Abstract
Purpose
Altered platelet aggregability has been implicated in the pathogenesis of glaucoma. This study aims to investigate the anti-platelet potential of intraocular pressure lowering drops, with the possibility of establishing it as an additional mechanism of anti-glaucomatous action.
Materials and methods
The anti-aggregating effects of a series of anti-glaucomatous eye drops were determined on human platelets in the platelet aggregation model, using four known aggregating factors (platelet activating factor [PAF], adenosine diphosphate [ADP], thrombin receptor-activating peptide [TRAP], and arachidonic acid [AA]).
Results
Almost all of the tested samples inhibited platelet aggregation induced by PAF, ADP, TRAP, and AA, except for Alphagan, which did not demonstrate inhibition of ADP- and TRAP-induced aggregation at a wide range of concentrations. Trusopt, Betoptic, and Azarga eye drops were the most potent inhibitors of all four aggregating factors, while Alphagan was the least potent (P<0.05).
Conclusion
This study shows that anti-glaucomatous eye drops possess anti-platelet effects, and this was shown for the first time by experimenting on human platelets.
Introduction
Glaucoma is a progressive degenerative disease of the retina resulting in the death of the retinal ganglion cells, and constitutes a leading cause of irreversible blindness worldwide. The pathophysiology behind anatomic and functional damage inflicted by glaucoma is still not completely understood. Increased intraocular pressure (IOP) is the most established risk factor for this condition.Citation1 However, occurrence of glaucomatous optic nerve damage is possible even in eyes with normal IOP, a condition characterized as normal- or low-tension glaucoma. This observation has led to the investigation of further possible risk factors, with endothelium-dependent vascular dysregulation being the most studied.Citation2–Citation7 Subsequent vascular insufficiency is believed to alter normal blood flow and generate damage to the optic nerve. It has also been hypothesized that episodes of transient ischemia and reperfusion add to the optic nerve injury.Citation8
Another factor which is believed to contribute to the pathogenesis of glaucomatous optic nerve damage is increased platelet aggregability, possibly triggering microthrombosis of the retinal capillaries and the short ciliary arteries, which result in vascular damage and generate defects in the microcirculation of the optic nerve head.Citation9–Citation16 From very early on, Drance et al noticed an increased platelet adhesiveness (over 60% adhesiveness) in a subgroup of 45 patients with low-tension glaucoma.Citation10 Hoyng et al observed a higher incidence of spontaneous platelet aggregation (SPA) in elderly patients with primary open angle glaucoma (POAG), independent of the presence or absence of vascular diseases,Citation11 while in a follow-up study, the same group observed a high percentage of SPA in POAG patients with visual field deterioration.Citation12 It also seems that these defects in platelet function are more pronounced in patients with low-tension glaucoma.Citation16 Circulating platelet aggregate values are also high in glaucoma patients compared to healthy individuals, indicating a prothrombotic state.Citation15
Considering the results of past studies showing that platelet hyperfunction may be associated with the pathogenesis of glaucoma, it is possible that anti-glaucomatous drugs may exert part of their action through interaction with the platelet aggregation process, in addition to their known pharmacological properties. This hypothesis motivated us to test the anti-platelet potential of a series of anti-glaucomatous eye drops, through inhibition of in vitro induced platelet aggregation. For the experiment, platelet-rich plasma (PRP) was isolated from healthy volunteers’ blood, and aggregation was induced in vitro using four known aggregating factors (platelet activating factor [PAF], adenosine diphosphate [ADP], thrombin receptor-activating peptide [TRAP], and arachidonic acid [AA]). In our previous study, we examined the inhibitory effects of a series of anti-glaucomatous eye drops on PAF-induced aggregation in washed rabbit platelets.Citation17 To our knowledge, the current study is the first to investigate this effect on human platelets, and the first to test ADP, TRAP, and AA, as well.
