Abstract
In the upcoming years, the proportion of elderly patients with chronic obstructive pulmonary disease (COPD) will increase, according to the progressively aging population and the increased efficacy of the pharmacological treatments, especially considering the management of chronic comorbidities. The issue to prescribe an appropriate inhalation therapy to COPD patients with significant handling or coordination difficulties represents a common clinical experience; in the latter case, the choice of an inadequate inhalation device may jeopardize the adherence to the treatment and eventually lead to its ineffectiveness. Treatment options that do not require particular timing for coordination between activation and/or inhalation or require high flow thresholds to be activated should represent the best treatment option for these patients. Nebulized bronchodilators, usually used only in acute conditions such as COPD exacerbations, could fulfill this gap, enabling an adequate drug administration during tidal breathing and without the need for patients’ cooperation. However, so far, only short-acting muscarinic antagonists have been available for nebulization. Recently, a nebulized formulation of the inhaled long-acting muscarinic antagonist glycopyrrolate, delivered by means of a novel proprietary vibrating mesh nebulizer closed system (SUN-101/eFlow®), has progressed to Phase III trials and is currently in late-stage development as an option for maintenance treatment in COPD. The present critical review describes the current knowledge about the novel nebulizer technology, the efficacy, safety, and critical role of nebulized glycopyrrolate in patients with COPD. To this end, PubMed, ClinicalTrials.gov, Embase, and Cochrane Library have been searched for relevant papers. According to the available results, the efficacy and tolerability profile of nebulized glycopyrrolate may represent a valuable and dynamic treatment option for the chronic pharmacological management of patients with COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by not fully reversible airflow obstruction due to structural derangement of the airways, sustained by an inflammatory response secondary to inhalation of noxious particles, mainly represented by cigarette smoke and air pollutants.Citation1 COPD represents an increasing social and health care problem, and a leading cause of disability. In current projections, mortality due to COPD will eventually increase by 2030 to become the third most common cause of death worldwide.Citation2 In COPD, both chronic bronchitis and emphysema can be present with different intensities and are responsible for airway narrowing, mucus hyper-secretion, loss of small conducting airways, and lung elastic recoil.Citation3 Starting from the peripheral airways, which represent the site of the initial damage, these anatomic changes eventually lead to expiratory airflow limitation, air trapping, dynamic hyperinflation, increased closing volume, and ventilation–perfusion mismatch.Citation4–Citation6 Long-acting muscarinic antagonists (LAMAs), such as tiotropium, aclidinium, glycopyrronium, and umeclidinium, and long-acting β2 agonists (LABAs), such as indacaterol, formoterol, salmeterol, and olodaterol, are able to reduce hyperinflationCitation7 and the extent of airway closure,Citation8 improving symptoms and exercise tolerance,Citation9 and thus represent the cornerstone of the long-term pharmacological treatment in COPD. Although inhaled therapy has the advantage to be administered directly to the site of action, the process interposed between the actuation of the device and the drug deposition in lung periphery relies on numerous variables, namely the drug formulation, patients’ inhalation technique, and lung mechanics.Citation10 So far, the most widespread hand-held inhalers’ devices are represented by dry powder inhalers (DPIs), pressurized metered dose inhalers (pMDIs), and soft mist inhalers (SMIs), while nebulizers are often left for the treatment of acute conditions such as COPD exacerbations or in patients with extremely limited self-sufficiency.Citation11 Although effective, technologically advanced and portable, the use of handheld devices has always been affected by numerous major mistakes that lead to an incorrect inhalation technique and eventually to the lack of treatment efficacy,Citation10 especially in older patients with COPD, with a severe disease and multiple comorbidities.Citation10 In the latter group of patients, drug delivery via nebulization may provide an effective alternative, since an optimal dose can be delivered during tidal breathing, regardless of disease severity or associated comorbidities, thus overcoming the need for coordination, specific handling and inspiratory maneuvers.Citation12
The present critical review will be focused on the nebulized delivery technology, developmental phases, efficacy and safety of nebulized glycopyrrolate (nebulized glycopyrronium bromide [GBn]) in patients with COPD. Medline, Embase, Cochrane Library, and ClinicalTrials.gov were searched for relevant full papers and conference abstracts up to August 2017. Only manuscripts in English language were considered.
Management issues in COPD treatment
The peculiarity of the pharmacology of the respiratory system is represented by the possibility to grant drug delivery directly to the desired site of action, allowing to bypass the majority of the intermediate metabolic steps usually inevitable for other administration routes.Citation13,Citation14 However, the efficacious access to the lung is jeopardized by complex variables that range from drug formulation and posology to the pathophysiology and stage of the disease. In this view, adherence becomes crucial for treatment efficacy. In clinical trials, adherence to inhaled treatments is usually >80%,Citation15 a result that does not usually reflect the real life context, in which adherence can be as low as 10%.Citation16,Citation17 Reduced adherence does not only impact the daily clinical management of patients with COPD but also increases the frequency of exacerbations, the number of hospitalizations, and emergency department visits, thus negatively affecting health care costs.Citation18
The device used to deliver the inhaled drug represents a fundamental step for the clinical decision process and may be as important as the choice of the drug itself. The inhalation devices more commonly prescribed are represented by pMDIs, DPIs, SMIs, and nebulizers. Despite a long-standing debate, there is no consensus on how to match patient requirements with criteria for selecting an alternative inhaler device. Rather than insisting with training a patient with a specific inhaler, it would be more appropriate to match the device with the needs and the skills of the patient.Citation10 Most of the inhalation devices currently available on the market have a comparable efficacy, provided they are used appropriately.Citation11,Citation19–Citation21 During drug administration, up to 75% of patients with COPD do not receive an optimal dose of the inhaled drug, mainly because of poor coordination or inhalation techniques.Citation22,Citation23
For pMDIs, the most common administration errors are represented by the patient’s poor coordination between the device activation and drug inhalation or by a short inhalation time. During DPIs’ use, patients are often unable to obtain a sufficient inspiratory peak flow necessary get the resistance required to activate the drug and dissociate the carrier from the active molecule; moreover, common mistakes regard dose preparation and exhalation through the device prior to inhalation.Citation24–Citation27 Even the use of SMI requires an accurate priming and breathing technique,Citation28 although the timing for inspiration and the required inspiratory flow are respectively longer and lower compared to pMDIs, since the delivered aerosol lasts three times longer than in pMDIs.Citation25 DPIs are often sensitive to humidity, which can reduce the delivered dose, while SMIs and pMDIs, when left unused for some time, must be re-primed.Citation29 Advantages and disadvantage of the devices currently available on the market are summarized in .
Table 1 Advantages and disadvantages of inhalation devices
Generally, patients that commit errors during inhalatory therapy hiring, tend to be older, more debilitated and to have a severe disease. In elderly patients, unintentional nonadherence to inhalation therapy often comes from cognitive impairment, hearing or visual loss, and other physical disabilities, such as arthritis and tremors resulting in poor coordination, which significantly affect their ability to understand and follow the suggested treatment. Complex pharmacological regimens are also secondary to multiple chronic comorbidities, representing a major risk factor for impaired adherence.Citation25
Glycopyrrolate by nebulizer
Taking into account the aging population and the projected increase in the prevalence of respiratory tract diseases, patients with COPD will become older, and treatment options that do not require an optimal coordination for activation/inhalation or require a flow threshold to be activated will become the preferable treatment option.
