Abstract
Leptomeningeal metastasis (LM) of high-grade glioma is a highly lethal disease requiring new effective therapeutic measures. For both de novo or relapsed glioma with LM, intrathecal cytarabine chemotherapy is not frequently used for first-line and relapse protocols. We encountered a clinical case demonstrating effective application of cytarabine in high-grade glioma with LM, prompting us to explore the effects of cytarabine on malignant glioma and molecular mechanisms of such effects through in vivo and in vitro experiments. The U87 cell line was selected to represent human glioma for studies. Cell viability was measured by MTT assay, plate colony formation assay, and trypan-blue dye exclusion test. Apoptosis was assessed by flow cytometry. Protein expression levels were detected by Western blot assay and immunohistochemistry. mRNA expression was examined by quantitative real-time reverse transcription polymerase chain reaction. Cytarabine inhibited tumor growth during the in vivo experiment. The present study confirmed that cytarabine inhibits proliferation and promotes apoptosis of U87 cells, and molecular analysis of this effect showed that cytarabine significantly reduces expression of phosphatidylinositol 3-kinase/serine/threonine kinase also known as the protein kinase B/mechanistic target of rapamycin (PI3K/Akt/mTOR) pathway, Ki-67, BCL2, and 4-1BB, and upregulates Bax and cleaved caspase-3. Our findings indicated that intrathecal administration of cytarabine manifests potential in prophylaxis and treatment of malignant glioma with LM. Effective medications for high-grade glioma with LM should contain cytarabine.
Introduction
Leptomeningeal metastasis (LM) has been described with increasing frequency in glioma in recent years as a result of improving survival rates and survival times in patients with high-grade glioma. LM occurs in 20%–28%Citation1–Citation3 of supratentorial and infratentorial glioblastoma (GBM), as described previously by autopsy series. Noh et alCitation4 reported that 23.4% of GMB patients (75 of 321) were diagnosed with LM; this observation was not significantly different from those in prior reports. Arita et alCitation5 reported that 15 (15.6%) out of 97 patients with malignant gliomas developed LM at least 2 years after their initial treatment. Among patients in the age group less than 20 years, the incidence of LM was higher (46%) in comparison with older groups (12%), and all patients died 2–24 weeks after diagnosis of LM. Arita et alCitation1 reported that 22 (14%) of 157 patients showed dissemination of malignant gliomas, and survival after dissemination was limited in all patients (mean, 19 weeks; range, 2–39 weeks). Prognosis in patients with LM is very poor and nearly always results in lethal outcomes. However, no consensus currently exists on treatment for LM in malignant glioma.
Risk factors of LM in patients with malignant gliomas include tumor location adjacent to the ventricular system,Citation6,Citation7 dispersion of tumor cells by recurrent operative treatment, ventricular entry during operation, and multiple resections.Citation6 LM in malignant gliomas is increasingly considered as progression in management strategies to better control primary tumors and to improve survival. Clinicians should be familiar with the potential of LM as well as treatment modalities to prevent severe neurological deficits and to provide the most appropriate treatment to affected patients. Treatment options for LM are not very satisfactory and mainly palliative. LM is usually not amenable to surgery because of the diffuse nature of gliomas. Systemic chemotherapy penetration works poorly for cerebrospinal fluid (CSF) or periventricular regions adjacent to the CSF channels.Citation8 One approach to targeting this region may be through CSF-directed chemotherapy. Thus, intracerebroventricular (ICV) or intracavitary (ICT) chemotherapy is an important and preferred method for directly addressing tumor cells.
Published studies showed activation or overexpression of p-serine/threonine kinase, also known as protein kinase B (Akt) and p-mechanistic target of rapamycin (mTOR), in human gliomas.Citation9,Citation10 The labeling index of the phosphatidylinositol 3-kinase (PI3K)/Akt/mTOR pathway increased with increasing grade of malignancy, with stronger expression found in higher grade gliomas.Citation9,Citation10 The important role of the PI3K/Akt/mTOR pathway in angiogenesis was confirmed in various malignancies, where inhibition of PI3K by LY294002 and downregulation of PI3KC2α were shown to block tumor vascularization.Citation11,Citation12 In GBM, the PI3K/Akt/mTOR pathway plays a crucial role in the induction of invasion, angiogenesis, and expression of vascular endothelial growth factor in cells.Citation13 Patients with GBM in whom the PI3K/Akt/mTOR pathway is activated show poorer prognosis than patients without oncogenic activation of the same pathway.Citation14 Therefore, inhibitors targeting the PI3K/Akt/mTOR pathway emerge as a potential treatment for malignant gliomas.
