Abstract
Community-acquired bacterial pneumonia (CABP) is a leading cause of death worldwide. However, antibacterial agents used to treat common pathogens in CABP are marked by adverse drug events and increasing antimicrobial resistance. Solithromycin is a new ketolide antibiotic, based on the macrolide antibiotic structure, being studied for use in CABP. It has efficacy in vitro against the common causative pathogens in CABP including Streptococcus pneumoniae, Haemophilus influenzae, and atypical pathogens. In Phase II and Phase III clinical trials, it has been demonstrated efficacious as a single agent for treatment of CABP with an apparently milder adverse event profile than alternative agents.
Introduction
Community-acquired bacterial pneumonia (CABP) is a lower respiratory tract infection acquired anywhere other than an acute care (hospital) or long-term care (nursing facility) setting.Citation1 In the USA alone, CABP may affect over 5 million patients and cause over 60,000 deaths annually.Citation2,Citation3 Worldwide, lower respiratory tract infections may be responsible for nearly 3 million deaths annually.Citation4 Patients ≥65 years and patients <5 years have a higher incidence of pneumonia, but pneumonia may affect patients of any age.Citation5 The most common causes of CABP are Streptococcus pneumoniae (pneumococcus), Haemophilus influenzae, and in some regions, atypical pathogens including Legionella, Mycoplasma, and Chlamydia species. Other pathogens, including Mycoplasma and Gram-negative bacilli, may also be involved.Citation1,Citation4,Citation6 Empiric recommendations are based on historical data and focus is on macrolide, fluoroquinolone, and beta-lactam antibiotics alone or in combination.Citation1 The high relative mortality of infections such as pneumonia coupled with problems with multi-drug resistant organisms, including macrolide-resistant S. pneumoniae, have prompted international calls for the design and development of novel antimicrobial agents.Citation7–Citation9
Solithromycin is a fluoroketolide “fourth-generation” macrolide antibiotic designed by Cempra®, Inc (Chapel Hill, NC, USA). Phase II and III trials have been conducted assessing solithromycin for use in CAPB and uncomplicated gonorrhea with promising results.Citation10–Citation13 The highlights of solithromycin use in CABP include 1) activity against the common agents S. pneumoniae, H. influenzae, and atypical pathogens, including those resistant to other macrolide antibiotics, 2) non-inferiority when compared to the respiratory fluoroquinolone antibiotic moxifloxacin in two Phase III trials, 3) an adverse event profile milder than that of other macrolide antibiotics, and 4) no association with Clostridium difficile infection as commonly seen with fluoroquinolones and many beta-lactam antibiotics.Citation8,Citation10,Citation11,Citation14,Citation15 Although solithromycin has been examined for potential uses in uncomplicated gonorrhea, Mycoplasma genitalium infections, bacterial infections in pregnancy, non-alcoholic fatty liver disease, and COPD, these uses are outside the scope of this review.Citation12,Citation16–Citation19