Materials and methods
Materials and instrumentation
Platelet aggregation assay was performed on a 490 X model (Chrono-Log, Havertown, PA, USA). All aggregation factors (PAF, TRAP, AA, and ADP), as well as bovine serum albumin (BSA), were purchased from Sigma-Aldrich Co. (St Louis, MO, USA).
Acid-citrate-dextrose (ACD) anticoagulant solution was prepared by dissolving in water: citric acid (PanReac AppliChem, Inc., Maryland Heights, MO, USA), sodium citrate (Thermo Fisher Scientific, Waltham, MA, USA), and dextrose (Sigma-Aldrich Co.) to final concentrations of 0.065 M, 0.085 M, and 0.0111 M, respectively.
In this study, the following eye drops were tested: Alphagan (Allergan, Inc., Irvine, CA, USA), Azarga (Alcon Laboratories, Inc., Fort Worth, TX, USA), Betoptic (Alcon Laboratories, Inc.), Cosopt (MSD-Chibret, Mirabel, France), Duotrav (Alcon Laboratories, Inc.), Trusopt (MSD-Chibret), and Xalaprost (Aspen Pharma Pty Ltd, St Leonards, NSW, Australia).
Methods
Every volunteer signed an informed written consent to participate in the study, and the Ethics Committee of the G Gennimatas General Hospital of Athens approved the protocol. Human blood was collected from the antecubital vein of healthy volunteers, and transferred to four polyethylene tubes containing anticoagulant (0.1 M buffered dextrose citrate, ACD) in a ratio of blood/anticoagulant: 9/1 (v/v) to a final volume of 15 mL. The isolation of PRP was obtained by centrifugation of blood specimens at 170× g for 18 min. PRP was then transferred to polypropylene tubes at room temperature for the biological assay, whereas poor platelet plasma (PPP) was obtained by further centrifuging the specimens at 1,500× g for 25 min. PRP was adjusted to 500,000 platelets/μL using the respective PPP. All procedures took place at 24°C (room temperature).
The samples were dissolved in BSA and the induced aggregation was examined with PRP according to the method of Demopoulos et al.Citation18 Each sample was added 1 min prior to the addition of the aggregation factor. The final concentration of each aggregating factor in the cuvette was 3.33 μM for PAF, 0.82 μM for ADP, 0.01 μM for TRAP, and 0.15 μM for AA. The induced platelet aggregation was measured before (considered as 0% inhibition), and after the addition of various concentrations of the examined sample. Consequently, the plot of percentage inhibition (ranging from 20% to 80%) versus different concentrations of the sample was linear. From this curve, the concentration of the sample that inhibited 50% factor-induced aggregation was calculated, and this value was defined as IC50. The minimum and maximum values of inhibition are demonstrated in . The experiments were performed in duplicate. IC50 results were reported in μL for each eye drop sample.
Table 1 The minimum and maximum values of inhibition against TRAP, PAF, ADP, and AA, along with the corresponding volume of sample in the parenthesis
Statistical analysis
The results are expressed as mean and standard deviation. Differences between samples were tested with one-way ANOVA with post hoc analysis for multiple comparisons. Statistical significance was considered as P<0.05. The analysis was performed using SPSS Statistics 20 (IBM Corporation, Armonk, NY, USA).
Results
Our study results showed that almost all of the tested samples inhibited platelet aggregation induced by PAF, ADP, TRAP, and AA. An exception to this was observed in the case of Alphagan, which could not demonstrate inhibitory effects on ADP- and TRAP-induced aggregation at a wide range of concentrations. Regarding Trusopt, IC50 in μL was 2.3±1.7, 4.1±0.3, 25.5±2.0, 25.6±1.8, Xalaprost, 15.9±2.1, 15.8±1.7, 16.5±1.6, 70±1.5, Betoptic, 2.1±0.2, 4.7±1.1, 25.4±0.9, 20.9±1.9, Cosopt, 3.2±0.8, 1.0±0.4, 58.8± 1.1, 46.7±1.3, Azarga, 0.1±0.1, 2.1±0.3, 35.1±1.2, 27.6±1.3, Duotrav, 23.6±0.9, 23.8±1.2, 176.3±21.8, 23.8±1.3, for PAF, ADP, TRAP, and AA, respectively. The IC50 in μL of Alphagan was 29±0.9 and 352.5±21.2 for PAF and TRAP, respectively. The aforementioned results are summarized in and illustrated in .