Cytarabine (1-β-arabinofuranosylcytosine) is a pyrimidine nucleoside analog that inhibits synthesis of DNA at the S phase of the cell cycle. Intrathecal liposomal cytarabine is indicated to be effective for prophylaxis and treatment of meningeal leukemia, brain tumors (medulloblastoma, meningioma, neuro-ectodermal tumors, germ cell tumors, and oligodendroglioma), lymphoma, and disseminated malignancies (breast).Citation15 Cytarabine manifests therapeutic efficiency in leukemic patients by inhibition of mTOR-dependent autophagic responses.Citation16 Previous studies indicated that GBM-isolated CD133+ stem cells demonstrate resistance to many agents, including temozolomide, carboplatin, paclitaxel, etoposide, and carmustine,Citation17,Citation18 but show relative sensitivity to cytotoxic cytarabine.Citation19,Citation20 However, the inhibitory mechanism of action of cytarabine in GBM remains unclear.
In the present study, we observed a clinical case showing effective application of cytarabine for high-grade glioma with LM, prompting us to explore the effects of cytarabine on malignant glioma and the molecular mechanism of such effects through in vivo and in vitro experiments. We defined a novel mechanism underlying the inhibitory function of cytarabine in malignant gliomas.
Materials and methods
Ethics statement
As specified in the case report, the patient in our study provided written informed consent for publication of this article and the accompanying images. Animal experiments were approved by the Capital Medical University and Beijing Shijitan Hospital and performed in accordance with the ethical guidelines for animal experiments of the Beijing Shijitan Hospital Scientific Research Ethics Committee.
Patient and treatment
The patient was enrolled at Beijing Shijitan Hospital from October 2013 to March 2015. We collected information, such as age, sex, initial symptoms, diagnosis, treatment, efficacy, safety, and other data, from the electronic database of the Beijing Shijitan Hospital. Evidence of recurrence or progression was confirmed by evaluably enhancing the recurrent tumor on contrast-enhanced magnetic resonance imaging (MRI) and comparing laboratory data (mainly CSF protein levels) before and after treatments.
The patient was an 18-year-old male, admitted to the Beijing Shijitan Hospital between October 2013 and March 2015. Initial symptoms in this patient included neck pain and right limb weakness, and an MRI showed neoplasms in 3–6 cervical cord. Surgical resection was conducted in March 2013 with histologically confirmed World Health Organization grade III malignant glioma, anaplastic astrocytoma, and was followed by three cycles of oral chemotherapies with temozolomide (5/28 scheme). Severe headaches, projectile vomiting, motor aphasia, and diplopia were observed in September 2013. Meningeal dissemination was confirmed by MRI scans and CSF assays. Craniospinal irradiation combined with methotrexate (MTX) intrathecal chemotherapy was undertaken seven times from September to November 2013. No obvious improvement occurred according to evaluable contrast-enhanced MRI, symptoms, and laboratory data (mainly CSF protein levels). Then, cytarabine was intrathecally injected twice a week, six times, from December 2013 to April 2014 and monthly, three times, from April 2014 to July 2014.
Cell culture
Human glioma cell line U87 cells were obtained from the ATCC and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum and 1% antibiotic mixture (penicillin and streptomycin) at 37°C in 5% CO2. DMEM was used as the drug carrier for all cytarabine (C1768; Sigma Aldrich, St Louis, MO, USA) treatments. A PI3K inhibitor, LY294002 (L9908; Sigma Aldrich, St Louis, MO, USA), was used at a final concentration of 10 µM for several experiments. An mTOR inhibitor, rapamycin (37094; Sigma Aldrich, St Louis, MO, USA), was used at a final concentration of 10 ng/mL. Subsequently, Western blot (WB) assay was applied to both inhibitors after 30 min.
MTT assay
U87 cells were seeded in 96-well plates at 2,000 and 500 cells/well in 200 µL of medium. Cells were cultured for 24 h and allowed to sediment. Subsequently, different concentrations of cytarabine (2,000, 1,000, 500, 125, 62.5, 15.6, and 3.9 nM) were added, and the plates were incubated for 24, 48, 72, 96, and 120 h after addition of drugs. Exactly 10 µL of MTT (5 mg/mL) was added to each well. Plates were incubated at 37°C for 4 h. Then, 110 µL of dimethyl sulfoxide was added to each well. Plates were agitated on a plate shaker for 10 min. Optical density at 570 nm was read with an Multiskan Spectrum Microplate Reader (Thermo Fisher Scientifc, Waltham, MA, USA).
Plate colony formation assay
A total of 400 U87 cells were seeded in 6 cm dishes in complete medium. Cells were cultured for 24 h and allowed to sediment; then, various concentrations of cytarabine (2,000, 1,000, 500, 250, 125, 62.5, 15.6, and 3.9 nM) were added. After 3 weeks of growth, colonies could be counted by the naked eye. Cells were fixed with 4% paraformaldehyde, stained with crystal violet (0.1% w/v in 20 nM 4-morpho linepropanesulfonic acid; Sigma Aldrich), and washed twice with phosphate-buffered saline (PBS). Efficiency of plating was determined as the number of clearly visible colonies (diameter >50 µm).