Design and development
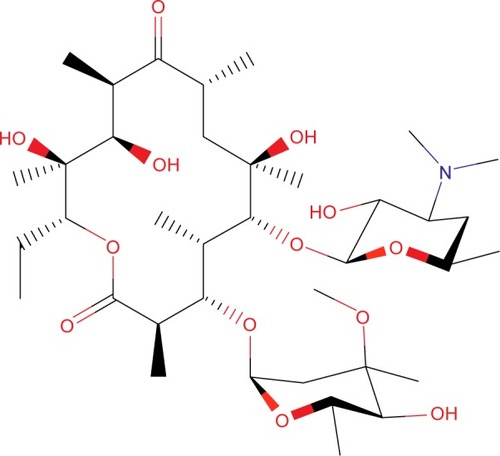
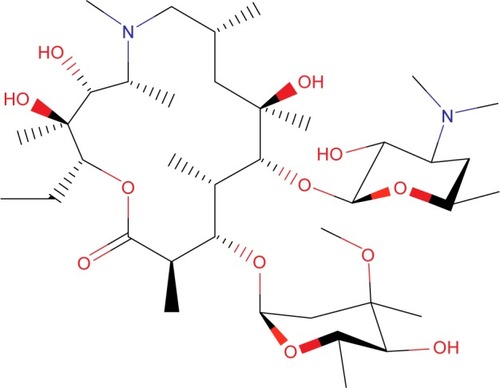
The first macrolide antibiotic, erythromycin (), was first released in 1954. It is associated with multiple adverse effects: diarrhea, hepatic dysfunction, QT prolongation, multiple drug interactions, and increasing resistance in target pathogens.Citation14,Citation20,Citation21 Azithromycin () was approved by the US Food and Drug Administration (FDA) in 1991 and has become a mainstay of outpatient therapy for respiratory infections, although its over- and under-use have been associated with emerging resistance. Azithromycin is also associated with hepatotoxicity, QT prolongation, and C. difficile infections, and carries a warning concerning exacerbation of myasthenia gravis symptoms. However, it does not appear to inhibit CYP3A4 and it does not carry as many interactions as other macrolide antibiotics.Citation14,Citation16,Citation22 Telithromycin () is the first ketolide antibiotic and was approved in Europe in 2001 and in America in 2004. The new ketolide was expected to overcome macrolide resistance by improving the strength of binding to 23S ribosomal RNA domain II while retaining binding to domain V (macrolides bind strongly to domain V and weakly to domain II); telithromycin is also considered bactericidal, where macrolides are considered bacteriostatic.Citation8,Citation14,Citation23,Citation24 Concerns over hepatotoxicity resulted in an additional labeled warning for telithromycin in 2006, and the indications for acute bacterial sinusitis and acute bacterial exacerbations of chronic bronchitis were removed in 2007, with a new boxed warning for patients with myasthenia gravis.Citation25–Citation27 Because of these and other adverse events, telithromycin is no longer actively marketed, underscoring the need for a new, tolerable antibiotic with bactericidal activity against macrolide-resistant organisms.Citation14,Citation28
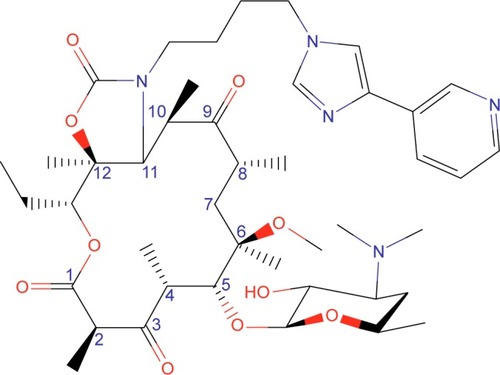
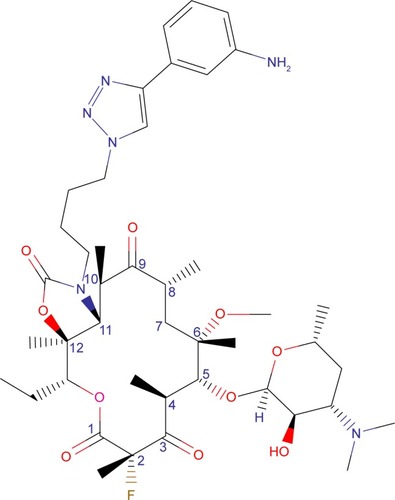
Solithromycin’s structure helps to overcome macrolide resistance as well as overcome problems with adverse events of telithromycin (). Key elements of solithromycin activity are described in and include resistance to induction of macrolide–lincosamide–streptogramin B (MLSB)-mediated modifications (shared with telithromycin), resistance to modifications in the domain V binding site (shared with telithromycin), improved hydrogen binding at the domain II binding site (similar to telithromycin), and a third 23S ribosomal RNA binding site accomplished by the C2 fluorine (unique to solithromycin).Citation9,Citation14,Citation29,Citation30 Solithromycin also lacks the pyridine moiety presumed to be associated with telithromycin-induced liver injury.Citation31 In short, the highlights of the differences between solithromycin and telithromycin include the aromatic side chain with an aminophenyl group allowing for a third binding site, compared with two for telithromycin, the lack of a pyridine moiety, presumably reducing hepatic toxicity, and the presence of a fluorine at C-2, improving drug binding and enhancing activity.