Table 2 IC50 values for each sample after stimulation with each of the four aggregating factors, compared using one-way ANOVA
Figure 1 Graphical depiction of the IC50 values for each sample after stimulation with each of the four aggregating factors.
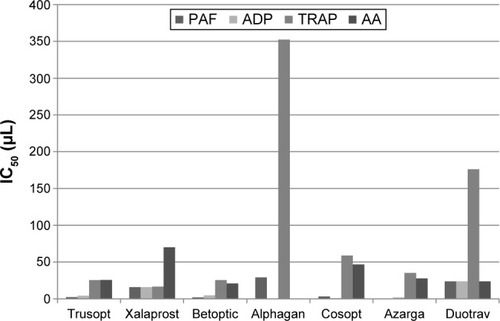
Concerning the statistical analysis, the results revealed that Trusopt, Betoptic, and Azarga were the most potent inhibitors in all four aggregation factors. On the other hand, Alphagan was the least potent. The rest of the eye drops, namely Xalaprost, Cosopt, and Duotrav showed an intermediate inhibitory action. In particular, regarding TRAP; Trusopt, Xalaprost, Betoptic, Cosopt, and Azarga displayed a similar inhibitory action, followed by Duotrav, and then Alphagan as the least potent inhibitor. For PAF; Azarga, Trusopt, Betoptic, and Cosopt were the most potent inhibitors, followed by Xalaprost, then Duotrav, and last Alphagan. Concerning ADP; Azarga, Cosopt, Trusopt, and Betoptic were the most potent inhibitors, followed by Xalaprost and then Duotrav, while Alphagan showed no inhibitory action at all. Regarding AA; Betoptic, Trusopt, Azarga, and Duotrav displayed the most potent inhibition, followed by Cosopt and then Xalaprost, while Alphagan showed no inhibitory action at all. In all cases P<0.05.
Discussion
A high IOP is generally accepted as a risk factor for glaucomatous optic nerve damage onset and progression. However, the increased IOP theory alone cannot explain the glaucomatous optic neuropathy observed in patients with normal IOP. Research has implicated vascular factors in the development of this type of injury. Past studies have demonstrated an association between glaucoma and a high degree of platelet aggregability.Citation9–Citation12,Citation15,Citation16 In the early 70s, Begg et al recognized that small vessel disease was associated with glaucomatous atrophy of the optic nerve head, and characterized a hypercoagulable state as a predisposition for glaucoma.Citation9 Two years later, Drance et al observed an increased platelet adhesiveness (over 60%) in the majority of patients with low-tension glaucoma that they examined.Citation10 A limitation of this study was that no group was used for statistical comparison regarding this outcome. The studies of Hoyng et al shed new light on the topic. Their team tested platelet aggregation in 79 patients with POAG, and in 81 patients with ocular hypertension but no glaucomatous damage, to find a higher incidence of SPA in patients with POAG over 70 years of age.Citation11 Remarkably, the high incidence of SPA in patients with POAG was observed to be independent of the presence or absence of vascular diseases.Citation11 In a follow-up study, the same group observed a higher percentage of SPA in POAG patients with visual field deterioration, compared to POAG patients without visual field loss and patients with ocular hypertension but no visual field defects.Citation12 A Croatian study reported a higher ratio of circulating platelet aggregates in patients with advanced glaucoma accompanied by visual loss, compared to healthy controls.Citation15 However, the same team failed to demonstrate a statistically significant difference in circulating platelet aggregates between patients with POAG and progression of visual field loss, and patients with POAG and non-progressive loss of visual fields, even though circulating platelet aggregate values were pathologic and higher in the former group.Citation19 Nevertheless, this observation does not exclude increased platelet aggregability as a risk factor for glaucomatous optic nerve injury. It is increasingly accepted that the pathogenesis of glaucoma is multifactorial, and indeed high platelet aggregability cannot solely explain visual field loss in glaucoma patients. A Japanese study confirmed the role of platelet hyper-aggregation in glaucoma. They observed that an increased platelet aggregation as defined by ADP- or collagen-induced abnormal secondary aggregation in-vitro is frequently associated with glaucoma patients.Citation16 Interestingly, this tendency is more apparent in patients with normal-tension glaucoma.Citation16
