Abstract
Objective
To investigate the effect of dexmedetomidine in the prevention of etomidate-induced myoclonus.
Methods
We searched for randomized controlled trials (RCTs) regarding the use of dexmedetomidine in preventing etomidate-induced myoclonus in the databases PubMed, EMBASE, the Cochrane Library, and CNKI. We extracted data and assessed the quality of the literature and adopted RevMan 5.2 to conduct meta-analysis on each effective index and employed funnel plot to test publication bias.
Results
The results showed that the incidence of etomidate-induced myoclonus in the dexmedetomidine treated groups was significantly lower than that of the control groups (risk ratio [RR]=0.27, 95% confidence interval [CI] [0.15, 0.47], P<0.00001). With regard to the severity of etomidate-induced myoclonus, incidences of etomidate-induced myoclonus in the dexmedetomidine treated groups resulting in mild myoclonus (RR=0.37, 95% CI [0.19, 0.75], P=0.006), moderate myoclonus (RR=0.21, 95% CI [0.12, 0.37], P<0.00001), or severe myoclonus (RR=0.18, 95% CI [0.08, 0.38], P<0.00001) were significantly lower than those of the control groups. No statistically significant difference was found (RR=0.70, 95% CI [0.47, 1.04], P=0.08) between etomidate-induced myoclonus in the dexmedetomidine treated groups and that of the midazolam treated groups.
Conclusion
Dexmedetomidine can effectively prevent the incidence of etomidate-induced myoclonus and reduce the severity of etomidate-induced myoclonus. In addition, there were no significant differences between the effects of dexmedetomidine and midazolam in preventing etomidate-induced myoclonus.
Introduction
Etomidate is a short-acting and non-barbiturate intravenous anesthetic with rapid induction and rapid awakening. Because of its low impact on hemodynamics and respiratory depression, etomidate is widely used in clinical practice, especially for patients with an unstable cardiovascular system. However, during the induction of anesthesia, etomidate can generate common adverse reactions, including injection pain, phlebitis, hemolysis, and myoclonus. The occurrence of phlebitis, hemolysis, injection pain, and other adverse reactions had almost entirely disappeared after etomidate emulsion was substituted for water-based etomidate. However, the incidence of myoclonus is still as high as 50%–80%.Citation1 Myoclonus can lead to muscle fiber damage, myalgia, elevated serum potassium, and the increase in the rate of reflux aspiration. Although etomidate-induced myoclonus in most patients is temporary, it is extremely harmful to patients with hypertension, aneurysms, and coronary heart disease. Myoclonus can make the patient feel unwell, and even endanger the patient’s life. Its mechanism is still not clear. How to reduce etomidate-induced myoclonus is one of the research directions of its application. Many agents have been used to prevent myoclonus. In recent years, there have been many clinical studies concerning the prevention of etomidate-induced myoclonus by dexmedetomidine. Therefore, this meta-analysis was designed to evaluate the effect of dexmedetomidine for the prevention of etomidate-induced myoclonus.
Materials and methods
Source of data
We searched the PubMed, CNKI, and EMBASE databases for randomized controlled trials (RCTs) using dexmedetomidine for the prevention of etomidate-induced myoclonus. The search time limit was May 2016. Index words included dexmedetomidine, etomidate, myoclonus, and RCT.
Studies were included in the analysis if they were RCTs using dexmedetomidine for the prevention of etomidate-induced myoclonus and published in a journal. In addition, they contained information regarding age, gender, weight, time of anesthesia induction, dose, and surgery time that was comparable between the groups tested, and the experimental group had received an injection of dexmedetomidine hydrochloride while the control group had received an equal volume of saline. The endpoint of the studies was the incidence of etomidate-induced myoclonus and its severity.
Studies were excluded if they had adopted a retrospective analysis method, the experimental designs were not rigorous, or inappropriate statistical methods had been used.
Quality assessment
We assessed the quality of the studies on the basis of the Jadad scale.Citation2 We evaluated whether the randomized method was correct, whether the allocation was concealed, and whether the allocation concealment method was correct. We also considered if a blinding method was used, and if there were withdrawal or lost to follow-up cases ().
Table 1 Characteristics of the studies included in the meta-analysis
Statistical methods
We employed RevMan 5.2 provided by the Cochrane Collaboration to conduct the meta-analysis. The total effect-size indices were represented by risk ratio (RR), mean difference, and 95% confidence intervals (CIs). Heterogeneity analysis was performed by Q test. When P>0.05, I2<50%, indicating that homogeneity was found among the studies, each clinical trial could employ a fixed effects model to conduct a combined analysis. However, when P<0.05, I2>50%, showing that heterogeneity was found, a random effects model could be applied. We used a funnel plot to assess publication bias.