Table 1 Key structures affecting solithromycin activity
Microbiology
Mechanisms of action and resistance
As with macrolides, solithromycin works by binding to the 50S ribosomal subunit near the peptide exit tunnel, resulting in premature termination of translation and frame shift errors in translation.Citation29 This mechanism is usually considered bacteriostatic, but ketolides are considered bactericidal, possibly due to their added ability to interfere with formation of the ribosomal 50S unit.Citation23,Citation32
Usual mechanisms of resistance involve modifying the target binding site (erythromycin ribosomal methylation) and efflux of the macrolide (macrolide efflux).Citation30
Erythromycin ribosomal methylation, erm, is the most common mechanism of resistance for MLSB organisms. Macrolides are further known to induce methylation in MLSB organisms, a mechanism known as inducible MLSB (iMLSB) as opposed to constitutive MLSB (cMLSB) where rRNA methylase is always produced.Citation30,Citation33 Solithromycin does not trigger iMLSB-mediated rRNA methylation and is still active against cMLSB organisms due to its third binding site and C3 ketone group.Citation14,Citation29
Macrolide efflux, mef, is an efflux pump for macrolides. Ketolides have been found to be less sensitive to mef, which may contribute to solithromycin’s restored activity against H. influenzae, but may require higher concentrations to remain efficacious.Citation34
L4/L22 mutations are rare modifications to ribosomal proteins which may confer solithromycin resistance. However, even with these phenotypes included, solithromycin performed well on resistance selection studies; so development of resistance may not be significant and will have to be determined clinically.Citation35
In vitro activity
Solithromycin has demonstrated in vitro efficacy against a wide variety of Gram-positive, Gram-negative, and atypical organisms known to cause pneumonia ().Citation10–Citation13,Citation16,Citation17,Citation32,Citation36–Citation43 Although clinical correlation is not complete, solithromycin appears to have a similar spectrum of activity to telithromycin and even some activity against telithromycin-resistant organisms. Highlights of solithromycin’s antibacterial activity include S. pneumoniae (especially macrolide-, penicillin-, and fluoroquinolone-resistant strains), H. influenzae, and pneumonia-causing atypical pathogens.
Table 2 Susceptible organisms from in vitro and limited clinical data
Pharmacokinetics and pharmacodynamics
Solithromycin is both a substrate and an inhibitor of cytochrome P450 CYP 3A4. As it autoinhibits its own clearance, solithromycin half-life extends with higher and cumulative doses, similar to macrolides: 7 days of 400 mg solithromycin per day yields half-lives ranging from 4.8 to 7.5 h.Citation44–Citation46 Because solithromycin’s major metabolic pathway involves CYP 3A4, it is likely to be subject to the same or similar drug interactions as erythromycin, clarithromycin, and telithromycin, but not azithromycin, which has a different metabolic pathway.Citation20,Citation22,Citation24,Citation45 Due to an uncertain mechanism, possibly uptake of the drug by polymorphonuclear neutrophils (PMNs), solithromycin achieves higher concentrations in epithelial lining fluid and alveolar macrophages than in plasma; this is similar to macrolides and other ketolides and may contribute to solithromycin’s utility in treating CABP.Citation47 Finally, solithromycin was studied in patients with mild, moderate, and severe chronic liver disease: renal excretion remained 5%–10%, indicating that renal dose reductions are not likely necessary, and no dose reduction was recommended for patients with chronic liver disease, although area under the curve (AUC) was lower in severe liver disease, possibly due to an expanded volume of distribution in these patients.Citation44
Clinical trials
Phase II trial
A Phase II, randomized, double-blind, controlled trial was conducted comparing 5 days of solithromycin (800 mg once on day 1, 400 mg once daily thereafter) to 5 days of levofloxacin (750 mg once daily) in patients with CABP. Patients had to be ≥18 years and with symptomatic pneumonia (pneumonia severity index score > 50 and ≤150) defined as three factors: cough with production of purulent sputum or a change in the character of sputum consistent with bacterial infection, dyspnea, or tachypnea; chest pain consistent with pneumonia; fever, rales, or radiographic evidence of consolidation. Patients were excluded if they received prior antimicrobial therapy (excepting documented treatment failure after 48 h), had known bronchial obstruction not related to pneumonia, or stage IV COPD, among other safety-related exclusions. Levofloxacin was chosen for its place in US guidelines as monotherapy for CABP.