Trypan-blue dye viability test
U87 cells were incubated with cytarabine (125, 500, and 1,000 nM), LY294002 (10 µM), and rapamycin (10 ng/mL) for 48 h, respectively. Exactly 100 µL of cell suspension was mixed with 100 µL 0.4% trypan blue solution, which was used to discriminate between viable and non-viable cells. Subsequently, cells were mixed intensively and incubated for 1 min at room temperature. A total of 10 µL mixed liquor was obtained, and the hemocytometer chamber was filled carefully. Cells were counted under the microscope in four 1×1 mm2 squares of one chamber, and the average number of cells per square was determined. Non-viable cells were blue, and viable cells remained unstained.
Apoptosis assay
Apoptotic cells were assessed using the PE Annexin V apoptosis detection kit (Becton, Dickinson and Company, NJ, USA). Cells in different groups were digested and resuspended in 100 µL of binding buffer containing 5 µL of PE Annexin V and 5 µL of 7-Amino-Actinomycin for 15 min at room temperature. Then, 400 µL of binding buffer was added for 1 h of incubation. Finally, cells were analyzed by flow cytometry on a FACSCalibur flow cytometer (Becton-Dickinson, Franklin-Lakes, NJ, USA).
Western blot analysis
U87 cells were incubated with cytarabine (15, 125, and 500 nM) for 0.5 h. Moreover, U87 cells were pretreated for 0.5 h with a PI3K inhibitor, LY294002 (10 µM), and the mTOR inhibitor, rapamycin (10 ng/mL). Cells were washed with PBS and lysed in Laemmli buffer to detect the PI3K/Akt/mTOR signal pathway. Akt (pan) (C67E7) Rabbit monoclonal antibody (mAb) (4691), Phospho-Akt (Ser473) (D9E) XP® Rabbit mAb (4060), mTOR (7C10) Rabbit mAb (2983), Phospho-mTOR (Ser2448) (D9C2) XP® Rabbit mAb (5536), p70S6 Kinase (49D7) Rabbit mAb (2708), and Phospho-p70S6 Kinase (Thr389) (108D2) Rabbit mAb (9234) were all acquired from Cell Signaling Technology (Danvers, MA, USA), and β-actin (A2228) was acquired from Sigma Aldrich (St Louis, MO, USA). Secondary antibodies were anti-rabbit antibodies (1:10,000; Cell Signaling Technology). Protein concentrations were detected by the Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA) according to manufacturer’s instructions. Forty microgram samples of each protein were loaded onto 10% sodium dodecyl sulfate-polyacrylamide gel. After electrophoresis, proteins were transferred to a hybridization nitrocellulose (NC) filter membrane (Millipore, Tullagreen, Carrigtwohill Co, Ireland). The NC membrane was blocked with blocking solution (Tris–HCl pH 7.4, NaCl, Tween 20, deionized water, and 5% bovine serum albumin [BSA]) for 1 h and reacted with antibodies overnight at 4°C. The membrane was then washed with blocking solution without skimmed milk and incubated with secondary antibodies for 1 h. After washing again, protein levels were analyzed by enhanced chemiluminescence (ECL) with an ECL and Western blotting detection system (Odyssey CLx; Gene Company Limited, Chai Wan, Hong).
Quantitative real-time reverse transcription polymerase chain reaction
Total RNA was extracted from cultured U87 cells using TRIzol reagent (Ambion; Thermo Fisher Scientific). High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carls-bad, CA, USA) was used to reverse transcribe 1 µg of RNA. Samples were prepared with PerfeCTa SYBR Green FastMix (Thermo Fisher Scientific, Waltham, MA, USA) and primer sets hBCL2, sense GAGCCACGACCCTTCTTAAGACAT, antisense CAGGGGTCAATTAATCCATGACA; h4-1BB, sense ATGACAATAAGCCACGAGGTGCAG, antisense AAAGGGAGCAGGACAAAGGCAGAA; hBax, sense TCCACCAAGAAGCTGAGCGAG, antisense GTCCAG CCCATGATGGTTCT; and hβ-actin, sense TGACGTGGACATCCGCAAAG, antisense CTGGAAGGTGGACAGC GAGG. Each RNA level was normalized by comparison with the corresponding housekeeping RNA level in the same sample. Results were calculated using the 2ΔCt method.
In vivo treatment studies
Experiments were approved by the ethics committee. Six-week-old nonobese diabetic/severe combined immunodeficiency (NOD-SCID) mice (n=18) were housed in groups of two to four in cages. Indoor temperature was maintained at 20°C and mice were subjected to a 12 h light–12 h darkness cycle. A week later, 0.7×107 U87 cells were injected subcutaneously into the back of mice after their adaptation to the experimental environment. Mice were monitored by palpation every 2 days until tumors were palpable at the 14th day. All mice were randomly divided into three groups, and two groups were intraperitoneally and peritumorally injected with 60 mg/kg cytarabine, respectively. The control group was not treated. Mice received two-cycle injections continually, and each cycle included 10 consecutive days of treatment followed by 7 days of rest. The dose and treatment period in this study were selected on the basis of directions for use of clinical cytarabine (Cytosar). Tumor volumes and mice weights were measured at different time points using a caliper and an electronic scale. Tumor volumes (longest diameter × shortest diameterCitation2)/2 were calculated. At the end of experiments, mice were narcotized by 10% chloral hydrate. Tumors were rapidly removed, and samples were preserved in formalin for subsequent hematoxylin and eosin staining for histopathological analysis and immunohistochemical (IHC) studies. Then, mice were sacrificed by cervical dislocation.
