL-1 exerted their effects uniquely on phosphorylation-dependent activation of ERK1/2, without affecting its total expression (data not shown).
Figure 2 Effects of IL-1 (1 ng/mL) in the presence or absence of either methylprednisone (10−5 M) or hydrocortisone (10−10 M) in a 24-h treatment on (A) p-ERK and (B) NF-κB evaluated by (C) Western blot analysis in the primary HBECs under the same treatments described in the graphs (lanes 1–6).
Abbreviations: HBECs, human bronchial epithelial cells; IL-1, interleukin-1; NF-κB, nuclear factor-κB; p-ERK, phosphorylated ERK 1/2.
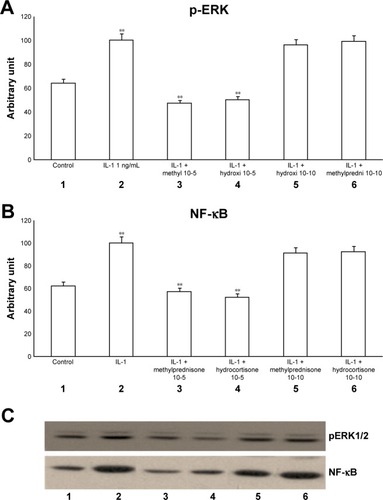
Figure 3 Effects of TNF-alpha (50 ng/mL) in the presence or absence of either methylprednisone (10−5 M) or hydrocortisone (10−10 M) in a 24-h treatment on (A) p-ERK and (B) NF-κB evaluated through (C) Western blot analysis in primary HBECs under the same treatments described in the graphs (lanes 1–7).
Abbreviations: HBECs, human bronchial epithelial cells; NF-κB, nuclear factor-κB; p-ERK, phosphorylated ERK 1/2; TNF, tumor necrosis factor.
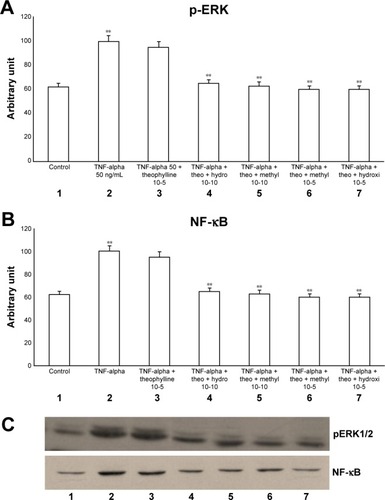
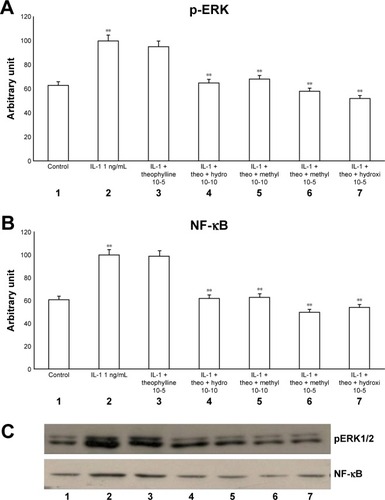
Figure 4 Effects of IL-1 (1 ng/mL) in the presence or absence of either methylprednisone (10−5 M) or hydrocortisone (10−10 M) in a 24-h treatment on (A) p-ERK and (B) NF-κB evaluated through (C) Western blot analysis in primary HBECs under the same treatments described in the graphs (lanes 1–7).
Abbreviations: HBECs, human bronchial epithelial cells; IL-1, interleukin-1; NF-κB, nuclear factor-κB; p-ERK, phosphorylated ERK 1/2.
Matrix metalloproteinases expression
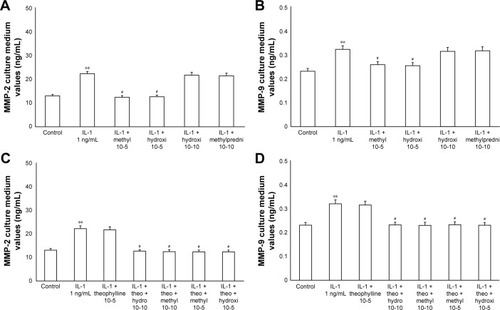
The exposure of HBECs for 24 h to TNF-alpha (50 ng/mL) induced a significant increase (P<0.01) in MMP-2 and MMP-9 expression. These effects were reversed by a 24-h treatment with methylprednisone and hydrocortisone at high dosage (10−5); in contrast, the treatment with corticosteroids at low dosage (10−10) did not modify the effects of TNF-alpha on MMPs expression (). Similarly, IL-1 (1 ng/mL) increased the expression of MMPs and this effect was significantly reversed (P<0.01) by a 24-h treatment with corticosteroids at high dosage (). Theophylline (10−5) potentiated the effects of methylprednisone and hydrocortisone on MMP-2 and MMP-9 expression (, ).