Patients were analyzed in four populations: 1) intention-to-treat (ITT) for all randomized patients, 2) clinically evaluable (CE) for patients who received at least two doses of the study drug (within 48 h) with clinical failure or at least four doses of the study drug with clinical success, 3) microbiological ITT (micro-ITT) as ITT patients with an identified bacterial pathogen associated with CABP, and 4) microbiologically evaluable (ME) as CE patients with an identified bacterial pathogen associated with CABP. The safety analysis included all patients who received at least one dose of the study drug. The primary endpoint was clinical success at test of cure (TOC) 4–11 days after last dose of study drug defined as: complete or nearly complete resolution of baseline symptoms, no new CABP symptoms, and radio-logic resolution, improvement, or stability. An early clinical response was added to comply with FDA and Foundation for the National Institutes of Health (FNIH) expectations, and required improvement in symptoms at day 3.
The study was not powered for inferential statistics, but efficacy outcomes were similar for both solithromycin and levofloxacin. In the safety analysis, gastrointestinal disorders were the most common adverse events for both groups. No patients withdrew from the solithromycin group due to adverse events, compared with six patients from the levo-floxacin group, but the study was not powered for inferential statistics. No patients in the solithromycin group reported nervous system or psychiatric disorders, compared with three patients from the levofloxacin group, and no patients in the solithromycin group showed elevated alanine aminotransferase (ALT) or aspartate aminotransferase (AST) levels. The authors concluded that solithromycin had efficacy comparable to levofloxacin with a favorable adverse event profile.Citation13
SOLITAIRE-ORAL
SOLITAIRE-ORAL was a Phase III, global, randomized, double-blind, controlled, non-inferiority trial comparing the same 5-day regimen of solithromycin to 7 days of moxifloxacin 400 mg once daily. Inclusion and exclusion criteria were similar to the Phase II trial except for changes made to comply with newer FDA expectations for such a trial, such as symptom inclusion criteria. The inclusion of Pneumonia Patient Outcomes Research Team score 2 (PORT 2) pneumonia patients was capped to ensure adequate inclusion of moderately severe patients (PORT 2 describes low risk for morbidity and mortality associated with CABP, compared to PORT 3 and 4, which describe moderate and high risk). Moxifloxacin was chosen as a comparator for its place in the guidelines as monotherapy and similar spectrum of activity to solithromycin.
Early clinical response was chosen as the primary outcome and defined as improvement in at least two cardinal symptoms (cough, chest pain, sputum production, dyspnea) and no worsening in any symptom at 72 h (evaluated at 71 to 108 h) after the first dose of the study drug. Clinical success at TOC visit was maintained as a secondary outcome to comply with European Medicines Agency expectations. For SOLITAIRE-ORAL, the CE population was defined as per-protocol, and a second microbiological ITT (mITT-2) population was limited to positive cultures from blood, pleural fluid, sputum, oropharyngeal Mycoplasma pneumoniae swab, or urine Legionella antigen.
Early clinical response in the ITT population was observed in 333/426 (78.2%) patients in the solithromycin group and 338/434 (77.9%) patients in the moxifloxacin group (difference 0.29%, 95% CI −5.5 to 6.1), meeting the pre-specified cutoff for non-inferiority. Early clinical response in the CE population also showed non-inferiority (solithromycin 326 [80.9%], moxifloxacin 330 [81.1%], difference −0.19, 95% CI −5.8 to 5.5). Non-inferiority was demonstrated at short-term follow-up also. As with the Phase II trial, the most common isolates in the microbiological populations were S. pneumoniae (including macrolide-resistant S. pneumoniae), H. influenzae, M. pneumoniae, and Legionella.
Forty-three (10%) patients in the solithromycin group and 54 (13%) in the moxifloxacin group experienced adverse events related to study treatment. Serious adverse events and discontinuations due to adverse events were similar in both groups. Two patients in the moxifloxacin group were found to have C. difficile associated diarrhea, compared with none in the solithromycin group, but this was not a pre-specified endpoint and not routinely tested. No patients experienced serious cardiovascular events attributed to the study drug or developed hepatobiliary abnormalities meeting Hy’s law, defined as ALT or AST greater than three times upper limit of normal, alkaline phosphatase less than two times upper limit of normal, and total bilirubin greater than two times upper limit of normal, without alternate explanation.Citation10 The study did report the rates of aminotransferase elevations: ALT was elevated greater than three times upper limit of normal in 22 (5.4%) patients receiving solithromycin and only in 14 (3.3%) patients receiving moxifloxacin; AST was elevated greater than three times upper limit of normal in 10 (2.5%) patients receiving solithromycin compared to 8 (1.9%) patients receiving moxifloxacin.