Immunohistochemistry and evaluation
Immunohistochemistry was performed, and diagnoses were confirmed. Percentages of positive cells were assessed and assigned to one of the following parameters: negative ≤10%, and ≥10% positive. Representative tissue blocks were cut at 4 mm thickness, deparaffinized with xylene rinse, and rehydrated with distilled water through graded alcohol. IHC staining was conducted on the other four slides using a two-step procedure. Slides were then incubated overnight at 4°C in humidified chambers with human Ki-67 (Abcam, Cambridge, UK; diluted 1:200), P-Akt (Cell Signaling Technology; diluted 1:200), and cleaved caspase-3 (Asp175; diluted 1:100; Cell Signaling Technology). Slides were washed thrice with PBS and further incubated with secondary antibodies for 30 min at room temperature. After washing with PBS, immunolabeled sections were incubated with biotin-conjugated secondary antibody for 20 min at room temperature, then with peroxidase-conjugated complex (Dako) for 20 min, visualized with 3,3′-diaminobenzidine, counterstained with hematoxylin, and then examined by light microscopy.
Statistical analysis
Each experiment was performed at least thrice, and representative data are presented as mean ± standard deviation. One-way analysis of variance was used to detect differences between control and experimental groups. Analyses were performed using SPSS 20.0 software. The standard value for statistical significance was P<0.05.
Results
Effects of treatment on patient
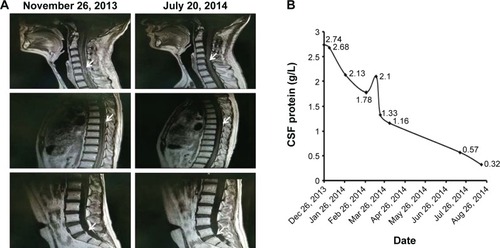
Tumor lesions in both cervical cord and meninges were eliminated in most parts according to radiographic assessment (). Levels of CSF protein gradually dropped to normal (). The patient remained stable until November 2014, and progression-free survival (PFS) was noted as 11 months.
Figure 1 Patient characteristics, treatment, and effect. (A) Changes of magnetic resonance imaging (MRI) appearance, before (left panel) and after (right panel) intrathecal cytarabine treatment, were compared. Tumor lesions in C3–C6 and meninges (white arrows) were partly eliminated in most regions after intrathecal cytarabine chemotherapy according to the radiographic assessment. (B) Values of cerebrospinal fluid (CSF) protein dropped to normal gradually with intrathecal cytarabine therapy from December 2013 to August 2014.
Cytarabine and PI3K/Akt/mTOR pathway inhibitors could inhibit human malignant glioma cell growth
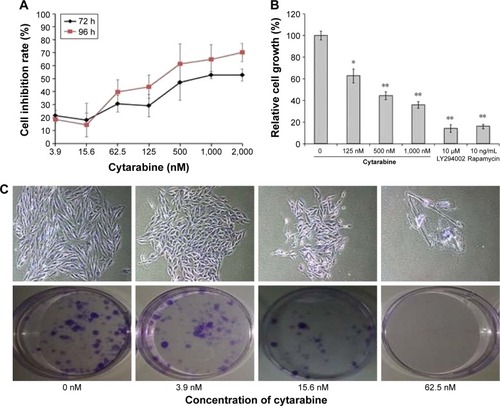
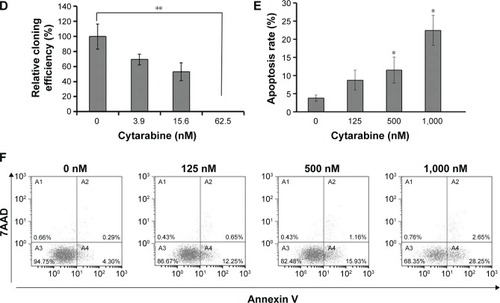
U87 cells were selected to represent human glioma for studies. Cell viability was measured by MTT assay, plate colony formation assay, and trypan-blue dye exclusion test. Apoptosis was assessed by flow cytometry. We discovered that cytarabine treatment resulted in decreased viability in U87 cells in a dose- and time-dependent manner, with IC50 values of 554.34±70.3 and 530.7±100.7 nM after 72 and 96 h of treatment, respectively (). Trypan-blue dye viability test showed that both cytarabine and LY294002 and rapamycin inhibited cell growth and decreased cell viability (). Plate colony formation assay indicated that cytarabine significantly decreased plating efficiency of U87 cells (). Flow cytometry showed that cytarabine induced cell apoptosis in a dose-dependent manner ().
Figure 2 Cytarabine and PI3K/Akt/mTOR pathway inhibitors could inhibit growth of human malignant glioma cells. (A) U87 cells grown in 96-well trays were treated with cytarabine for 72 and 96 h. Cell viability was detected using the MTT assay. (B) Both cytarabine and LY294002 and rapamycin decreased cell viability. (C) and (D) U87 cells were observed with the number of colonies formed. (E) and (F) Cytarabine induced cell apoptosis in a dose-dependent manner. *P<0.05; **P<0.01.