To date, nebulized muscarinic antagonists are limited to short-acting formulations (SAMAs), while β-agonists are available both as short-acting (SABAs) and as long-acting formulations (aformoterol tartrate), the latter approved only in the USA. SAMA and SABA, in addition to being the treatment choice in acute conditions, are also often suggested for maintenance treatment in selected COPD patients. However, to maintain an optimal bronchodilation coverage, three or four doses per day are needed when SAMA and SABA are delivered via a general purpose jet nebulizer. The latter are usually bulky, characterized by limited portability and with dispensing times of 10–15 minutes per dose, aspects that likely affect patients’ compliance. In view of the aforementioned limitations, the lack of long-acting molecules that would reduce the daily posology and a shorter delivery time appear desirable.Citation30
The GOLD document recommends nebulizers to be used in specific populations, such as patients with inspiratory flow rates as low as <30 L/s or patients with poor hand-eye coordination.Citation1 Furthermore, the benefits of nebulizer treatment over DPI and MDI formulations should be evaluated symptomatically and the treatment should be continued as long as similar benefits are not achievable by simpler, cheaper, and more portable alternatives. Indeed, recent surveys reported both patients and their caregivers to be increasingly satisfied with nebulized drug delivery, in terms of symptom relief, ease to use, and improved quality of life.Citation10,Citation11,Citation31
Nebulizers can convert a liquid into aerosol droplets suitable for patient inhalation. Currently, the technologies available are mainly represented by air-jet nebulizers, the most widely used, and ultrasonic nebulizers. There are also two pysicochemical categories of drugs available for nebulization: solutions and suspensions. Solutions contain a drug dissolved in saline or other liquids. Suspensions contain a drug that is not soluble in water or other respirable liquids but exist as a mixture of small drug particles suspended in liquid, which jeopardizes their presence in each droplet generated during the nebulization process. Conventional ultrasonic nebulizers cannot be used to administer suspensions.Citation30
Due to their safety profile and to a better exacerbation-preventing effect compared to LABAs,Citation32,Citation33 LAMAs are usually the preferred starting therapy for COPD patients, but no nebulized LAMA is actually available on the market. As a part of the maintenance treatment of patients with COPD, in last years, glycopyrronium bromide (GB) has been developed and available as a single agent, in combination with LABAs,Citation34–Citation36 or in triple therapy in fixed dose combination with LABAs and inhaled corticosteroids (ICSs).Citation37,Citation38 The effects of GBn have been studied since 1984 in patients affected by asthma and since 1995 in patients with COPD.Citation39,Citation40 Interestingly, probably due to its limits in administering time and portability, nebulization was the first to be studied, but the least to be proposed on the market. In the last years, several clinical trials have investigated a nebulized formulation of GBn delivered by a high efficiency nebulizer, which is currently awaiting for the US Food and Drug Administration (FDA) approval as maintenance therapy in COPD patients.
GBn has been investigated as a soluble molecule named EP-101 in 2015.Citation41 Later on, under the name of SUN-101, it was part of the Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer (GOLDEN) development program, consisting of a total of seven trials.Citation42–Citation48
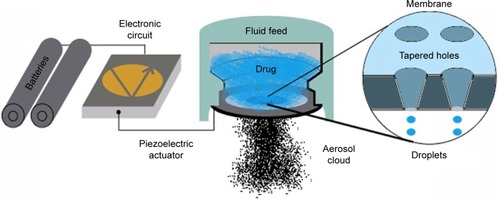
GBn was studied to be delivered by the eFlow® Closed System (eFlow® CS) device (PARI Pharma GmbH, Starnberg, Germany). The latter is characterized by the possibility to deliver a wide range of drug volumes (0.5–5 mL) and dosages (0.001–1,000 mg). In addition, the hole sizes can be adjusted from just <2 μm upward, allowing this system to deliver drugs during consecutive breaths. The eFlow® is a vibrating mesh nebulizer, an evolution of 2005 ultrasonic wave nebulizers, in which a mesh/membrane with 1,000–7,000 laser-drilled holes vibrate at the top of the liquid reservoir, pressuring out a mist of very fine droplets through the holes. The latter technology appears to have solved some important issues existent with previous nebulizers, such as having too much liquid waste and undesired heating of the medical liquid. Portability for these devices is also enhanced by the battery-power and lightweight design. A disadvantage of vibrating mesh nebulizers consists of the little information concerning the ideal dose of the bronchodilator solution to be added to the nebulizer; consequently, the potential of over-dosing exists if the same dose for conventional jet nebulizers is used. To address this concern, a new generation of closed system mesh nebulizers has been conceived that will accept only the ampule containing the specific drug approved for use.Citation49
In successive trials, a modified version of the eFlow® nebulizer was adopted: the eFlow® CS. The latter is a handheld, portable, battery-operated, electronic vibrating membrane nebulizer, modified from an FDA-cleared open-system device used to deliver cystic fibrosis medications. Like other vibrating mesh nebulizers, the eFlow® is virtually silent, designed to deliver the medication in two to three minutes during tidal breathing. It has been shown to improve patient compliance due to its comparatively short treatment time since it is more efficient than having a vibrating piezoelectric element at the bottom of the liquid reservoir ().Citation50 The most common jet nebulizers are usually cumbersome, not portable, noisy (up to 60 dB) and require 10–15 minutes to deliver the drug.Citation51
Fundamental for the destiny of an aerosolized particle is its aerodynamic diameter, determined by geometrical diameter, density, and form.Citation52 Only particles with a diameter between 5 and 0.5 μm can reach and stay in the lung: bigger particles impact on upper airways’ mucosa, while smaller ones usually reach alveoli but are also exhaled away since they fail to lay on the epithelium.Citation52,Citation53 The mass median aerodynamic diameter (MMAD), geometric standard deviation (GSD), and fine particle fraction (FPF, %, <5 μm) of the eFlow® CS system have values independent of formulation strength. For both strengths tested, the mean MMAD was 3.7 μm, the mean GSD was 1.7, and the mean FPF was 72%. As expected in tidal breathing conditions, aerosol is expelled to waste during the exhalation phase. Compared to general purpose nebulizers, which can nebulize different drugs, to avoid incorrect dosing of the medication, the eFlow® CS uses a unique ready-to-use unit-dose vial, designed to mitigate misuse and ensure dose uniformity.Citation3,Citation52,Citation54 The device requires to be disassembled and cleaned after each use to prevent clogging of the mesh openings. At the time of this review, the eFlow® system used for cystic fibrosis is being sold for $800–1,000.Citation51
Traditional nebulization route has some limits indeed, especially in regard to the output variation between different nebulizers of the same type. In fact, factors affecting nebulizer output are inextricably linked with those affecting particle size and nebulization time.Citation55 Output can be diminished if the jet is blocked with dirt or drug crystals. Repeated uses may increase the diameter of the air orifice thus increasing the mean diameter of the droplets generated and reducing the air speed. This aging may be due to mechanical wear from the compressed air source or to excessive cleaning. Nebulizers designed to deliver small particles may have increased residual volume, decreasing drug output and increasing recirculation of the nebulizer solution, thus lengthening nebulization time. Increasing the volume fill improves drug output but lengthens nebulization time. Increasing driving gas flow rate may help, but for home use a higher performance compressor may be needed.Citation56 Another disadvantage is represented by the need of the end-user to load the medication prior to each administration.Citation57
Despite some drawbacks associated with nebulizers, current evidence suggests that the efficacy of treatments administered to patients with moderate-to-severe COPD via nebulizers is similar to that observed with pMDIs and DPIs.Citation20–Citation23 Furthermore, for some patients, the use of both a nebulizer as maintenance therapy and a handheld inhaler as rescue medication may provide the best combination of efficacy and convenience.