Figure 5 Primary HBECs under TNF-alpha (50 ng/mL) stimuli in the presence or absence of either methylprednisone (10−5 M) or hydrocortisone (10−10 M) in 24-h treatment. (A) Expression of MMP-2 and (B) MMP-9 in the absence of theophylline. (C) Expression of MMP-2 and (D) MMP-9 in the presence of theophylline (10−5 M). Protein levels were determined by Western blot analysis.
Abbreviations: HBECs, human bronchial epithelial cells; MMP, matrix metalloproteases; TNF, tumor necrosis factor.
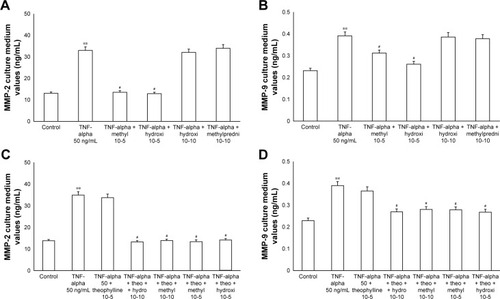
Figure 6 Primary HBECs under IL-1 (1 ng/mL) stimuli in the presence or absence of either methylprednisone (10−5 M) or hydrocortisone (10−10 M) in a 24-h treatment. (A) MMP-2 and (B) MMP-9 expression in the absence of theophylline. (C) MMP-2 and (D) MMP-9 expression in the presence of theophylline (10−5 M). Protein levels were determined by Western blot analysis.
Abbreviations: HBECs, human bronchial epithelial cells; IL-1, interleukin-1; MMP, matrix metalloproteases.
Discussion
TNF-alpha plays an important role in defense of the body against foreign pathogens such as viruses, bacteria, and fungi. TNF-alpha production within the lung (from several cells, ie, neutrophils, T cells, macrophages, monocytes, epithelial cells, fibroblasts, and smooth muscle cells) plays a central role in both inducing the expression of adhesion molecules (eg, intercellular adhesion molecule [ICAM]-1, vascular cell adhesion molecule-1, and E-selectin) and cytokines (eg, IL-1, IL-6, IL-8, platelet-derived growth factor, granulocyte macrophage colony stimulating factor, monocyte chemoattractant protein-1, and macrophage inflammatory protein 2).Citation36
Increased levels of chemokines, cytokines, and adhesion molecules induce both the activation and recruitment of neutrophils and macrophages to the lung and lead to tissue destruction.Citation37 In agreement, it has been documented that blood levels of TNF-alpha and IL-1 correlate with COPD severity.Citation38,Citation39 In this view, we treated primary HBECs with TNF-alpha and IL-1 to mimic organ conditions in COPD. In our study, both TNF-alpha and IL-1 induced a significant increase in pERK and NF-κB suggesting that these proinflammatory molecules play a role in the inflammatory process. These data are in agreement with other authors who documented that in human lung cells, TNF-alpha and IL-1beta induce the activation of NF-κB and MAPK signaling pathways.Citation40,Citation41
Previously in HBECs, we documented that TNF-alpha stimulates, via activation of p38 MAPK signaling pathway, IL-8 release and airway epithelial cell apoptosis, an effect that was inhibited by budesonide.Citation42
In agreement, Ito et alCitation43 showed both in vitro and in vivo that low-dose theophylline enhances HDAC activity in epithelial cells and macrophages via p38 MAPK activation and this effect increases the activity of corticosteroids.
Now, we have documented the involvement of ERK pathway in this mechanism. In fact in our study both methylprednisone and hydrocortisone reduced the effects of both TNF-alpha and IL-1 on both pERK and NF-κB, suggesting a suppressive effect at high concentration. In contrast, low concentration of theophylline did not counteract this effect, but significantly inhibited these mechanisms in the presence of both corticosteroids at low dosage, suggesting a synergic mechanism.