The study authors concluded that solithromycin was non-inferior to moxifloxacin for CABP without an increase in adverse events, and with a lower risk for precipitating C. difficile colitis. They did voice a desire to extend follow-up to measure the effect of CABP on long-term outcomes.Citation10
SOLITAIRE-IV
SOLITAIRE-IV was a Phase III, randomized, double-blind, controlled study comparing solithromycin to moxifloxacin. In this trial, the study drug was initially delivered via the intravenous (IV) route, and prescribers had the option to switch to oral (PO) therapy based on pre-defined IV-to-PO criteria. IV doses for both drugs were 400 mg once daily; PO therapy was the same as in SOLITAIRE-ORAL, including the 800 mg loading dose given as the first PO dose. Therapy continued for a total of 7 days, regardless of when, or if, an IV-to-PO conversion was performed. Inclusion and exclusion criteria were similar to SOLITAIRE-ORAL.
Early clinical response was the primary endpoint for SOLITAIRE-IV, with end of treatment, short-term follow-up, and late follow-up evaluations as secondary endpoints. Analysis populations were defined as in SOLITAIRE-ORAL. For the ITT population, early clinical response was achieved in 344/434 (79.3%) patients in the solithromycin group and in 342/429 (79.7%) patients in the moxifloxacin group (difference −0.46, 95% CI −6.1 to 5.2), meeting the criteria for non-inferiority. Mean duration of IV treatment was the same in both groups.
Infusion reactions were much more common in the solithromycin group (31.3%) compared to the moxifloxacin group (5.4%). Adverse events not related to infusion reactions were similar for both groups. Solithromycin was associated with elevations in ALT and AST, but these were asymptomatic and generally resolved by short-term follow-up. The study authors’ discussion recognized the increases in hepatic aminotransferase levels and increased incidence of infusion site reactions, which are common with macrolide antibiotics, and maintained, as with SOLITAIRE-ORAL, that solithromycin is non-inferior to moxifloxacin for the treatment of CABP regardless of dose formulation.Citation11
Additional safety trials
The Phase II and Phase III trials for solithromycin did not report QT intervals associated with solithromycin as an outcome. Macrolide antibiotics have been known to prolong QTc, making this an important safety endpoint for the new fluoroketolide. A specially conducted, randomized, crossover, double-blind trial assessed the effects of placebo, solithromycin, and moxifloxacin on echocardiography. Using a difference of 10 ms as a cutoff, the study concluded that solithromycin, unlike macrolide antibiotics, does not prolong QTc.Citation48
A Phase I trial was performed examining the use and pharmacokinetics of solithromycin in adolescents. Thirteen adolescents, aged 12–17, were given solithromycin in addition to appropriate antimicrobial therapy for suspected or known infections. The authors concluded that kinetics in adolescents was similar to healthy adults, but the sample size was small and confounded by disease state (cystic fibrosis), concurrent therapy with CYP 3A4 inducers, and one blood transfusion. Two adolescents experienced mild headaches and one displayed elevated transaminase levels less than three times upper limit of normal, but this patient was on other medications known to raise transaminase levels. A Phase II trial will be needed to further characterize the utility of solithromycin for CABP in children and adolescents.Citation49
Discussion
In light of the incidence of CABP, the mortality rate associated with pneumonia, and the increasing antimicrobial resistance in organisms most commonly seen in CABP, solithromycin is a promising new drug. It has increased activity against common CABP organisms compared to both macrolide and ketolide antibiotics, and an apparent adverse event profile which is much safer than that of the only currently FDA-approved ketolide antibiotic, and both of these traits are supported by solithromycin’s medicinal chemistry. It is easy to use for outpatient treatment, and the dosing regimen is similar to azithromycin; so its proper use will be familiar to many healthcare providers. In addition, solithromycin does not carry warnings against C. difficile colitis or tendon rupture associated with fluoroquinolones, both of which may be magnified in elderly patients who are more likely to need treatment for pneumonia, and it has a spectrum of activity, which includes likely pathogens, unlike beta-lactam antibiotics.