Cytarabine could downregulate PI3K/Akt/mTOR pathway in human malignant glioma cells
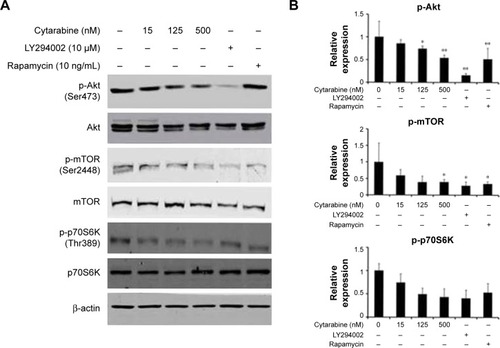
Subsequent studies aimed at elucidating the underlying molecular mechanisms of cytarabine. To further delineate mechanisms by which cytarabine decreased cell survival and proliferation in U87 cells, WB assay was performed and revealed that cytarabine significantly decreased phosphorylation of Akt, mTOR, and p70S6K in a dose-dependent manner (). These results revealed that cytarabine inhibited cell proliferation and survival in human malignant glioma cells through downregulation of the PI3K/Akt/mTOR pathway.
Figure 3 Cytarabine could downregulate the PI3K/Akt/mTOR pathway in human malignant glioma cells. (A) and (B) U87 cells were treated with cytarabine at the indicated doses for 30 min. Protein expression was determined by Western blot assay. Cytarabine inhibits the pI3K/Akt/mTOR/p70S6K signaling pathway in U87 cells. *P<0.05; **P<0.01.
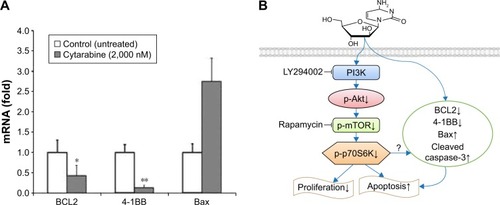
Cytarabine could downregulate antiapoptotic molecules BCL2 and 4-1BB and upregulate proapoptotic molecule Bax
To explore mechanisms by which cytarabine induced apoptosis in U87 cells, we examined expression of antiapoptotic molecules BCL2 and 4-1BB and proapoptotic molecule Bax by quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) in cytarabine-treated cells. As shown in , after cytarabine treatment, BCL2 and 4-1BB mRNA levels decreased and Bax levels increased in U87 cells as compared with those in controls. Significant differences were observed in 4-1BB and BCL2. These findings defined a novel signaling cascade controlling survival of glioma cells and helped delineate mechanisms underlying antitumoral properties of cytarabine ().
Figure 4 Cytarabine could downregulate antiapoptotic molecules BCL2 and 4-1BB and upregulate the proapoptotic molecule Bax. (A) The mRNA expression of BCL2, Bax, and 4-1BB were examined by quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR). (B) To delineate the mechanisms underlying the antitumoral properties of cytarabine in malignant glioma. *P<0.05; **P<0.01.
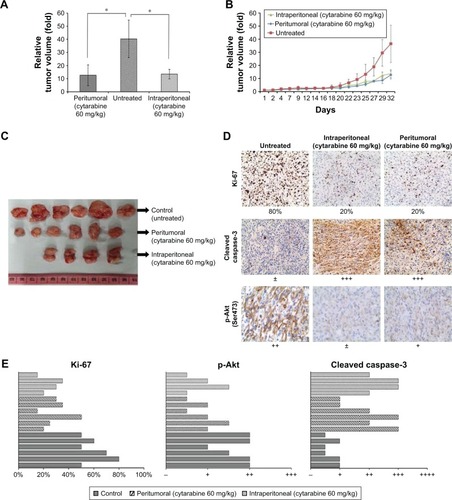
Cytarabine reduced tumor volume and growth of U87 cell xenografts in vivo
Cytarabine inhibited cell proliferation and induced apoptosis in glioma cells in vitro. Tests were conducted to determine whether cytarabine can inhibit progression of glioma cells in vivo. To test the effects of cytarabine on progression of human glioma cells, NOD-SCID mice inoculated with U87 cells were continuously treated with cytarabine (60 mg/kg/d) in two cycles until termination of the study. A total of 60 mg/kg cytarabine was intraperitoneally and peritumorally injected into mice with U87 tumor xenografts for two cycles; each cycle included a 10-day treatment and 7-day rest. Tumor growth in treated groups was significantly inhibited (P<0.05) compared with controls (). No significant differences occurred in tumor volume between intraperitoneal and peritumoral injections and mean body weight between control and treatment groups. However, two mice died after one cycle of treatment in the intraperitoneally injected group, indicating that intraperitoneal treatment resulted in severe adverse effects. Then, IHC staining of Ki-67, p-Akt, and cleaved caspase-3 was performed in xenograft tumor sections. We observed downregulation of the proliferation marker Ki-67 and p-Akt and upregulation of cleaved caspase-3 in cytarabine-treated groups (). Collectively, these results suggested that cytarabine inhibited glioma growth in vivo.