Pharmacology of glycopyrrolate via nebulizer
Chemistry and pharmacokinetics
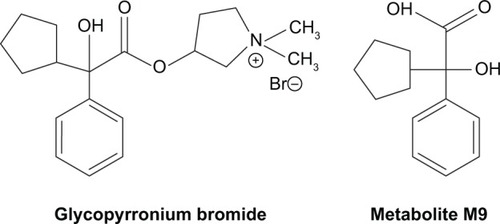
Glycopyrronium, the active moiety of its bromide salt, also known as glycopyrrolate, is an antimuscarinic drug that was initially approved by the FDA for systemic administration for the reduction of sialorrhea due to preoperative stages, neuromuscular, and drug-related diseasesCitation58,Citation59 and due to the property of reducing gastric secretions, as a treatment for peptic ulcer.Citation60 It is a quaternary ammonium derivative – (1,1-dimethylpyrrolidin-1-ium-3-yl) 2-cyclopentyl-2-hydroxy-2-phenylacetatethatCitation61 – with a peripheral effect similar to atropine. The chemical structure of GB is reported in .
Figure 2 Chemical structure of glycopyrronium bromide (left) and its plasma metabolite M9 (right).
GB is rapidly absorbed into the systemic circulation after inhalation, ≈90% via lung and ≈10% via gastrointestinal absorption.Citation62 The mean terminal elimination half-life (t1/2) of GB-DPI, is half that reported for GB-MDI (from 13 to 22 hours for a dose range of 25–200 μgCitation63 and from 6.3 to 9.6 hours for a dose range of 14.4–115.2 μg), leaving open discussion on whether these pharmacokinetic features should be interpreted as a signal favoring the once daily (OD) or a bis in die (BID) administration. During the Phase II study by Leaker et al,Citation41 a formulation of GBn delivered by the eFlow® nebulizer was rapidly absorbed with peak plasma concentrations within 15–30 minutes. There were only two measurable plasma GBn concentrations available with the 12.5 μg dose. Therefore, 12.5 μg was not included in the dose proportionality analysis. The elimination half-life (t1/2) of GBn was calculated for the 0–1 and 0–12 hours intervals. The median t1/2, 0−1 hours ranged from 1.10 to 1.15 hours, and median t1/2, 0–12 hours ranged from 2.30 to 7.45 hours, following administration of 50, 100, 200, and 400 μg of GB, respectively.Citation41
Metabolism and elimination
GB is nonenzymatically hydrolyzed to form the major circulating metabolite, M9, which is a racemic carboxylic acid derivative, formed from the swallowed fraction of inhaled GB ().Citation62 In animal models, the primary biotransformation pathways for GB have been demonstrated to include the addition of one or two oxygen atoms to the cyclopentane and phenyl rings and dehydrogenation on the cyclopentane ring.Citation64 Further hydrolysis of the ester linkage forms the M9 metabolite. About 10% of systemic exposure is due to the swallowed drug, oral bioavailability of GB being 5%. The metabolite M9 has a plasma concentration that is approximately the same as that of the parent drug after inhalation but not after intravenous administration.Citation64 Biliary excretion contributes ~5% to total clearance. GB also forms various mono and bis-hydroxylated metabolites in vitro. Glucuronide and sulfate conjugates of GB were recovered in urine of subjects receiving multiple dosages of inhaled drug.Citation64 The most quantitatively important cytochrome that contributes to the metabolism of GB is CYP2D6.Citation64
Pharmacodynamics
Muscarinic antagonists promote the relaxation of airway smooth muscle by blockade of the acetylcholine receptor.Citation65 GB is a competitive muscarinic receptor antagonist that bronchodilates the airways by inhibiting acetylcholine induced bronchoconstriction in bronchial smooth muscle cells.Citation66 The three main muscarinic receptors related with human lung bronchial dynamics are the M1, M2, and M3 receptors. M2 receptor protects against bronchoconstriction and its block may increase heart rate.Citation36,Citation66 The bronchoconstrictor effect of acetylcholine is mediated mainly through M3 receptors located on airway smooth muscle cells, whereas M1 receptors enhance cholinergic reflexes. In contrast, M2 receptors on cholinergic nerve endings inhibit acetylcholine release, thus reducing bronchoconstriction, and should not be blocked.Citation67 Mucus secretion is largely mediated by M3 receptors and M3 receptor blockade may also reduce mucus hypersecretion.Citation68,Citation69 In vitro, GB binds with high affinity to all the bronchial muscarinic receptors but has four- to fivefold higher selectivity for human M1 and M3 receptors than for the human M2 receptor (equilibrium binding affinity constants of 9.60–9.81 and 9.47–9.64 vs 8.70–9.25).Citation6,Citation70 GB has also different dissociation kinetics for the three muscarinic receptors, with dissociation half-life of 11.4 and 13.9 for M3 and M1, respectively, vs 1.07 min for M2 and kinetic off rate of 0.061 and 0.05 vs 0.646 per minute, respectively.Citation62,Citation66 GB showed greater equilibrium binding selectivity (M3 selectivity ratio [ratio of the affinity constant for the M3 receptor vs that for the M2 receptor] of 7.76-fold vs 2.09-fold) and kinetic selectivity (M3 kinetic selectivity ratio [ratio of the area under the simulated association and dissociation curves for the M3 receptor vs that for the M2 receptor] of 11.41-fold vs 4.30-fold) for M3 versus M2 than tiotropium bromide, indicating the potential for an improved therapeutic index.Citation62,Citation66
Selective M1 and M3 receptors’ blockers such as tiotropium and GB are therefore preferable to nonselective muscarinic antagonists, such as atropine and ipratropium bromide, which express their effects also on the M2 receptor subtype.Citation68,Citation71 Both GB and tiotropium are in fact selective for M1 and M3 receptors and have a similar duration of action with slow dissociation for these receptor subtypes.Citation72 The slow dissociation profile of GB contributes to the long duration of action seen in clinical studies.Citation73 Accordingly, GB rapidly dissociates from M2 receptors, thus avoiding their prolonged presynaptic blockade and reducing the antagonizing effect of acetylcholine release.Citation72,Citation74
Compared to tiotropium bromide, GB has a faster onset of action both in vitro and in vivo animal models.Citation70 In fact, GB has shown to have both lower equilibrium binding affinity constants and a faster kinetic off rate (9.59 and 0.061 vs 10.37 and 0.015 per minute, respectively), which justifies its faster onset of action also in patients with moderate-to-severe COPD when compared with tiotropium bromide at a dosage of 18 μg.Citation75 The onset of action, however, appeared slower both in terms of relief from airflow obstruction and in reduction of static hyperinflation when compared with aclidinium bromide 400 μg in a double-blind cross-over study in severe and very severe patients with COPD.Citation76
GB has been shown to be more potent than ipratropium bromide and tiotropium bromide in terms of concentration drug necessary to inhibit the contractile bronchial smooth muscle response by 50%.Citation77 In a recent report that compared in vivo and in vitro effects of GB-DPI, aclidinium bromide DPI, and tiotropium bromide DPI, GB-DPI resulted to be the most potent of the three LAMAs investigated, inducing, with aclidinium, a faster bronchodilation compared to tiotropium.Citation78 However, given the fact that so far, the approved marketed doses are not isoeffective for the two LAMAs, to make precise pharmacodynamics speculations of their effect in vivo remains a difficult task. A potential disadvantage brought by the GB fixed-dose combinations currently available on the market is represented by the GB dosage (≤50 μg); in fact, from the analysis of the forced expiratory volume in the first second (FEV1) dose–response in pharmacodynamic studies, the optimal dose of GB appeared to be at least 100 μg, to be reflected in a FEV1 difference of >100 mL.Citation11
Glycopyrrolate-approved formulations
GB has been initially approved by the European Medicines AgencyCitation79 and the Pharmaceuticals and Medical Devices AgencyCitation80 in 2012 and by the FDACitation81 in 2015, as a standalone treatment for COPD as a dry-powder inhalation formulation. Subsequently, while in Europe it has been approved with OD posology, FDA approved its usage as BID long-acting bronchodilator both as a standalone treatment and as a fixed-dose DPI combination with indacaterol. An MDI formulation containing a fixed-dose combination of GB and formoterol fumarate has been available in USA and Japan since 2016. A DPI formulation containing a triple combination of GB, formoterol fumarate, and beclomethasone has been approved for marketing in EU in May 2017.Citation82
At the time of the present review, a nebulized formulation of GB is awaiting approval from FDA as a maintenance treatment for patients with COPD.Citation83
Efficacy studies
Phase II and Phase III efficacy and safety clinical trials involving GBn are summarized in .