FDA response letter
On December 29, 2016, the FDA released its complete response letter (CRL) to Cempra, Inc., regarding their new drug application for solithromycin. The CRL states that <1,000 patients treated with solithromycin in their submitted studies is too few to adequately characterize the risk of hepatic adverse events or a possible relationship to drug-induced liver injury. The FDA is asking for a study of 9,000 patients to better characterize the risk, and also suggests that even if no serious adverse events are found, the labeling will not only contain warnings about potential hepatotoxicity, but require that solithromycin be used only in patients who have limited therapeutic options.Citation50 This may be concerning to some who think the FDA is being too strict with novel antibiotics and see this as a problem when combating drug-resistant organisms.Citation28 While limiting use of a novel antibiotic may be useful from an antimicrobial stewardship perspective, it might also be premature to require specific labeling before collecting adequate information to characterize the risk. Either way, azithromycin carries a label warning for hepatotoxicity, which has not curtailed its use, and the strength of a comparison between solithromycin and telithromycin from a safety perspective will increase after the larger trial is conducted and reported.
Potential place in therapy
Solithromycin is a novel antimicrobial with bactericidal activity against the most common pathogens associated with CABP, useful in both outpatient and inpatient settings, and subject to few effective mechanisms for resistance. If safety concerns about solithromycin and hepatotoxicity can be resolved, solithromycin may find a place as a first-line therapy for CABP or as a second-line therapy for patients who fail to show early clinical response to other first-line therapies.
Disclosure
The authors report no conflicts of interest in this work.
References
- MandellLAWunderinkRGAnzuetoAAmerican Thoracic SocietyInfectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adultsClin Infect Dis200744Suppl 2 S27 S7217278083
- BrarNKNiedermanMSManagement of community-acquired pneumonia: a review and updateTher Adv Respir Dis201151 61 7820935033
- KaysinAVieraAJCommunity-acquired pneumonia in adults: diagnosis and managementAm Fam Physician2016949 698 70627929242
- GBD 2015 Mortality and Causes of Death CollaboratorsGlobal, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015Lancet201638810053 1459 154427733281
- BrouletteJYuHPyensonBIwasakiKSatoRThe incidence rate and economic burden of community-acquired pneumonia in a working-age populationAm Heal Drug Benefits201368 494 503
- NiedermanMSLunaCMCommunity-acquired pneumonia guidelines: a global perspectiveSemin Respir Crit Care Med2012333 298 31022718216
- BoucherHWTalbotGHBradleyJSBad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of AmericaClin Infect Dis2009481 1 1219035777
- FarrellDJFlammRKSaderHSJonesRNResults from the Solithromycin International Surveillance Program (2014)Antimicrob Agents Chemother2016606 3662 366827044551
- Van BambekeFRenaissance of antibiotics against difficult infections: focus on oritavancin and new ketolides and quinolonesAnn Med2014467 512 52925058176
- BarreraCMMykietiukAMetevHSOLITAIRE-ORAL Pneumonia TeamEfficacy and safety of oral solithromycin versus oral moxifloxacin for treatment of community-acquired bacterial pneumonia: a global, double-blind, multicentre, randomised, active-controlled, non-inferiority trial (SOLITAIRE-ORAL)Lancet Infect Dis2016164 421 43026852726
- FileTMJrRewerskaBVucinic-MihailovicVSOLITAIRE-IV: A Randomized, Double-Blind, Multicenter Study comparing the efficacy and safety of intravenous-to-oral solithromycin to intravenous-to-oral moxifloxacin for treatment of community-acquired bacterial pneumoniaClin Infect Dis2016638 1007 101627448679