Figure 5 Cytarabine inhibits tumor growth and reduces tumor volume in vivo. (A) and (C) The comparison of relative tumor volume in three groups after two cycles of cytarabine (60 mg/kg) treatment. (B) The growth of tumors was monitored in terms of tumor volume every 2–3 days. (D) and (E) Immunohistochemical (IHC) stainings of Ki-67, cleaved caspase-3, and p-Akt in untreated and cytarabine-treated U87 xenografts. *P<0.05.
Discussion
Cytarabine is a deoxycytidine nucleoside analog. This antimetabolite drug incorporates into human DNA and consequently kills cells by interfering with DNA and RNA synthesis.Citation21 This drug is most commonly used for ICV administration and possesses a prominent position in treatment of hematological malignancies (eg, leukemia and lymphomas).Citation22 Liposomal cytarabine is a slow-release formulation of cytarabine. This compound is manufactured through encapsulating aqueous cytarabine in spherical multi-vesicular particles known as DepoFoam (DepoCyte; SkyePharma, San Diego, CA, USA).Citation23 Exposure to cytarabine in the CSF is prolonged in this formulation. Liposomal formulation exerts its effect in CSF for a week in children and almost 2 weeks in adults, whereas conventional cytarabine exerts its effect in CSF for less than 24 h.
Few published reports discussed the role of liposomal cytarabine in LM from malignant glioma. Feasibility and safety of intrathecal liposomal cytarabine administration to neoplastic meningitis were demonstrated by Bomgaars et alCitation24 in a Phase I trial and reported by Peyrl et alCitation25 in central nervous system (CNS) tumors. Glantz et alCitation26 conducted a randomized controlled trial comparing intrathecal DepoCyte to MTX in patients (n=14) with LM in malignant glioma and confirmed that DepoCyte produced a response rate comparable to that of MTX and significantly increased the time before neurological progression while offering the benefit of a less demanding dose schedule. Slavc et alCitation27 reported intrathecal therapy consisting of alternating cycles of liposomal cytarabine and etoposide in 57 patients with various malignant brain tumors and concluded the feasibility of alternating intraventricular liposomal cytarabine and etoposide. In the present study, we reported a clinical case showing evidence of advantage that can be attributed to the use of cytarabine, which is generally well tolerated and results in longer remission. In our clinical case, PFS was 11 months, which was longer than the published survival time of 19Citation1 or 24 weeks following LM.Citation5
The present study elucidated cytotoxic antitumoral effects of cytarabine in both in vitro cell research and in vivo mice tests and demonstrated, for the first time, the ability of cytarabine to inhibit growth of the glioma cell line through the PI3K/Akt/mTOR pathway. Akt belongs to a family of serine/threonine protein kinases that participate in cell proliferation and survival, as demonstrated by several downstream cellular targets that it regulates; these cellular targets include Bad, caspase-9, glycogen synthase kinase 3, Forkhead transcription factors, and nuclear factor-kappa B.Citation28 Aberrant activation of Akt is frequently observed in malignant gliomasCitation29 and was reported in approximately 70% of gliomasCitation30,Citation31 and 80% of GBM,Citation30 in correlation with PI3K/Akt signaling being altered in 88% of GBM.Citation32 The Akt pathway was reported to inhibit cell growth, induce radiosensitizing effects on GBM,Citation33 and stimulate apoptosis in a range of mammalian cells.Citation34,Citation35 In our study, cytarabine significantly decreased phosphorylation of Akt in a dose-dependent manner in WB assay and IHC staining. mTOR regulated multiple cell growth and cellular functions by controlling mRNA translation, ribosome biogenesis, autophagy, and metabolism.Citation36 mTOR is activated by Akt. Furthermore, mTOR is required for maintenance of astrocytic character in mice glioma cells, and inhibition of mTOR results in regional apoptosis in these tumors.Citation37 Rapamycin and its synthetic analogs, Torisel and Afinitor, were assessed in clinical trials of recurrent malignant gliomas, proving their modest efficacy.Citation38 mTOR is the primary autophagy repressor.Citation39 mTORC1 is a negative regulator of autophagy and a downstream target of the PI3K/Akt pathway.Citation40 Anticancer agents that target this pathway induce autophagy and inhibit growth. Our research confirmed that cytarabine can repress phosphorylation of mTOR, showing similarity to actions of rapamycin. Activated mTOR phosphorylated p70S6K, which induces GBM formation in mice.Citation30 S6K1 correlated with Ki67 labeling index, indicating that p-p70S6K is correlated with proliferation of tumor cells.Citation9 After WB assay, we confirmed that cytarabine significantly decreased phosphorylation of p70S6K in a dose-dependent manner.