MMP-2 and MMP-9 are members of gelatinases, which start the degradation components of the endothelial basal lamina including type IV collagen, fibronectin, laminin, and heparan sulfate. In a recent clinical trial performed in patients with post-thrombotic syndrome, we documented a correlation between MMPs, TNF-alpha, and ILs suggesting that these molecules are involved in inflammatory pathways.Citation44 Moreover, previous experimental studies documented in cerebral endothelial cells show that TNF-alpha and IL-1 beta were able to increase the MMP-9 levels and this effect was reduced by dexamethasone treatment.Citation45,Citation46 In agreement, in the present study, TNF-alpha and IL-1-induced MMPs release that were inhibited by methylprednisone and hydrocortisone. The effects of these corticosteroids on MMPs secretion, seems to be related to two mechanisms: 1) block of the activator protein (AP)-1 site in the MMP-9 gene, and 2) induction of the expression of tissue inhibitor of metalloproteinase-1 (TIMP-1).Citation46 AP-1 is involved in the coordinated expression of several genes that control inflammation, cell proliferation, and apoptosis, while TIMP-1 is the controller of MMPs.Citation47 An imbalance between MMPs and TIMP is involved in airway diseases.Citation48,Citation49 Yigit et alCitation50 documented in 27 patients with inflammatory nasal polyposis that the treatment with oral corticosteroid reduces the tissutal expression of MMP2, while increases the tissutal expression of TIMP-1. Previously, in patients with COPD as well as in patients with asthma it has been documented that a combination therapy with corticosteroid and low-dose theophylline may reduce airway inflammation, improving clinical symptoms.Citation51,Citation52 An explanation of this synergic mechanism has been postulated by Sun et al,Citation13 that in an experimental study it is documented that a low-dose theophylline might improve the anti-inflammatory effects of steroids by increasing HDAC-2 activity.
In our study, theophylline used at low dosage potentiated the inhibitory effects of methylprednisone and hydrocortisone on MMP-2 and MMP-9, suggesting that theophylline may be able to improve the effects of steroids also through its anti-inflammatory activity.
Our study has several limitations. First, these data must be confirmed in clinical trials. Second, we measured the immunoreactivity of MMPs in the cells but these values are not related to the activity of the enzymes, which would have been better and more reflective of their actual enzymatic functional activity. In fact, although we used antibodies against active MMPs in this study, we did not measure activity or localize the activity. However, this study could increase the knowledge related to the synergic effects of these compounds supporting the rationale for the use of theophylline at low dosage with corticosteroid in respiratory diseases with an inflammatory component. This synergy (theophylline-corticosteroids at low dosages) resulting in improved anti-inflammatory effect, could improve the clinical efficacy of the compounds, reducing their side effects.
Acknowledgments
The authors thank Dr Erika Cione and Dr Manuela Sgambato for significant technical help and Dr Domenico Sturino for the careful English review of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- D’AgostinoBAdvenierCde PalmaRThe involvement of sensory neuropeptides in airway hyper-responsiveness in rabbits sensitized and challenged to Parietaria judaicaClin Exp Allergy2002323 472 47911940080
- D’AgostinoBMarroccoGDe NardoMActivation of the nociceptin/orphanin FQ receptor reduces bronchoconstriction and microvascular leakage in a rabbit model of gastroesophageal refluxBr J Pharmacol20051446 813 82015685213
- D’AgostinoBOrlottiDCalòGNociceptin modulates bronchoconstriction induced by sensory nerve activation in mouse lungAm J Respir Cell Mol Biol2010422 250 25419448152
- SulloNRoviezzoFMatteisMNociceptin/orphanin FQ receptor activation decreases the airway hyperresponsiveness induced by allergen in sensitized miceAm J Physiol Lung Cell Mol Physiol201330410 657 664
- GallelliLD’AgostinoBMarroccoGRole of tachykinins in the bronchoconstriction induced by HCl intraesophageal instillation in the rabbitLife Sci20037210 1135 114212505544