Table 2 Available studies on nebulized GB for COPD treatment
Phase II studies
GBn was studied in a Phase II, two-center, randomized, placebo-controlled, double-blind, dose ranging, single-dose, six-way cross-over trial in 35 patients with moderate-to-severe COPD (GOLD II/III), aged 40–75 years.Citation41 All patients were required to have at least 12% and 150 mL reversibility to inhaled ipratropium bromide as inclusion criteria. Eligible patients were randomized to receive a single dose of GBn via eFlow® nebulizer (12.5, 25, 50, 100, and 200 μg) or placebo in six treatment visits separated by washout periods of 5–12 days; each dose was followed by an onset of response on placebo-adjusted FEV1 in <5 minutes (60, 80, 100, 130, and 140 mL). The bronchodilating effect progressively decreased over 24 hours period with a dose–response relationship, so that the higher the initial dose, the longer the duration of bronchodilation. The placebo-adjusted FEV1 improvement at 24 h postdose was >100 mL with both the 100 μg (104 mL increase over placebo effect) and 200 μg (118 mL increase) doses; only the latter doses produced a significant improvement over placebo.Citation41
The eFlow® was able to deliver all doses of GBn in <2 minutes (range 1.6−1.9 minutes). GBn peak plasma concentrations were measured within 15–30 minutes.Citation41
The GOLDEN-1 study was a Phase II, multicenter, randomized, double-blind, placebo-controlled, four-period, incomplete block, cross-over study.Citation42 The primary outcomes were the mean change in 24-hour postdose trough FEV1, and the standardized change in FEV1 area under the curve (AUC) (0–12 and 12–24 hours) on days 1 and 7.Citation42 Secondary outcome measures were peak FEV1 (maximum FEV1 during the first 4 hours postdose on days 1 and 7); time to onset of action (time necessary to obtain a ≥10% improvement in postdose FEV1); treatment responders (proportion of subjects with clinically meaningful change from predose in trough FEV1 on days 1 and 7); and safety and tolerability.Citation42
A total of 140 patients with moderate-to-severe COPD were randomized to four of seven treatments: GBn (25, 50, 100, and 200 μg) or placebo OD via eFlow® nebulizer, open-label tiotropium 18 μg OD and open-label ipratropium 500 μg three times a day delivered via jet nebulizer. All doses of GBn demonstrated dose-related and significant improvements in FEV1 AUC (0–24 hours) on day 7 compared to placebo, with estimated differences between GBn doses and placebo ranging between 110 and 169 mL.Citation84
Following the pharmacokinetic profile of GBn, in the subsequent Phase II studies, only the BID posology has been maintained.
Two randomized, double-blind, placebo- and active- controlled Phase II studies, GOLDEN-2Citation43,Citation85 and GOLDEN-6,Citation47 further characterized the efficacy of GBn in terms of dose–response relationship recruiting a total of 378 patients with moderate-to-severe COPD (GOLDEN-2, N=282; GOLDEN-6, N=96). The GOLDEN-2 study had a parallel group design of 28 days, while the GOLDEN-6 was a 7-day crossover trial. It should be underlined that for the GOLDEN-2 study eligible patients were <40 years old.Citation47
The primary endpoint of both studies was the change from baseline in trough FEV1 on day 7 or day 28. Safety and tolerability were evaluated based on the incidence of treatment-emergent adverse events (TEAEs), serious AEs (SAEs), and discontinuations due to TEAEs. FEV1 AUC 0–12 was a secondary endpoint. The data were pooled for the lung function assessments, common to both studies.Citation31,Citation47
In both studies, increasing doses of GBn (3–100 μg BID via eFlow® CS nebulizer) were compared with placebo or aclidinium bromide 400 μg BID (Tudorza® Pressair®). GBn produced rapid onset (≤5 minutes) dose-related bronchodilation following a single-dose administration. Improvements were maintained over a 24-hour period at all doses >50 μg.Citation41,Citation47 The primary endpoints of both studies were reached obtaining on days 7 and 28 a significant improvement of the placebo-adjusted trough FEV1, with dose-related improvements ranging from 82 mL for the 6.25 μg dose to 177 mL for 100 μg BID, with corresponding changes in FEV1 AUC0–12, ranging from 84 to 183 mL. The improvements in lung function for the 25 and 50 μg BID doses were comparable to those obtained with aclidinium bromide (trough FEV1 =157 mL, FEV1 AUC0–12 =190 mL). The GBn dose of 3 μg BID was identified as the “no-effect” dose.Citation43,Citation46,Citation86
The GOLDEN-2 and GOLDEN-6 dose-finding studies supported the selection of GBn 25 and 50 μg BID doses to advance in the subsequent Phase III GOLDEN trials.Citation43,Citation45,Citation86
Phase III studies
GOLDEN-3Citation44 and GOLDEN-4Citation45 were twin Phase III, 12 weeks, randomized, double-blind, placebo-controlled, parallel-group, multicenter, efficacy, and safety trials.
The primary endpoint was change from baseline at week 12 in trough FEV1. Key secondary endpoints included forced vital capacity (FVC), St George’s Respiratory Questionnaire (SGRQ), and rescue medication use. Safety was also assessed throughout the study.