The exact mechanism of glaucomatous damage due to platelet hyper-aggregation is not clear. It has been postulated that microcirculatory defects, such as vasoconstriction and retention of blood, damage endothelial cells, leading to sub-endothelial collagen exposure which triggers platelet aggregation, subsequently causing ischemic injury of the optic nerve.Citation12,Citation16,Citation20 The Ocular Hypertension Treatment Study demonstrated that occurrence of disc hemorrhage predisposes to the development of POAG,Citation21 while later Shim et alCitation22 claimed that micro-infarctions within the optic nerve head and retinal circulatory disorders may be the cause of disc hemorrhages in patients with normal-tension glaucoma.Citation9,Citation22–Citation24 Platelets indeed seem to play a role in this process. However, the primary risk factor for disc hemorrhages in these experiments was found to be delayed – instead of increased – platelet aggregation.Citation22 A possible cause of platelet hyper-reactivity in patients with glaucoma may involve the pigment epithelium-derived factor (PEDF). PEDF is a multifunctional secreted protein that has been found to possess antithrombogenic, anti-angiogenic, and vasculoprotective properties in vivo, thereby protecting against vascular events.Citation25–Citation28 Also, PEDF suppresses occlusive thrombus formation by inhibiting platelet activation and aggregation in rats through its anti-oxidative properties.Citation29 In glaucomatous eyes, PEDF is significantly reduced,Citation30 a fact which may predispose to increased platelet aggregability and the establishment of a thrombogenic state. A final explanation could be that platelet aggregates exclusively promote an elevated IOP by blocking the physiological pores of Schlemm’s canal.Citation31 Platelets’ role is fundamental in the function of the inner wall of Schlemm’s canal.Citation31 A disruption or alteration in their function may result in extensive pore occlusion and decreased aqueous humor drainage. However, this does not explain low-tension glaucoma.
This study examined, for the first time in scientific literature, the anti-platelet properties of anti-glaucomatous drugs as determined by inhibition of in vitro induced human platelet aggregation using four known aggregating factors (PAF, ADP, TRAP, and AA). Our previous study determined the anti-aggregating effects of anti-glaucomatous drugs on washed rabbit platelets, and after stimulation by PAF only.Citation17 Our results indicate that anti-glaucomatous drugs exhibit anti-platelet properties. In general, the most potent inhibition was seen when aggregation was induced by PAF and ADP, and it was less apparent with TRAP and AA. Concerning the drugs, Azarga, Betoptic, and Trusopt demonstrated the most potent inhibitory action, while Alphagan was the least potent. It is quite interesting that each tested eye drop displayed a nearly similar effect on the four different aggregation factors. This fact indicates that general/non-specific, instead of specific, inhibition exists that may be crucial at a clinical practice level.