- Miraglia Del GiudiceMMaielloNDecimoFAirways allergic inflammation and L. reuterii treatment in asthmatic childrenJ Biol Regul Homeost Agents2012261 Suppl S35 S4022691248
- StoltzDJJacksonDJEvansMDSpecific patterns of allergic sensitization in early childhood and asthma & rhinitis riskClin Exp Allergy2013432 233 24123331564
- GallelliLFerreriGColosimoMRetrospective analysis of adverse drug reactions to bronchodilators observed in two pulmonary divisions of Catanzaro, ItalyPharmacol Res2003476 493 49912742002
- Morfín MacielBMCastillo MorfínBMTheophylline, a new look to an old drugRev Alerg Mex2010574 112 12221255523
- FoukasLCDanieleNKtoriCAndersonKEJensenJShepherdPRDirect effects of caffeine and theophylline on p110 delta and other phosphoinositide 3-kinases: differential effects on lipid kinase and protein kinase activitiesJ Biol Chem200227740 37124 3713012145276
- ItoKCaramoriGAdcockIMTherapeutic potential of phosphatidylinositol 3-kinase inhibitors in inflammatory respiratory diseaseJ Pharmacol Exp Ther20073211 1 817021257
- ToYItoKKizawaYTargeting phosphoinositide-3-kinase-delta with theophylline reverses corticosteroid insensitivity in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med20101827 897 90420224070
- SunXLiQGongYRenLWanHDengWLow-dose theophylline restores corticosteroid responsiveness in rats with smoke-induced airway inflammationCan J Physiol Pharmacol2012907 895 90222708526
- HuangAXLuLWLiuWJHuangMPlasma inflammatory cytokine IL-4, IL-8, IL-10, and TNF-α levels correlate with pulmonary function in patients with asthma-chronic obstructive pulmonary disease (COPD) overlap syndromeMed Sci Monit201622 280027501772
- MontañoMSansoresRHBecerrilCFEV1 inversely correlates with metalloproteinases 1, 7, 9 and CRP in COPD by biomass smoke exposureRespir Res201415 7424980707
- OstridgeKWilliamsNKimVRelationship between pulmonary matrix metalloproteinases and quantitative CT markers of small airways disease and emphysema in COPDThorax2016712 126 13226645414
- BarnesPJInflammatory mechanisms in patients with chronic obstructive pulmonary diseaseJ Allergy Clin Immunol20161381 16 2727373322
- ChangLKarinMMammalian MAP kinase signalling cascadesNature20014106824 37 4011242034
- HolgateSTThe sentinel role of the airway epithelium in asthma pathogenesisImmunol Rev20112421 205 21921682747
- CulpittSVRogersDFShahPImpaired inhibition by dexamethasone of cytokine release by alveolar macrophages from patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med20031671 24 3112406856
- BarnesPJRole of HDAC2 in the pathophysiology of COPDAnnu Rev Physiol200971 451 46418817512
- BarnesPJCorticosteroid resistance in patients with asthma and chronic obstructive pulmonary diseaseJ Allergy Clin Immunol20131313 636 64523360759
- PelaiaGCudaGVatrellaAEffects of hydrogen peroxide on MAPK activation, IL-8 production and cell viability in primary cultures of human bronchial epithelial cellsJ Cell Biochem2004931 142 15215352171
- CioneETucciPSenatoreVSynthesized esters of ferulic acid induce release of cytochrome c from rat testes mitochondriaJ Bioenerg Biomembr2008401 19 2617899337
- CioneEGenchiGCharacterization of rat testes mitochondrial retinoylating system and its partial purificationJ Bioenerg Biomembr2004362 211 21715224971
- BonofiglioDCioneEVizzaDBid as a potential target of apoptotic effects exerted by low doses of PPARγ and RXR ligands in breast cancer cellsCell Cycle20111014 2344 235421654191
- KaneMAFoliasAEPingitoreACrbpI modulates glucose homeostasis and pancreas 9-cis-retinoic acid concentrationsMol Cell Biol20113116 3277 328521670153
- PelaiaGGallelliLD’AgostinoBEffects of TGF-beta and glucocorticoids on map kinase phosphorylation, IL-6/IL-11 secretion and cell proliferation in primary cultures of human lung fibroblastsJ Cell Physiol20072102 489 49717044077
- GallelliLFalconeDPelaiaGInterleukin-6 receptor superantagonist Sant7 inhibits TGF-induced proliferation of human lung fibroblastsCell Prolif2008413 393 40718435790
- PelaiaGGallelliLRendaTEffects of statins and farnesyl transferase inhibitors on ERK phosphorylation, apoptosis and cell viability in non small lung cancer cellsCell Prolif2012456 557 56523045963