Included patients were 40–75 years old, with a ≥10 pack-year smoking history and a moderate-to-very-severe COPD (postbronchodilator FEV1 <80% of predicted normal or FEV1/FVC ratio <0.70). To include a closer-to-real-life COPD population, patients with maintenance treatment with LABA (31%) and ICS (29%) and co-existing cardiovascular disease were not excluded in both studies; patients were stratified by cardiovascular history, 64% being at high risk.Citation87 The administration of both doses of GBn/eFlow® (25 and 50 μg BID) resulted in statistically and clinically significant improvements compared to baseline in placebo-adjusted trough FEV1 (GOLDEN-3: +105 and +126 mL; P≤0.0001; GOLDEN-4: +84 and +82 mL; P≤0.0001) and placebo-adjusted trough FVC (GOLDEN-3: +149 and +167 mL, P<0.001; GOLDEN-4: +130 and +113 mL, P<0.01) at week 12.Citation87,Citation88
GOLDEN-5 study was a Phase III, randomized, open-label, active-controlled, parallel-group, multicenter, long-term safety trial of 48 weeks of treatment with GBn delivered by the eFlow® CS system or tiotropium 18 μg OD in 1,087 moderate-to-very-severe COPD patients.Citation46 The population included patients with background use of LABA and history of significant cardiovascular disease.Citation46
The primary endpoints were the incidence of TEAEs, SAEs, and discontinuations due to TEAEs. Secondary endpoints included patient-reported outcomes, rescue medication use, and the mean change from baseline over 48 weeks in trough FEV1. Lung function results confirmed the findings from GOLDEN-3 and -4 studies. Considering patients with and without background LABA therapy, improvements in trough FEV1 after GBn administration were not different, 99 and 105 mL, respectively.Citation46,Citation89
GOLDEN-7 is a Phase I randomized, open-label, single-dose per dosing period, five-way cross-over study in subjects aged 40–70 years with a diagnosis of moderate-to-severe COPD designed to evaluate the total systemic exposure and lung bioavailability of GBn compared to GB delivered by Breezhaler® (Seebri®). GB was administered with and without activated charcoal. Unfortunately to date, no results are available for this study, as the available abstracts have been withdrawn.Citation47,Citation90
Safety and tolerability
During the Phase II trial conducted by Leaker et al, single doses of GBn were well tolerated with AE profiles comparable to placebo. After all dosages, there were no clinically relevant changes in vital signs and ECG parameters, including QTc, and no typical muscarinic side effects, such as dry mouth and increased heart rate.Citation41
In the GOLDEN-1, all doses of GBn were well tolerated with similar AE rates between placebo and GBn (31.2, 29.7, 26.9, 35.5, and 30.7% for placebo, 25, 50, 100, and 200 μg, respectively). There was no apparent dose–response relationship for incidence and severity of AEs. Mean changes in vital signs and ECG parameters from baseline to day 7 were comparable between the treatment groups.Citation41 No clinically significant differences compared to placebo were observed in terms of heart rate, blood pressure, and ECG parameters.Citation42 The GBn safety profile was similar to placebo, tiotropium, and ipratropium in regard to cardiovascular AEs.Citation91
GBn was well tolerated in GOLDEN-3 and -4 trials, with a combined overall incidence of TEAEs being numerically lower with GBn 25 and 50 μg BID doses compared to placebo (43.4, 50.7, and 52.3% for GBn 25 and 50 μg doses and placebo, respectively). The most frequent AEs were represented by skin injury, headache, acute COPD exacerbations, cough, and dyspnea. Discontinuations due to TEAEs were numerically higher in the placebo group. There was one cardiovascular-related death in the 50 μg BID group.Citation88
A safety analysis of the 48 week GOLDEN-5 trial, in which the primary objectives were the number and percentage of patients with TEAE, demonstrated comparable and consistent results with GOLDEN-3 and GOLDEN-4 trials at 12 weeks.Citation46,Citation89 Discontinuation rate was 10% for GBn and 2.8% in patients receiving tiotropium; the most frequent TEAEs leading to discontinuation for GBn were cough (2.3%), dyspnea (1.5%), and COPD (1.9%). The incidence rate of major adverse cardiovascular events (MACE) was 6.4 per thousand person-years for GBn and 20.3 for tiotropium.Citation89 There were seven deaths during the study, none of which were considered treatment related: three in the GBn group (0.5%) and four in the tiotropium group (0.9%).Citation89 Results from GOLDEN-5 trial, focused on long-term safety of GBn compared to placebo, showed that GBn BID was well tolerated, with a similar overall incidence of adverse events compared to the standard of care (48.6% for patients treated with GBn and 51.2% for tiotropium).Citation89 Compared to tiotropium, the incidence of MACE was lower in the GBn group, although not significantly different compared to patients treated with tiotropium (6.4/1,000 persons per year for GBn and 20.3/1,000 per year for tiotropium), finding consistent with results from the GOLDEN-2 trial.Citation85 The pooled analysis of GOLDEN-3 and -4 trials demonstrated a very low incidence of anticholinergic-related events such as dry mouth, with an incidence ranging between 0.5 and 2.3% in patients treated with GBn; only one gastrointestinal obstruction was reported in both studies, while glaucoma-related adverse events were very rarely observed (0.5 and 0.2% for GBn 25 and 50 μg doses, respectively).Citation88 As for urinary tract adverse events, urinary tract infection was reported in 2.3 and 1.9% for the GBn 25 μg dose and 3.2 and 2.3% for the highest dose across the GOLDEN-3 and -4 trials, respectively. Urinary retention was not observed throughout the studies.
Quality of life, patient satisfaction, and acceptability
Pooled data including respiratory symptoms from GOLDEN-3 and -4 studies (1,293 patients in total) and data from the long-term trial GOLDEN-5 (1,086 patients) were analyzed. Patients had the same inclusion criteria across all trials.Citation87–Citation89
Clinically meaningful changes (≤4.0 unit reduction) in SGRQ scores at week 12 were observed in 46.8, 41.7, and 34.5% of patients treated with GBn 25 and 50 μg BID dosages and placebo, respectively. Improvements were largely driven by changes in the SGRQ symptom domain.Citation87 During GOLDEN-5, administration of GBn 25 and 50 μg BID resulted in statistically significant improvements from baseline in health-related quality of life, using the Evaluating Respiratory Symptoms in COPD (E-RS™: COPD) electronic diary. E-RS™: COPD total score was collected starting from week 2 (P<0.05 vs placebo for both doses), with no significant effect on rescue medication use. E-RS responders at week 12 were 48.1, 40.3, and 36.9% of patients in the GBn 25 μg, 50 μg, and placebo groups, respectively; the placebo group scored −0.6 from the baseline while GBn −1.76 and −1.52 for doses of 25 and 50 μg, respectively. In GOLDEN-5, improvements in E-RS total scores were seen across all visits in the GBn and tiotropium groups, with similar changes in the score (from −1.30 to −1.86 on the baseline score at week 12 and −1.5 for both tiotropium and GBn at week 48).Citation92
A survey conducted among patients participating in the GOLDEN-5 study, which collected 473 questionnaires about patient-reported satisfaction with the investigational nebulizer and the ability to use it, suggested that regardless of previous nebulizer use, 75% of patients were “satisfied” or “very satisfied” with the novel delivery system. A total of 83% of patients also reported being “confident” to “very confident” that the study drug was being efficiently delivered to their lungs by the eFlow® CS nebulizer. Additionally, >70% of the patients also rated the investigational nebulizer as “easy” or “very easy” to assemble, operate and clean.Citation93
Conclusion and place in therapy
The number of elderly patients with COPD will increase as we become more efficient treating the disease and their chronic morbidities. It is not uncommon during everyday clinical practice to be unable to prescribe an adequate inhalation therapy for an elderly COPD patient due to his inability to handle the device or his low adherence to the suggested treatment. Treatment options that do not require coordination for activation and inhalation or necessitate an inspiratory peak flow threshold to be activated should represent the best treatment option for these patients. Nebulizers seem to fulfill that missing spot, although no nebulized bronchodilators are currently available for the treatment of stable COPD. The use of high efficiency nebulizers may bring together the highest number of advantages and the lowest number of disadvantages in terms of treatment efficacy and tolerability. GBn might be the first nebulized LAMA to be approved for use in COPD patients. The eFlow® CS nebulizer has also some limits that should be considered: it is expensive and its maintenance may be complicated due to the need of cleaning the device after each administration. The latter aspect should not be forgotten, especially in patients that may be a priori selected for their inability to handle properly traditional inhalation devices.