Unfortunately, there are no large studies examining the role of platelet hyper-aggregation in glaucoma, and most of our reviewed studies have included a limited number of patients and controls. Another limitation of our study, is that we are not able to conclude whether the anti-aggregating effect of the tested eye drops is due to the active ingredients, or due to included excipients in the drugs. Finally, in our experiments, we did not examine the role of the coagulation cascade and the fibrinolytic system, which are concomitantly activated with the platelet aggregation process, and have also been mentioned in studies investigating the vascular mechanisms behind glaucomatous optic nerve damage.Citation10,Citation16,Citation32–Citation34 We hope that this information will be a topic of interest in future studies launched by our results.
It is important that we investigate the pathophysiology behind glaucomatous optic damage with a broad and holistic approach. Currently, increased IOP is the best-established risk factor.Citation1 Yet, it does not explain glaucomatous optic damage observed in patients with normal IOP. Altered platelet aggregability is a potential risk factor for this disorder. Preliminary results of our project show that IOP lowering eye drops can have an effect on platelets and inhibit their aggregation process. This anti-platelet potential may be another mechanism through which these drugs halt glaucoma progression. Known anti-platelet agents, and their effect on glaucoma optic neuropathy progression should be the topic of research in future targeted studies. For instance, a long-term anti-platelet therapy (eg, low-dose aspirin) trial in patients with glaucoma – a suggestion made by other research teams as wellCitation16,Citation35 – is a good idea to study this topic at the level of the intervention.
Disclosure
The authors report no conflicts of interest in this work.
References
- AgarwalRGuptaSKAgarwalPSaxenaRAgrawalSSCurrent concepts in the pathophysiology of glaucomaIndian J Ophthalmol2009574 257 26619574692
- SugiyamaTMoriyaSOkuHAzumaIAssociation of endothelin-1 with normal tension glaucoma: clinical and fundamental studiesSurv Ophthalmol199539Suppl 1 S49 S567660312
- GassAFlammerJLinderLRomerioSCGasserPHaefeliWEInverse correlation between endothelin-1-induced peripheral microvascular vasoconstriction and blood pressure in glaucoma patientsGraefes Arch Clin Exp Ophthalmol199723510 634 6389349947
- NoskeWHensenJWiederholtMEndothelin-like immunoreactivity in aqueous humor of patients with primary open-angle glaucoma and cataractGraefes Arch Clin Exp Ophthalmol19972359 551 5529342603
- TezelGKassMAKolkerAEBeckerBWaxMBPlasma and aqueous humor endothelin levels in primary open-angle glaucomaJ Glaucoma199762 83 899098815
- OrgulSPrunteCFlammerJEndothelium-derived vasoactive substances relevant to normal-tension glaucomaCurr Opin Ophthalmol199892 88 9410180520
- CioffiGASullivanPThe effect of chronic ischemia on the primate optic nerveEur J Ophthalmol19999Suppl 1 S34 S3610230604
- DranceSMSome factors in the production of low tension glaucomaBr J Ophthalmol1972563 229 2425032759
- BeggISDranceSMSweeneyVPIschaemic optic neuropathy in chronic simple glaucomaBr J Ophthalmol1971552 73 905550539
- DranceSMSweeneyVPMorganRWFeldmanFStudies of factors involved in the production of low tension glaucomaArch Ophthalmol1973896 457 4654706442
- HoyngPFGreveELFrederikseKGeijssenCOostingHPlatelet aggregation and glaucomaDoc Ophthalmol1985612 167 1734075960
- HoyngPFde JongNOostingHStilmaJPlatelet aggregation, disc haemorrhage and progressive loss of visual fields in glaucoma. A seven year follow-up study on glaucomaInt Ophthalmol1992162 65 731587697