- PreianòMPasquaLGallelliLSimultaneous extraction and rapid visualization of peptidomic & lipidomic body fluids fingerprints by using mesoporous aluminosilicate and MALDI-TOF MSProteomics20121222 3286 329422997056
- RocciaFCampoloBGallelliLEffects of ambrisentan in a patient affected by combined pulmonary fibrosis and emphysema and by severe pulmonary hypertension: clinical, functional and biomolecular findingsClin Drug Invest2013336 451 457
- SerraRGallelliLContiAThe effects of sulodexide on both clinical and molecular parameters in patients with mixed arterial and venous ulcers of lower limbsDrug Des Devel Ther20148 519 527
- SerraRBuffoneGFalconeDChronic venous leg ulcers are associated with high levels of metalloproteinases-9 and neutrophil gelatinase-associated lipocalinWound Repair Regen2013213 395 40123531093
- SerraRGrandeRBuffoneGGallelliLDe FranciscisSThe effects of minocycline on extracellular matrix in patients with chronic venous leg ulcersActa Phlebol2013143 99 107
- CromwellOHamidQCorriganCJExpression and generation of interleukin-8, IL-6 and granulocyte-macrophage colony-stimulating factor by bronchial epithelial cells and enhancement by IL-1 beta and tumour necrosis factor-alphaImmunology1992773 330 3371478679
- DemirjianLAbboudRTLiHDuronioVAcute effect of cigarette smoke on TNF-alpha release by macrophages mediated through the erk1/2 pathwayBiochim Biophys Acta200617626 592 59716777389
- von HaehlingSHopkinsonNSPolkeyMINiethammerMAnkerSDGenth-ZotzSElevated TNFalpha production in whole blood in patients with severe COPD: the potential link to disease severityWien Klin Wochenschr20091219–10 303 30819562291
- HammadDina RElgazzarAhmed GEssawyTarek SAbd El SameieSohair AEvaluation of serum interleukin-1 beta as an inflammatory marker in COPD patientsEgypt J Chest Dis Tuberc201564 347 352
- ChunlianWHeyongWJiaXJieHXiCGentaoLMagnolol inhibits tumor necrosis factor-α-induced ICAM-1 expression via suppressing NF-κB and MAPK signaling pathways in human lung epithelial cellsInflammation2014376 1957 196724893579
- LinFSLinCCChienCSLuoSFYangCMInvolvement of p42/p44 MAPK, JNK, and NF-κB in IL-1β-induced ICAM-1 expression in human pulmonary epithelial cellsJ Cell Physiol2005202 464 47315389584
- GallelliLPelaiaGFrattoDEffects of budesonide on P38 MAPK activation, apoptosis and IL-8 secretion, induced by TNF-alpha and haemophilus influenzae in human bronchial epithelial cellsInt J Immunopathol Pharmacol2010232 471 47920646342
- ItoKLimSCaramoriGA molecular mechanism of action of theophylline: Induction of histone deacetylase activity to decrease inflammatory gene expressionProc Natl Acad Sci U S A20029913 8921 892612070353
- de FranciscisSGallelliLAmatoBPlasma MMP and TIMP evaluation in patients with deep venous thrombosis: could they have a predictive role in the development of post-thrombotic syndrome?Int Wound J Epub2015924
- HarknessKAAdamsonPSussmanJDDavies-JonesGAGreenwoodJWoodroofeMNDexamethasone regulation of matrix metalloproteinase expression in CNS vascular endotheliumBrain20001234 698 70910734001
- FörsterCKahlesTKietzSDrenckhahnDDexamethasone induces the expression of metalloproteinase inhibitor TIMP-1 in the murine cerebral vascular endothelial cell line cENDJ Physiol20075803 937 94917317742
- ChungKFAdcockIMMultifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destructionEur Respir J2008316 1334 135618515558
- GuanWJGaoYHXuGSputum matrix metalloproteinase-8 and -9 and tissue inhibitor of metalloproteinase-1 in bronchiectasis: clinical correlates and prognostic implicationsRespirology2015207 1073 108126122009
- ChaudhuriRMcSharryCBradyJLow sputum MMP-9/TIMP ratio is associated with airway narrowing in smokers with asthmaEur Respir J2014444 895 90424993912
- YigitOAcioğluEGelişgenRServerEAAzizliEUzunHThe effect of corticosteroid on metalloproteinase levels of nasal polyposisLaryngoscope20111213 667 67321305560
- FordPADurhamALRussellREGordonFAdcockIMBarnesPJTreatment effects of low-dose theophylline combined with an inhaled corticosteroid in COPDChest20101376 1338 134420299628
- MarkhamAFauldsDTheophylline. A review of its potential steroid sparing effects in asthmaDrugs1998566 1081 10919878995