- HookEW3rdGoldenMJamiesonBDA phase 2 trial of oral solithromycin 1200 mg or 1000 mg as single-dose oral therapy for uncomplicated gonorrheaClin Infect Dis2015617 1043 104826089222
- OldachDClarkKSchranzJRandomized, Double-Blind, Multicenter Phase 2 Study comparing the efficacy and safety of oral solithromycin (CEM-101) to those of oral levofloxacin in the treatment of patients with community-acquired bacterial pneumoniaAntimicrob Agents Chemother2013576 2526 253423507282
- FernandesPMartensEBertrandDPereiraDThe solithromycin journey – it is all in the chemistryBioorg Med Chem20162424 6420 642827595539
- Communication FDSFDA Drug Safety Communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together Available from: https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Published 2016Accessed January 31, 2017
- CouldwellDLLewisDAMycoplasma genitalium infection: current treatment options, therapeutic failure, and resistance-associated mutationsInfect Drug Resist20158 147 16126060411
- KeelanJAPayneMSKempMWIrelandDJNewnhamJPA new, potent, and placenta-permeable macrolide antibiotic, solithromycin, for the prevention and treatment of bacterial infections in pregnancyFront Immunol20167 11127066004
- RotmanYSanyalAJCurrent and upcoming pharmacotherapy for non-alcoholic fatty liver diseaseGut2017661 180 19027646933
- KobayashiYWadaHRossiosCA novel macrolide solithromycin exerts superior anti-inflammatory effect via NF-kB inhibitionJ Pharmacol Exp Ther20133451 76 8423359665
- ANI PharmaceuticalsErythromycin ethylsuccinate – erythromycin ethylsuccinate granule, for suspension. [Updated Sept 27, 2016]DailyMed [homepage on the Internet]Bethesda MD, USNational Library of Medicine Available from: https://dailymed.nlm.nih.gov/dai-lymed/drugInfo.cfm?setid=3de1e0b9-473b-44c0-a644-5fa14ad69a83Accessed October 9, 2017
- CamilleriMParkmanHPShafiMAAbellTLGersonLClinical guideline: management of gastroparesisAm J Gastroenterol20131081 18 3823147521
- Pfizer Laboratories Div Phizer IncZithromax – azithromycin dihydrate tablet, film coated; Zithromax – azithromycin dihydrate powder, for suspension. [Updated May 18, 2017]DailyMed [Internet]Bethesda MD, USNational Library of Medicine Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=db52b91e-79f7-4cc1-9564-f2eee8e31c45Accessed October 9, 2017
- ShiJMontayGBhargavaVOClinical pharmacokinetics of telithromycin, the first ketolide antibacterialClin Pharmacokinet2005449 915 93416122280
- Sanofi-Aventis U.S. LLCKetek – telithromycin tablet, film-coated. [Updated May 2, 2012]DailyMed [Internet]Bethesda MD, USNational Library of Medicine Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ba1cca98-f350-4655-88e3-6-ef990779fb9Accessed October 9, 2017
- ClayKDHansonJSPopeSDRissmillerRWPurdumPP3rdBanksPMBrief communication: severe hepatotoxicity of telithromycin: three case reports and literature reviewAnn Intern Med20061446 415 42016481451
- FDA News ReleasesFDA completes safety assessment of Ketek new safety information to be added to product labeling Available from: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108682.htm. Published 2006Accessed January 7, 2017
- FDA News ReleasesFDA announces label and indication changes for the antibiotic Ketek Available from: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucm108842.htm. Published 2007Accessed January 7, 2017
- JarvisLMThe Ketek Effect: biotechs worry that FDA has gotten tougher on approving new antibioticsChem Eng News Available from: http://cen.acs.org/articles/86/i15/Ketek-Effect.html. Published 2008Accessed January 4, 2017
- Llano-SoteloBDunkleJKlepackiDBinding and action of CEM-101, a new fluoroketolide antibiotic that inhibits protein synthesisAntimicrob Agents Chemother20105412 4961 497020855725
- JudaMChudzik-RzadBMalmAThe prevalence of genotypes that determine resistance to macrolides, lincosamides, and streptogramins B compared with spiramycin susceptibility among erythromycin-resistant Staphylococcus epidermidisMem Inst Oswaldo Cruz20161113 155 16027008373