BCL2, Bax, and caspase-3 are apoptosis-related genes. In addition, 4-1BB is a known regulator of cell apoptosis and growth.Citation41 Caspase-3 has been shown to induce apoptosis in microglia and astrocytes of developing brains.Citation42,Citation43 It exists in either an active cleaved caspase form, or an inactive pro-caspase form. Results of our study showed that cytarabine could downregulate expression of BCL2 and 4-1BB, and upregulate Bax and cleaved caspase-3, suggesting that this compound could cause apoptosis of glioma cells.
Results of our in vitro and in vivo experiments suggest a novel mechanism underlying cytarabine antiglioma function through downregulation of the PI3K/Akt/mTOR pathway and antiapoptotic molecules BCL2 and 4-1BB, and upregulation of cleaved caspase-3 and proapoptotic molecule Bax. However, we lack information on how cytarabine regulates expression of BCL-2, Bax, 4-1BB, and caspase-3 through PI3K/Akt/mTOR. Further studies are required to assess the relationship between these factors.
Incorporation of intraventricular chemotherapy into initial treatment of medulloblastoma yielded promising results. In the German HIT-SKK’92 trial, 10-year PFS rate was 82% for patients who had no postoperative residual tumor and 50% for patients with residual tumors.Citation44 These findings were favorable compared with 29%–41% 5-year PFS rates observed in previous studies that excluded CSF-directed chemotherapy.Citation45 Based on encouraging results of CSF-directed chemotherapy at the initial treatment of medulloblastoma, similar benefits may be observed by incorporating CSF-directed therapy into the initial treatment of high-grade glioma for LM prevention and therapy. Our research confirmed that intrathecal cytarabine can be used as standard prophylaxis and treatment for LM.
Conclusion
Inhibition of the PI3K/Akt/mTOR pathway and regulation of apoptosis-related genes such as BCL2, Bax, cleaved caspase-3, and 4-1BB involved cytarabine in glioma. Our research suggests that using intrathecal cytarabine may be a valid strategy to control LM occurrence and progression in malignant gliomas.
Acknowledgments
The authors gratefully acknowledge all the staff in the laboratory at the Beijing Shijitan Hospital for their enthusiastic assistance. The authors thank the enrolled patient and his family for cooperating with follow-up requirements.
Disclosure
The authors report no conflicts of interest in this work.
References
- AritaNTanedaMHayakawaTLeptomeningeal dissemination of malignant gliomas. Incidence, diagnosis and outcomeActa Neurochir (Wien)19941262–4 84 928042560
- OndaKTanakaRTakahashiHTakedaNIkutaFCerebral glioblastoma with cerebrospinal fluid dissemination: a clinicopathological study of 14 cases examined by complete autopsyNeurosurgery1989254 533 5402797391
- ErlichSSDavisRLSpinal subarachnoid metastasis from primary intracranial glioblastoma multiformeCancer1978426 2854 2864215298
- NohJHLeeMHKimWSOptimal treatment of leptomeningeal spread in glioblastoma: analysis of risk factors and outcomeActa Neurochir (Wien)20151574 569 57625663100
- AritaNHayakawaTMogamiHUshioYMeningeal gliomatosis – clinical features and results of treatmentGan No Rinsho19893511 1308 1312 Japanese [with English abstract]2478733
- GrabbPAAlbrightALPangDDissemination of supratentorial malignant gliomas via the cerebrospinal fluid in childrenNeurosurgery1992301 64 711738457
- LawtonCDNagasawaDTYangIFesslerRGSmithZALeptomen-ingeal spinal metastases from glioblastoma multiforme: treatment and management of an uncommon manifestation of diseaseJ Neurosurg Spine2012175 438 44822958073
- LimDAChaSMayoMCRelationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotypeNeuro Oncol200794 424 42917622647
- AnnovazziLMellaiMCalderaVmTOR, S6 and AKT expression in relation to proliferation and apoptosis/autophagy in gliomaAnticancer Res2009298 3087 309419661320
- LiXYZhangLQZhangXGAssociation between AKT/mTOR signalling pathway and malignancy grade of human gliomasJ Neurooncol20111033 453 45820878445
- YoshiokaKYoshidaKCuiHEndothelial PI3K-C2alpha, a class II PI3K, has an essential role in angiogenesis and vascular barrier functionNat Med20121810 1560 156922983395
- HuLHofmannJJaffeRBPhosphatidylinositol 3-kinase mediates angiogenesis and vascular permeability associated with ovarian carcinomaClin Cancer Res20051122 8208 821216299254
- GaleticIAndjelkovicMMeierRBrodbeckDParkJHemmingsBAMechanism of protein kinase B activation by insulin/insulin-like growth factor-1 revealed by specific inhibitors of phosphoinositide 3-kinase – signifcance for diabetes and cancerPharmacol Ther1999822–3 409 42510454216