The pathophysiology and functional impairment in patients with COPD guides the current technology dedicated to bronchodilator delivery, which should strive to assure the best efficiency of drug deposition within the lung, obtained with the lowest chance of critical errors possible. Nebulized glycopyrronium may represent the starting point of an alternative tool to increase the efficiency of long term bronchodilator therapy, especially in patients who present issues with the employment of devices containing predosed drugs.
Disclosure
PS has received financial support for research from Pfizer, Almirall, Chiesi Farmaceutici, and AirLiquide. He has received honoraria for lectures at national meetings from Chiesi Farmaceutici, Novartis, Zambon Italia, AstraZeneca, Almirall, GlaxoSmithKline, Boehringer Ingelheim, Menarini, and Malesci-Guidotti. He has served as consultant for Zambon Italia, AstraZeneca, Novartis, Chiesi Farmaceutici, and Boehringer Ingelheim. DR has received honoraria for lectures from Astra Zeneca and Boehringer Ingelheim. AC, VV, and MR report no conflicts of interest in this work.
Notes
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) [homepage on the Internet]Global Strategy for Diagnosis, Management, and Prevention of COPD2016 Available from: http://goldcopd.org/Accessed August 25, 2017
- MathersCDLoncarDProjections of global mortality and burden of disease from 2002 to 2030PLoS Med2006311 e44217132052
- McDonoughJEYuanRSuzukiMSmall-airway obstruction and emphysema in chronic obstructive pulmonary diseaseN Engl J Med201136517 1567 157522029978
- SeniorRAtkinsonJChronic obstructive pulmonary disease: epidemiology pathophysiology, and pathogenesisAlfred FishmanMFishman’s Pulmonary Diseases and DisordersI4th edNew York, NYMcGraw Hill Medical2008 707 728
- O’DonnellDELavenezianaPPhysiology and consequences of lung hyperinflation in COPDEur Respir Rev200615100 61 67
- PecchiariMRadovanovicDSantusPD’AngeloEAirway occlusion assessed by single breath N2 test and lung P-V curve in healthy subjects and COPD patientsRespir Physiol Neurobiol2016234 60 6827612586
- SantusPRadovanovicDDi MarcoSEffect of indacaterol on lung deflation improves cardiac performance in hyperinflated COPD patients: an interventional, randomized, double-blind clinical trialInt J Chron Obstruct Pulmon Dis201510 1917 192326392766
- PecchiariMMSantusPRadovanovicDD’AngeloEGThe acute effects of long acting bronchodilators on small airways detected in COPD patients by single breath N2 test and lung P-V curveJ Appl Physiol Epub2017
- O’DonnellDEFlugeTGerkenFEffects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPDEur Respir J2004236 832 84015218994
- ScichiloneNBenfanteABocchinoMWhich factors affect the choice of the inhaler in chronic obstructive respiratory diseases?Pulm Pharmacol Ther201531 63 6725724817
- DolovichMBAhrensRCHessDRAmerican College of Chest PhysiciansAmerican College of Asthma, Allergy, and ImmunologyDevice selection and outcomes of aerosol therapy: evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and ImmunologyChest20051271 335 37115654001
- BartaSKCrawfordARobertsCMSurvey of patients’views of domiciliary nebulizer treatment for chronic lung diseaseRespir Med2002966 375 38112117035
- SantusPRadovanovicDPaggiaroPWhy use long acting bronchodilators in chronic obstructive lung diseases? An extensive review on formoterol and salmeterolEur J Intern Med2015266 379 38426049917
- HickeyAJPharmaceutical Inhalation Aerosol Technology2nd edNew YorkMarcel Dekker2004
- SabatéEAdherence to Long-Term Therapies: Evidence for ActionGenevaWorld Health Organization 2003 Available from: http://www.who.int/chp/knowledge/publications/adherence_full_report.pdf?ua=1Accessed August 18, 2017
- SulaimanICushenBGreeneGObjective assessment of adherence to inhalers by patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med201719510 140728504606
- RauJLDeterminants of patient adherence to an aerosol regimenRespir Care20055010 1346 135916185370
- FerroniEBelleudiVCasciniSOUTPUL Study GroupRole of tiotropium in reducing exacerbations of chronic obstructive pulmonary disease when combined with long-acting β2-agonists and inhaled corticosteroids: the OUTPUL studyPharmacoepidemiol Drug Saf20162511 1295 130427396695
- RamFSBrocklebankDMMuersMWrightJJonesPWPressurised metered-dose inhalers versus all other hand-held inhalers devices to deliver bronchodilators for chronic obstructive pulmonary diseaseCochrane Database Syst Rev20021 CD002170
- TurnerMOPatelAGinsburgSFitzGeraldJMBronchodilator delivery in acute airflow obstruction. A meta-analysisArch Intern Med199715715 1736 17449250235
- DhandRDolovichMChippsBMyersTRRestrepoRFarrarJRThe role of nebulized therapy in the management of COPD: evidence and recommendationsCOPD201291 58 7222292598
- WedzichaJADecramerMFickerJHAnalysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group studyLancet Respir Med201313 199 20924429126
- SestiniPCappielloVAlianiMAssociazione Italiana Pneumologi Ospedalieri Educational GroupPrescription bias and factors associated with improper use of inhalersJ Aerosol Med2006192 127 13616796537
- BlasiFCanonicaGWCentanniSGenuair® usability test: results of a national public survey of the elderlyCOPD2016133 367 37126645660
- LavoriniFManniniCChelliniEFontanaGAOptimising inhaled pharmacotherapy for elderly patients with chronic obstructive pulmonary disease: the importance of delivery devicesDrugs Aging2016337 461 47327216613
- CromptonGKBarnesPJBroederMAerosol Drug Management Improvement TeamThe need to improve inhalation technique in Europe: a report from the Aerosol Drug Management Improvement TeamRespir Med20061009 1479 149416495040
- PriceDBosnic-AnticevicSBriggsAInhaler competence in asthma: common errors, barriers to use and recommended solutionsRespir Med20131071 37 4623098685
- AndersonPUse of Respimat® Soft Mist™ Inhaler in COPD patientsInt J Chron Obstruct Pulmon Dis200613 251 25918046862
- RauJLPractical problems with aerosol therapy in COPDRespir Care2006512 158 17216441960
- BoeJDennisJHO’DriscollBREuropean Respiratory Society Task Force on the use of nebulizersEuropean Respiratory Society Guidelines on the use of nebulizersEur Respir J2001181 228 24211510796