- SchroerHScheurerGBehrens-BaumannWVascular occlusion of the retina – an experimental model. II. Platelet aggregatesGraefes Arch Clin Exp Ophthalmol19922303 281 2851597296
- BoerrigterRMSiertsemaJVKemaIPSerotonin (5-HT) and the rat’s eye. Some pilot studiesDoc Ophthalmol1992821–2 141 1501305018
- BojicLSkare-LibrenjakLCirculating platelet aggregates in glaucomaInt Ophthalmol1998223 151 15410548459
- MatsumotoMMatsuhashiHNakazawaMNormal tension glaucoma and primary open angle glaucoma associated with increased platelet aggregationTohoku J Exp Med20011934 293 29911453537
- MoschosMMChatziralliIPStamatakisGPapakonstantinouVDTsatsosMDemopoulosCAIn vitro effects of anti-glaucomatous eye drops on platelet-activating factor and its metabolismSemin Ophthalmol2017322 198 20326270771
- DemopoulosCAPinckardRNHanahanDJPlatelet-activating factor. Evidence for 1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphorylcholine as the active component (a new class of lipid chemical mediators)J Biol Chem197925419 9355 9358489536
- BojicLMandicZBukovicDKarelovicDStrinicTCirculating platelet aggregates and progression of visual field loss in glaucomaColl Antropol2002262 589 59312528286
- YauJWTeohHVermaSEndothelial cell control of thrombosisBMC Cardiovasc Disord201515 13026481314
- BudenzDLAndersonDRFeuerWJDetection and prognostic significance of optic disc hemorrhages during the Ocular Hypertension Treatment StudyOphthalmology200611312 2137 214316996592
- ShimSHKimJMWooHYShinKUKohJWParkKHAssociation between platelet function and disc hemorrhage in patients with normal-tension glaucoma: a prospective cross-sectional studyAm J Ophthalmol20151606 1191 119926384167
- SonnsjoBDokmoYKrakauTDisc haemorrhages, precursors of open angle glaucomaProg Retin Eye Res2002211 35 5611906810
- KrakauCEBengtssonBHolminCThe glaucoma theory updatedActa Ophthalmol (Copenh)1983615 737 7416659881
- YamagishiSMatsuiTPigment epithelium-derived factor (PEDF) and cardiometabolic disordersCurr Pharm Des20142014 2377 238623844817
- YamagishiSIMatsuiTAnti-atherothrombogenic properties of PEDFCurr Mol Med2010103 284 29120236055
- YamagishiSMatsuiTNakamuraKTakenakaKPigment epithelium-derived factor (PEDF) inhibits collagen-induced platelet activation by reducing intraplatelet nitrotyrosine levelsInt J Cardiol20101401 121 12219046781
- YamagishiSMatsuiTNakamuraKTakenakaKAdministration of pigment epithelium-derived factor prolongs bleeding time by suppressing plasminogen activator inhibitor-1 activity and platelet aggregation in ratsClin Exp Med200991 73 7618815870
- TakenakaKYamagishiSMatsuiTPigment epithelium-derived factor (PEDF) administration inhibits occlusive thrombus formation in rats: a possible participation of reduced intraplatelet PEDF in thrombosis of acute coronary syndromesAtherosclerosis20081971 25 3317850801
- OgataNMatsuokaMImaizumiMArichiMMatsumuraMDecreased levels of pigment epithelium-derived factor in eyes with neuroretinal dystrophic diseasesAm J Ophthalmol20041376 1129 113015183804
- HamanakaTBillAPlatelet aggregation on the endothelium of Schlemm’s canalExp Eye Res1994593 249 2567821369
- MehraKSDubeBMikuniIDubeRKReduced fibrinolytic activity in aqueous humor of chronic simple glaucomaTokai J Exp Clin Med198491 33 346535299
- MehraKSDubeBDubeRKReduced fibrinolytic activity in aqueous humour in glaucomaIndian J Ophthalmol1983315 592 5936671769
- MehraKSDubeBDubeRKFibrinolytic activity in blood and aqueous humour in glaucomaIndian J Ophthalmol198331Suppl 827 8296544262
- PacheMFlammerJA sick eye in a sick body? Systemic findings in patients with primary open-angle glaucomaSurv Ophthalmol2006513 179 21216644363