- ChakravartiAZhaiGSuzukiYThe prognostic significance of phosphatidylinositol 3-kinase pathway activation in human gliomasJ Clin Oncol20042210 1926 193315143086
- BeneschMSieglerNHoffKVSafety and toxicity of intrathecal liposomal cytarabine (depocyte) in children and adolescents with recurrent or refractory brain tumors: a multi-institutional retrospective studyAnticancer Drugs2009209 794 79919617818
- BosnjakMRisticBArsikinKInhibition of mTOR-dependent autophagy sensitizes leukemic cells to cytarabine-induced apoptotic deathPLoS One201494 e9437424714637
- KangMKKangSKTumorigenesis of chemotherapeutic drug-resistant cancer stem-like cells in brain gliomaStem Cells Dev2007165 837 84717999604
- LiuGYuanXZengZAnalysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastomaMol Cancer20065 6717140455
- FolkinsCManSXuPShakedYHicklinDJKerbelRSAnticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumorsCancer Res2007678 3560 356417440065
- DoetschFGarcía-VerdugoJMAlvarez-BuyllaARegeneration of a germinal layer in the adult mammalian brainProc Natl Acad Sci U S A19999620 11619 1162410500226
- LambaJKGenetic factors influencing cytarabine therapyPharmacogenomics20091010 1657 167419842938
- GalmariniCMJordheimLDumontetCPyrimidine nucleoside analogs in cancer treatmentExpert Rev Anticancer Ther200335 717 72814599094
- MurryDJBlaneySMClinical pharmacology of encapsulated sustained-release cytarabineAnn Pharmacother20003410 1173 117811054987
- BomgaarsLGeyerJRFranklinJPhase I of intrathecal liposomal cytarabine in children with neoplastic meningitisJ Clin Oncol20042219 3916 392115459213
- PeyrlASauermannRTraunmuellerFPharmacokinetics and safety of intrathecal liposomal cytarabine in children aged <3 yearsClin Pharmacokinet2009484 265 27119492871
- GlantzMJJaeckleKAChamberlainMCA randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumorsClin Cancer Res1999511 3394 340210589750
- SlavcIPeyrlAAziziAALong-term intraventricular therapy alternating etoposide and liposomal cytarabine is feasible and safe: experience in 57 children and adolescents with malignant brain tumorsNeuro Oncol201618Suppl 6 vi27 vi28
- TestaJRBellacosaAAKT plays a central role in tumorigenesisProc Natl Acad Sci U S A20019820 10983 1098511572954
- KitaDYonekawaYWellerMOhgakiHPIK3CA alterations in primary (de novo) and secondary glioblastomasActa Neuropathol20071133 295 30217235514
- HollandECCelestinoJDaiCSchaeferLSawayaREFullerGNCombined activation of Ras and Akt in neural progenitors induces glioblastoma formation in miceNat Genet2000251 55 5710802656
- RajasekharVKVialeASocciNDWiedmannMHuXHollandECOncogenic Ras and Akt signaling contribute to glioblastoma formation by differential recruitment of existing mRNAs to polysomesMol Cell2003124 889 90114580340
- Cancer Genome Atlas Research NetworkComprehensive genomic characterization defines human glioblastoma genes and core pathwaysNature20084557216 1061 106818772890
- KoulDShenRBerghSInhibition of Akt survival pathway by a small-molecule inhibitor in human glioblastomaMol Cancer Ther200653 637 64416546978
- AhnJYHuYKrollTGAllardPYeKPIKE-A is amplified in human cancers and prevents apoptosis by up-regulating AktProc Natl Acad Sci U S A200410118 6993 699815118108
- ChengJQAltomareDAKleinMATransforming activity and cell cycle-dependent expression of the AKT2 oncogene: evidence for a link between cell cycle regulation and oncogenesisOncogene19971423 2793 28019190895
- ShawRJCantleyLCRas, PI(3)K and mTOR signalling controls tumour cell growthNature20064417092 424 43016724053
- HuXPandolfiPPLiYKoutcherJARosenblumMHollandECmTOR promotes survival and astrocytic characteristics induced by Pten/AKT signaling in glioblastomaNeoplasia200574 356 36815967113
- GalanisEBucknerJCMaurerMJNorth Central Cancer Treatment GroupPhase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group StudyJ Clin Oncol20052323 5294 530415998902
- YangZKlionskyDJMammalian autophagy: core molecular machinery and signaling regulationCurr Opin Cell Biol20102222 124 13120034776
- ManningBDCantleyLCAKT/PKB signaling: navigating downstreamCell20071297 1261 127417604717
- CheukATMuftiGJGuinnBARole of 4-1BB:4-1BB ligand in cancer immunotherapyCancer Gene Ther2004113 215 22614671675
- ZhangYGoodyerCLeBlancASelective and protracted apoptosis in human primary neurons microinjected with active caspase-3, -6, -7 and -8J Neurosci20002022 8384 838911069945
- DalmauIVelaJMGonzálezBFinsenBCastellanoBDynamics of microglia in the developing rat brainJ Comp Neurol20034582 144 15712596255
- RutkowskiSBodeUDeinleinFTreatment of early childhood medulloblastoma by postoperative chemotherapy aloneN Engl J Med200535210 978 98615758008
- RutkowskiSCurrent treatment approaches to early childhood medulloblastomaExpert Rev Neurother200668 1211 122116893348