- SharafkhanehAWolfRAGoodnightSHananiaNAMakeBJTashkinDPPerceptions and attitudes toward the use of nebulized therapy for COPD: patient and caregiver perspectivesCOPD2013104 482 49223875742
- VogelmeierCHedererBGlaabTPOET-COPD InvestigatorsTiotropium versus salmeterol for the prevention of exacerbations of COPDN Engl J Med201136412 1093 110321428765
- DecramerMChapmanKDahlRINVIGORATE InvestigatorsOnce-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group studyLancet Respir Med201317 524 53324461613
- BuhlRBanerjiDProfile of glycopyrronium for once-daily treatment of moderate-to-severe COPDInt J Chron Obstruct Pulmon Dis20127 729 74123118536
- KerwinEHébertJGallagherNEfficacy and safety of NVA237 versus placebo and tiotropium in patients with COPD: the GLOW2 studyEur Respir J2012405 1106 111423060624
- BeehKMSinghDDi ScalaLDrollmannAOnce-daily NVA237 improves exercise tolerance from the first dose in patients with COPD: the GLOW3 trialInt J Chron Obstruct Pulmon Dis20127 503 51322973092
- RadovanovicDManteroMSferrazza PapaGFFormoterol fumarate + glycopyrrolate for the treatment of chronic obstructive pulmonary diseaseExpert Rev Respir Med20161010 1045 105527552524
- VestboJPapiACorradiMSingle inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trialLancet201738910082 1919 192928385353
- JohnsonBESurattPMGalTJWilhoitSCEffect of inhaled glycopyrrolate and atropine in asthma. Precipitated by exercise and cold air inhalationChest1984853 325 3286697786
- CydulkaRKEmermanCLEffects of combined treatment with glycopyrrolate and albuterol in acute exacerbation of chronic obstructive pulmonary diseaseAnn Emerg Med1995254 470 4737710150
- LeakerBRBarnesPJJonesCRTutuncuASinghDEfficacy and safety of nebulized glycopyrrolate for administration using a high efficiency nebulizer in patients with chronic obstructive pulmonary diseaseBr J Clin Pharmacol2015793 492 50025243340
- Sunovion Respiratory Development IncStudy to investigate the dose response, safety and efficacy of nebulized ep-101(sun101) in patients with chronic obstructive pulmonary disease (COPD): GOLDEN-1 Study Available from: https://clinicaltrials.gov/ct2/show/NCT01426009?term=NCT01426009. NLM identifier: NCT01426009Accessed July 15, 2017
- Sunovion Respiratory Development IncA study of the efficacy and safety of EP-101 (SUN101) in subjects with moderate to severe chronic obstructive pulmonary disease (GOLDEN-2) Available from: https://clinicaltrials.gov/ct2/show/NCT01706536. NLM identifier: NCT01706536Accessed July 15, 2017
- Sunovion Respiratory Development IncEfficacy and safety trial of 12 weeks of treatment with nebulized SUN-101 in patients with COPD (GOLDEN-3) Available from: https://clinicaltrials.gov/ct2/show/NCT02347761. NLM identifier: NCT02347761Accessed July 15, 2017
- Sunovion Respiratory Development IncEfficacy and safety trial of 12 weeks of treatment with nebulized SUN-101 in patients with COPD (GOLDEN-4) Available from: https://clinicaltrials.gov/ct2/show/NCT02347774. NLM identifier: NCT02347774Accessed July 15, 2017
- Sunovion Respiratory Development IncA long-term safety trial of treatment with nebulized SUN-101 in patients with COPD (GOLDEN-5) Available from: https://clinicaltrials.gov/ct2/show/NCT02276222. NLM identifier: NCT02276222Accessed July 15, 2017
- Sunovion Respiratory Development IncA dose-range finding study of SUN-101 in subjects with moderate to severe COPD (GOLDEN 6) Available from: https://clinicaltrials.gov/ct2/show/NCT02038829. NLM identifier: NCT02038829Accessed July 15, 2017
- Sunovion Respiratory Development IncStudy to determine the amount of glycopyrrolate absorbed in the lungs after taking the medicine with a eFlow nebulizer and Seebri® Breezhaler® with and without activated charcoal in subjects with moderate to severe chronic obstructive pulmonary disease (COPD) (GOLDEN7) Available from: https://clinicaltrials.gov/ct2/show/NCT02512302. NLM identifier: NCT02512302Accessed July 15, 2017
- BerlinskiAAssessing new technologies in aerosol medicine: strengths and limitationsRespir Care2015606 833 84726070578
- LenneyWEdenboroughFKhoPKovarikJMLung deposition of inhaled tobramycin with eFlow rapid/LC Plus jet nebulizer in healthy and cystic fibrosis subjectsJ Cyst Fibros2011101 9 1420884302
- AriAJet, ultrasonic, and mesh nebulizers: an evaluation of nebulizers for better clinical outcomesEurasian J Pulmonol2014161 1 7
- HeyderJSvartengrenMUBasic principles of particle behaviour in the human respiratory tractBisgaardHO’CallaghanCSmaldoneGCDrug Delivery to the LungNew York, NYMarcel Dekker2002 21 45
- de BoerAHGjaltemaDHagedoornPFrijlinkHWCan “extrafine” dry powder inhalers improve lung deposition?Eur J Pharm Biopharm201596 143 15126220014
- PhamSFergusonGTKerwinEMGoodinTWheelerABauerAIn-vitro characterization of the Eflow® Closed-System (eFlow® Cs) nebulizer with glycopyrrolate inhalation solution (SUN-101)Am J Respir Crit Care Med2017195 A5472
- MerkusPJvan Essen-ZandvlietEEParlevlietEChanges of nebulizer output over the yearsEur Respir J199254 488 4911563507
- AlvineGFRodgersPFitzsimmonsKMAhrensRCDisposable jet nebulizers: how reliable are they?Chest19921012 316 3191735247
- O’CallaghanCBarryPWThe science of nebulised drug deliveryThorax199752suppl 2 S31 S449155849
- BanfiPTicozziNLaxAGuidugliGANicoliniASilaniVA review of options for treating sialorrhea in amyotrophic lateral sclerosisRespir Care2015603 446 45425228780
- BlissitKTTilleryELathamCPacheco-PerezJGlycopyrrolate for treatment of clozapine-induced sialorrhea in adultsAm J Health Syst Pharm20147115 1282 128725027535
- Robinul® (Glycopyrrolate) oral tablets [prescribing information]Atlanta, GAShionogi Pharma2010
- National Center for Biotechnology Information [webpage on the Internet]PubChem Compound Database; CID=116932017 Available from: https://pubchem.ncbi.nlm.nih.gov/compound/11693Accessed July 25, 2017
- CarterNJInhaled glycopyrronium bromide. A review of its use in patients with moderate to severe chronic obstructive pulmonary diseaseDrugs2013737 741 75323677802
- Bevespi Aerosphere™ [prescribing information]WilmingtonAstra-Zeneca Pharmaceuticals LP2017