Results
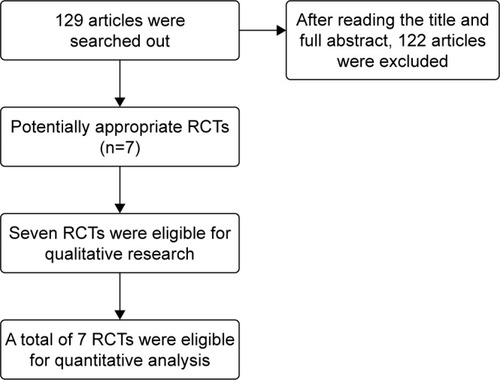
Study features
Initially, 129 related articles were found, and 7 of themCitation3–Citation9 were chosen based on the described inclusion and exclusion criteria (). The 7 included articles all adopted randomization methods, but 3 of them did not illustrate the specific method. These 7 articles did not mention allocation concealment, but only 3 of them adopted a double-blind method.
Incidence of etomidate-induced myoclonus
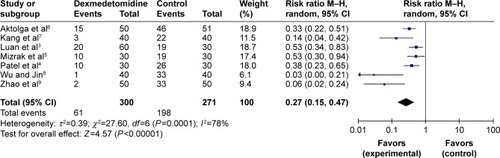
All RCTs, totaling 571 patients, reported the incidence of etomidate-induced myoclonus. Statistical heterogeneity (P=0.0001, I2=78%) was found among all studies; thus, a random effects model was adopted to conduct meta-analysis. The results showed that the effect of dexmedetomidine in preventing etomidate-induced myoclonus was significantly greater than that found in the control groups (RR=0.27, 95% CI [0.15, 0.47], P<0.00001; ).
Figure 2 Effect of dexmedetomidine in preventing etomidate-induced myoclonus.
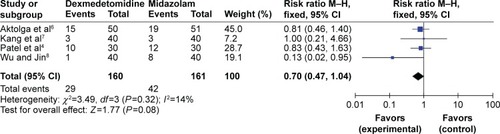
Only 4 RCTs, totaling 321 patients, reported the incidence of etomidate-induced myoclonus in both dexmedetomidine-treated and midazolam-treated groups. No statistical heterogeneity (P=0.32, I2=14%) was found among all studies, and a fixed effects model was employed to conduct the meta-analysis. The results suggested that there were no distinctive differences (RR=0.70, 95% CI [0.47, 1.04], P=0.08) between the dexmedetomidine treated groups and midazolam treated groups in preventing mild myoclonus that was induced by etomidate ().
Severity of etomidate-induced myoclonus
Mild myoclonus
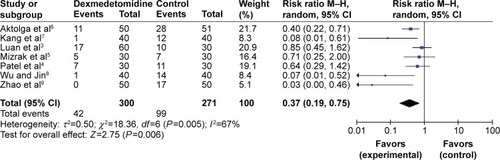
All RCTs, totaling 571 patients, reported the degree of mild myoclonus that was induced by etomidate. Statistical heterogeneity (P=0.005, I2=67%) was found among all studies; thus, a random effects model was employed for the meta-analysis. The results showed that there were distinctive differences (RR=0.37, 95% CI [0.19, 0.75], P=0.006) between the dexmedetomidine treated groups and the control groups regarding the incidence of mild etomidate-induced myoclonus ().
Moderate myoclonus
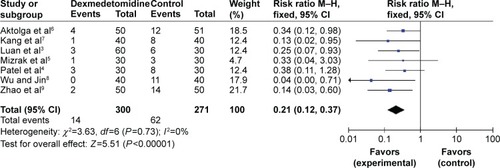
All RCTs, totaling 571 patients, reported the degree of moderate myoclonus that was induced by etomidate. No statistical heterogeneity (P=0.73, I2=0%) was found, and a fixed effects model was adopted for meta-analysis. The results showed that there were distinctive differences (RR=0.21, 95% CI [0.12, 0.37], P<0.00001) between the dexmedetomidine groups and the control groups regarding the incidence of moderate etomidate-induced myoclonus ().
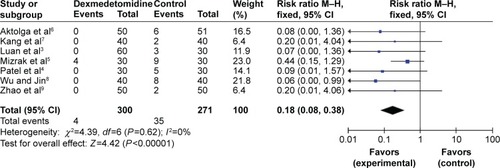
Severe myoclonus
All RCTs, totaling 571 patients, reported the degree of severe myoclonus that was induced by etomidate. No statistical heterogeneity (P=0.62, I2=0%) was found, and a fixed effects model was adopted for meta-analysis. The results showed that there were distinctive differences (RR=0.18, 95% CI [0.08, 0.38], P<0.00001) between the dexmedetomidine treated groups and the control groups regarding the incidence of severe etomidate-induced myoclonus ().
Sensitivity analysis
In the random effects model, we excluded 3 studies,Citation7–Citation9 before conducting the meta-analysis, and no statistical heterogeneity (P=0.43, I2=0%) was found. The results did not change, indicating that in this study, the evaluation of corresponding effect size was stable and reliable. Three studiesCitation7–Citation9 were excluded due to the total heterogeneity in the process of, respectively, performing combined funnel plot analysis. After the studies were excluded, we repeated the funnel plot analysis. All scatters in the figure were substantially symmetrical, and basically fell within the confidence limits; they were distributed in the upper funnel plot, showing no significant publication bias.