- BertrandDBertrandSNeveuEFernandesPMolecular characterization of off-target activities of telithromycin: a potential role for nicotinic acetylcholine receptorsAntimicrob Agents Chemother20105412 5399 540220855733
- RodgersWFrazierADChampneyWSSolithromycin inhibition of protein synthesis and ribosome biogenesis in Staphylococcus aureus, Streptococcus pneumoniae, and Haemophilus influenzaeAntimicrob Agents Chemother2013574 1632 163723318809
- SaderiHEmadiBOwliaPPhenotypic and genotypic study of macrolide, lincosamide and streptogramin B (MLS B) resistance in clinical isolates of Staphylococcus aureus in Tehran, IranMed Sci Monit2011172 BR48 BR5321278685
- SutcliffeJAAntibiotics in development targeting protein synthesisAnn N Y Acad Sci20111241 122 15222191530
- McgheePClarkCKosowska-shickKMIn vitro activity of CEM-101 against Streptococcus pneumoniae and Streptococcus pyogenes with defined macrolide resistance mechanismsAntimicrob Agents Chemother2010541 230 23819884376
- PiccinelliGFernandesPBonfantiCCaccuriFCarusoADe FrancescoMAIn vitro activity of solithromycin against erythromycin-resistant Streptococcus agalactiaeAntimicrob Agents Chemother2014583 1693 169824379197
- FarrellDJMendesREJonesRNAntimicrobial activity of solithromycin against serotyped macrolide-resistant Streptococcus pneumoniae isolates collected from U.S. medical centers in 2012Antimicrob Agents Chemother2015594 2432 243425605359
- FarrellDJCastanheiraMSaderHSJonesRNThe in vitro evaluation of solithromycin (CEM-101) against pathogens isolated in the United States and Europe (2009)J Infect2010616 476 48320831882
- JensenJSFernandesPUnemoMIn vitro activity of the new fluoroketolide solithromycin (CEM-101) against macrolide-resistant and - susceptible Mycoplasma genitaliumAntimicrob Agents Chemother2014586 3151 315624637681
- MallegolJFernandesPMelanoRGGuyardCAntimicrobial activity of solithromycin against clinical isolates of legionella pneumophila serogroup 1Antimicrob Agents Chemother2014582 909 91524277019
- FurfaroLLSpillerOBKeelanJAPayneMSIn vitro activity of solithromycin and its metabolites, CEM-214 and N-acetyl-CEM-101, against 100 clinical Ureaplasma spp. isolates compared with azithromycinInt J Antimicrob Agents2015463 319 32426141231
- HardyDJVicinoDFernandesPIn vitro activity of solithromycin against Bordetella pertussis, an emerging respiratory pathogenAntimicrob Agents Chemother20166012 7043 704527620481
- WeintraubARashidMUNordCEIn-vitro activity of solithromycin against anaerobic bacteria from the normal intestinal microbiotaAnaerobe201642 119 12227725229
- JamiesonBDCiricSFernandesPSafety and pharmacokinetics of solithromycin in subjects with hepatic impairmentAntimicrob Agents Chemother2015598 4379 438625870056
- AbduljalilKKinzigMBulittaJModeling the autoinhibition of clarithromycin metabolism during repeated oral administrationAntimicrob Agents Chemother2009537 2892 290119414584
- StillJGSchranzJDegenhardtTPPharmacokinetics of solithromycin (CEM-101) after single or multiple oral doses and effects of food on single-dose bioavailability in healthy adult subjectsAntimicrob Agents Chemother2011555 1997 200321282444
- RodvoldKAGotfriedMHStillJGClarkKFernandesPComparison of plasma, epithelial lining fluid, and alveolar macrophage concentrations of solithromycin (CEM-101) in healthy adult subjectsAntimicrob Agents Chemother20125610 5076 508122802254
- DarpoBSagerPTFernandesPSolithromycin, a novel macrolide, does not prolong cardiac repolarization: a randomized, three-way crossover study in healthy subjectsJ Antimicrob Chemother2017722 515 52127798210
- GonzalezDPalazziDLBhattacharya-MithalLSolithromycin pharmacokinetics in plasma and dried blood spots and safety in adolescentsAntimicrob Agents Chemother2016604 2572 257626883693
- Compra, IncCempra receives complete response letter for solithromycin NDAs Available from: http://investor.cempra.com/releasedetail.cfm?ReleaseID=1005708. Published 2016Accessed January 4, 2017