- Ema.europa.euSeebri® Breezhaler® (GB): European Medicines Agency Assessment ReportLondon, UKEuropean Medicines Agency2012 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002430/WC500133771.pdfAccessed Jul 5, 2017
- CazzolaMPageCPCalzettaLMateraMGPharmacology and therapeutics of bronchodilatorsPharmacol Rev2012643 450 50422611179
- UlrikCSOnce-daily glycopyrronium bromide, a long-acting muscarinic antagonist, for chronic obstructive pulmonary disease: a systematic review of clinical benefitInt J Chron Obstruct Pulmon Dis20127 673 67823055716
- HaddadEBPatelHKeelingJEYacoubMHBarnesPJBelvisiMGPharmacological characterization of the muscarinic receptor antagonist, glycopyrrolate, in human and guinea-pig airwaysBr J Pharmacol19991272 413 42010385241
- RestrepoRDUse of inhaled anticholinergic agents in obstructive airway diseaseRespir Care2007527 833 85117594728
- MoultonBCFryerADMuscarinic receptor antagonists, from folklore to pharmacology; finding drugs that actually work in asthma and COPDBr J Pharmacol20111631 44 5221198547
- SykesDADowlingMRLeighton-DaviesJThe influence of receptor kinetics on the onset and duration of action and the therapeutic index of NVA237 and tiotropiumJ Pharmacol Exp Ther20123432 520 52822854200
- HanselTTBarnesPJTiotropium bromide: a novel once-daily anti-cholinergic bronchodilator for the treatment of COPDDrugs Today (Barc)2002389 585 60012582447
- BuelsKSFryerADMuscarinic receptor antagonists: effects on pulmonary functionHandb Exp Pharmacol2012208 317 341
- HanselTTNeighbourHErinEMGlycopyrrolate causes prolonged bronchoprotection and bronchodilation in patients with asthmaChest20051284 1974 197916236844
- RadovanovicDSantusPBlasiFManteroMThe evidence on tiotropium bromide in asthma: from the rationale to the bedsideMultidiscip Respir Med201712 1228484598
- VerkindreCFukuchiYFlémaleASustained 24-h efficacy of NVA237, a once-daily long-acting muscarinic antagonist, in COPD patientsRespir Med201010410 1482 148920541381
- SantusPRadovanovicDDi MarcoFRaccanelliRValentiVCentanniSFaster reduction in hyperinflation and improvement in lung ventilation inhomogeneity promoted by aclidinium compared to glycopyrronium in severe stable COPD patients. A randomized crossover studyPulm Pharmacol Ther201535 42 4926549785
- VillettiGBergamaschiMBassaniFPharmacological assessment of the duration of action of glycopyrrolate vs tiotropium and ipratropium in guinea-pig and human airwaysBr J Pharmacol20061483 291 29816565730
- RoglianiPCalzettaLOraJPharmacological assessment of the onset of action of aclidinium and glycopyrronium versus tiotropium in COPD patients and human isolated bronchiEur J Pharmacol2015761 383 39025952728
- Ema.europa.eu [webpage on the Internet]London, UKEuropean Medicines Agency2017 Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002430/human_med_001580.jsp&mid=WC0b01ac058001d124Accessed Oct 16, 2017
- Pmda.go.jpJCN 301000500740Tokyo, JapanPharmaceuticals and Medical Devices Agency2012 Available from: http://www.pmda.go.jp/files/000153897.pdfAccessed Oct 15, 2017
- Accessdata.fda.gov [webpage on the Internet]AdministrationSilver Spring, MDU.S. Food and Drug Administration2015 Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=207923Accessed Oct 15, 2017
- Ema.europa.euTrimbow ® Summary of Opinion (Initial Authorisation)London, UKEuropean Medicines Agency2017 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/004257/WC500228080.pdfAccessed Oct 11, 2017
- Sunovion.usMarlborough, MASunovion Press Release2017 Available from: http://www.sunovion.us/featured-news/press-releases/20170526.pdfAccessed on Oct 10, 2017
- FogartyCKerwinEDunnKSinghDTutuncuAThe GOLDEN-1 study: safety and bronchodilatory effects of nebulized glycopyrrolate (EP-101) using high efficiency nebulizer in patients with COPDEur Respir J201240 2194
- TashkinDPA review of nebulized drug delivery in COPDInt J Chron Obstruct Pulmon Dis201611 2585 259627799757
- DonohueJFGoodinTTosielloRWheelerADose selection for SUN-101/eFlow® phase 3 clinical studies: results from GOLDEN (Glycopyrrolate for obstructive lung disease via electronic nebulizer) phase 2 dose-finding studiesAm J Respir Crit Care Med2017195 A3591
- FergusonGTKerwinEMDonohueJFOzol-GodfreyAGanapathyVHealth-related quality of life (HRQoL) in moderate-to-very severe chronic obstructive pulmonary disease (COPD) patients treated with SUN-101 (glycopyrrolate/eFlow®): findings from the phase 3 GOLDEN studiesAm J Respir Crit Care Med2017195 A1402
- KerwinEMDonohueJFGoodinTEfficacy and safety of glycopyrrolate/eFlow® (nebulized glycopyrrolate) in moderate-to-very-severe COPD: Results from the glycopyrrolate for obstructive lung disease via electronic nebulizer (GOLDEN) 3 and 4 randomized controlled trialsRespir Res2017181 828061907
- FergusonGTGoodinTTosielloRWheelerAKerwinEMLong-term safety of SUN-101/eFlow® in moderate-to-very-severe COPD: results from the glycopyrrolate for obstructive lung disease via electronic nebulizer (GOLDEN) 5 studyAm J Respir Crit Care Med2017195 A3588
- AliFYLeakerBRNicholsonGCSinghDBarnesPJA five-way crossover study to compare systemic absorption and bronchodilator effect of glycopyrrolate after a single dose delivered by nebulizer (SUN-101) or a dry powder inhaler (seebri) and in patients with COPD (GOLDEN-7)Am J Respir Crit Care Med2017195 A3604
- KerwinEMFogartyCDunnKSinghDTutuncuACardiovascular safety of nebulized glycopyrrolate (SUN-101) compared with tiotropium, ipratropium and placebo in patients with COPDAm J Respir Crit Care Med2013187 A1483
- DonohueJFFergusonGTKerwinEMOzol-GodfreyAGanapathyVImprovement in respiratory symptoms in moderate-to-very severe chronic obstructive pulmonary disease patients treated with SUN-101 (glycopyrrolate/eFlow®) – patient-reported outcomes from the phase 3 GOLDEN studiesAm J Respir Crit Care Med2017195 A5738
- KerwinEMDonohueJFFergusonGTOzol-GodfreyAGanapathyVPatient device satisfaction with eFlow closed system nebulizer: results from the GOLDEN-5 study in patients with moderate-to-very-severe chronic obstructive pulmonary disease (COPD)Am J Respir Crit Care Med2017195 A1416