Discussion
This meta-analysis included a total of 571 patients with different types of surgery. Observable effect indicators included the incidence of etomidate-induced myoclonus and its severity. Dexmedetomidine can effectively prevent the incidence of etomidate-induced myoclonus and reduce the severity of etomidate-induced myoclonus. No differences were found between dexmedetomidine and midazolam on preventing etomidate-induced myoclonus.
Etomidate is an intravenous anesthetic derivative of imidazole. Etomidate-induced myoclonus can make intraocular pressure increase, thereby increasing the risk of vitreous prolapse.Citation10 However, to date, no conclusion has been reached on the reason. Some studies have suggested that myoclonus may be some form of seizure, while other studies hypothesized that the reason may be that a large dose of etomidate inhibits the cerebral cortex but without inhibiting subcortical neurons.Citation11,Citation12 In addition, studies have shownCitation13,Citation14 that etomidate-induced myoclonus is similar to convulsive seizures and similar to mechanisms of certain forms of epilepsy.
Dexmedetomidine is a highly selective α2 agonist. Its affinity with adrenergic receptors is 8× than that of clonidine. In addition, its half-life is shorter than that of clonidine; its half-life of distribution is about 6 minutes, and its half-life of elimination ~2 hours. The pharmacokinetics is also more predictable. The α2 adrenergic receptor is commonly found in synapses, postsynaptic parts of the central nervous system, peripheral nerves, and autonomic ganglia. Stimulating synaptic α2 receptors in sympathetic nerve endings can inhibit the release of norepinephrine. Before anesthesia, intravenous injection of the drug can significantly reduce the stress responses of laryngoscope and endotracheal intubation and can reduce the dose of propofol and opioids.Citation15 Therefore, the effect of dexmedetomidine in relieving myoclonus may be related to its sedative and analgesic effects.
Conclusion
In this meta-analysis, all basic and comparable features both in the experimental group and the control group were distributed in a balanced way. However, the overall sample size was small, and unpublished data and other non-traditional sources of evidence were deficient. All these may affect the objectivity and reliability of the system evaluation. Consequently, more multicenter, randomized, and double-blind, controlled trials with larger samples are needed to confirm the above conclusions.
Disclosure
The authors report no conflicts of interest in this work.
References
- HoldcroftAMorganMWhitwamJGLumleyJEffect of dose and premedication on induction complications with etomidateBr J Anaesth1976483 199 2051259885
- JadadARMooreRACarrollDAssessing the quality of reports of randomized clinical trials: is blinding necessary?Control Clin Trials1996171 1 128721797
- LuanHFZhaoZBFengJYPrevention of etomidate-induced myoclonus during anesthetic induction by pretreatment with dexmedetomidineBraz J Med Biol Res2015482 186 19025351237
- PatelMHRajeshCRamaniMNA comparison of dexmedetomidine and midazolam for the prevention of myoclonic movements and pain following etomidate injectionRes J Pharm Biol Chem Sci20156 161 168
- MizrakAKorukSBilgiMPretreatment with dexmedetomidine or thiopental decreases myoclonus after etomidate: a randomized, double-blind controlled trialJ Surg Res20101591 e11 e1620018300
- AktolgaSGunesYGunduzMA comparison of midazolam and dexmedetomidine for the prevention of myoclonic movements and pain following etomidate injectionJ Anaesthesiol Clin Pharmacol2010263 162 166
- KangZXieWXieWA comparison of midazolam and dexmedetomidine for the prevention of myoclonic movements and pain following etomidate injection under general anesthesia inductionChinese Med Innovat20131005 11 13
- WuLJinGPretreatment with dexmedetomidine or midazolam for the prevention of myoclonic movements and following etomidate injection undergoing general anesthesia inductionShand Med J20125218 92 93
- ZhaoXGaoCWangJPretreatment with dexmedetomidine decreases myoclonus after etomidateShanxi Med J20114012 1220 1221
- FragenRJCaldwellNComparison of a new formulation of etomidate with thiopental – side effects and awakening timesAnesthesiology1979503 242 244434512
- DoenickeAWRoizenMFKuglerJKrollHFossJOstwaldPReducing myoclonus after etomidateAnesthesiology1999901 113 1199915320
- GancherSLaxerKDKriegerWActivation of epileptogenic activity by etomidateAnesthesiology1984615 616 6186497005
- Herrera-PecoIWix-RamosRDominguez-GadeaLCambios en la perfusión cerebral inducidos por etomidato en pacientes con epilepsia del lóbulo temporal. [Changes in cerebral perfusion induced by etomidate in patients with temporal lobe epilepsy]Rev Neurol20094911 561 565 Spanish19921619
- VossLJSleighJWBarnardJPThe howling cortex: seizures and general anesthetic drugsAnesth Analg20081075 1689 170318931234
- YildizMTavlanATuncerSEffect of dexmedetomidine on haemodynamic responses to laryngoscopy and intubation: perioperative haemadynamics and anaesthetic requirementsDrugs200671 43 52