Abstract
Objective
To discover novel isoquinoline derivatives for inhibition of inhibitor of apoptosis proteins (IAP) for the treatment of ovarian cancer.
Methods
We first synthesized 533 isoquinoline derivatives, and screened them using CCK-8 to measure their antiproliferative activity. These compounds were further tested by Hoechst staining and flow cytometric analysis to assess proapoptotic activity. The in vivo antitumor efficacy and safety of the screened compounds were evaluated on the xenograft mouse model. Ki-67 staining and TUNEL assay were used to evaluate proliferation and apoptosis in the resected tumors, respectively. Western blot and polymerase chain reaction (PCR) were conducted to evaluate the levels of proliferating cell nuclear antigen (PCNA), caspase-3, PARP, and IAP in resected tumors.
Results
Compound B01002 and C26001 displayed antiproliferative and proapoptotic activity on SKOV3 ovarian cancer with an IC50 of 7.65 and 11.68 µg/mL, respectively. Both compounds inhibited tumor growth in a xenografted mouse model with good safety profiles, and tumor growth inhibition (TGI) of B01002 and C26001 was 99.53% and 84.23%, respectively. Resected tumors showed that both compounds inhibited tumor cell proliferation and induced apoptosis in vivo. Caspase-3 and PARP were activated, whereas IAP proteins were downregulated at the protein level.
Conclusion
Compound B01002 and C26001 could inhibit ovarian tumor growth and promote tumor apoptosis, partly by downregulating the IAPs, and, thus, might be promising candidates for treatment of ovarian cancer.
Introduction
Malignant tumors could fend off anticancer therapies in several ways. One strategy involves the evasion of apoptosis by upregulating the inhibitors of apoptosis proteins (IAP).Citation1 IAP comprise a family of functionally and structurally related proteins that share the presence of one or more baculoviral IAP repeat (BIR) domains.Citation2 The property of apoptosis inhibition has been described for at least four IAP family members – cIAP1, cIAP2, XIAP, and survivin.Citation3–Citation5 IAP subverts apoptosis through binding and inactivating caspases via one or more BIR domains,Citation6 which are approximately 80-amino acid zinc-binding domains and are essential for the antiapoptotic functions of the IAP. For example, the second BIR domain (BIR2) of XIAP is essential for potent inhibition of caspase-3.
Elevated IAP have been identified in numerous neoplasms, and have been proved to be correlated with poor prognosis. In several recent reports, it has been demonstrated that IAP play an active role in promoting tumor maintenance through the inhibition of cellular death, thus enhancing tumor’s resistance to radiotherapy and chemotherapy.Citation7,Citation8 Furthermore, IAP participate in signaling pathways associated with tumor proliferation, invasion, metastasis, and angiogenesis.Citation9–Citation11 Therefore, agents targeting IAP might be an effective approach for the treatment of cancer.
Different IAP-targeting therapies have been evaluated in preclinical and clinical studies in a variety of tumors recently.Citation12 Among those therapeutic strategies, one appealing approach is based on the occupation of the IAP–BIR peptide-binding pocket by certain reagents and, thus, interference with the IAP–caspases interactions.Citation13 An endogenous inhibitor of the BIR peptide, the four amino-acid N-terminus of the second mitochondrial activator of caspases (Smac; AVPI), can antagonize IAPs, with high binding affinity against the BIR2/3 domains of XIAP, the BIR3 domains of cIAP1/2, and the single BIR domain of survivin. This AVPI tetrapeptide sequence has been regarded as the lead structure for designing small-molecule BIR inhibitors for antagonizing IAPs by many researchers. Moreover, efforts to design new compounds capable of inhibiting BIR domains have focused on high-throughput-screening bioactive Smac mimetics that are based on those AVPI tetrapeptides, and subsequently evaluate their anti-cancer activity and underlying mechanism. Preclinical studies have demonstrated the capacity of those small-molecule BIR inhibitors to inhibit tumor growth and to sensitize cells to conventional therapeutic agents, such as radiation or cisplatin, in multiple solid tumors. To date, at least six BIR inhibitors have entered human clinical trials. Preliminary data indicate good tolerance and predicted biomarker modulation, such as cIAP1 downregulation and increased levels of processed caspase-3. However, it appears that those reported BIR inhibitors either have low potency or poor metabolic stability. For instance, results from phase I clinical trials indicated that AT406 (Ascenta Therapeutics) did not show equivalent efficacy as previously tested in mice, and HGS1029 (Aegera Therapeutics/Human Genome Sciences) required intravenous administration owing to large molecular size.Citation12,Citation14 Therefore, efforts need to be made to develop more efficient BIR inhibitors with improved potency and better pharmacokinetics.
Molecules with an isoquinoline skeleton have been reported to have the capacity to antagonize IAP with a high binding affinity to the BIR2 domain, highlighting their utility as a potential drug lead.Citation11 Compounds with an isoquinoline skeleton are reported to be capable of antagonizing XIAP. In this study, we firstly designed and synthesized series of small compounds, with an isoquinoline skeleton and different chemical groups, and then evaluated their antitumor activities on ovarian cancer cells in vivo. Two compounds screened out were further evaluated on the nude mouse xenograft model, and showed good antitumor efficacy and in vivo safety. They inhibited tumor cell proliferation mainly in an apoptotic way, as caspase-3 and PARP were activated, whereas IAP were downregulated at the protein level.
Materials and methods
Synthesis
We synthesized 533 molecules with an isoquinoline skeleton as described previously.Citation15,Citation16
Unless otherwise stated, all commercial reagents were used as received. All solvents were dried and distilled according to standard procedures. Flash column chromatography was performed using silica gel (60-Å pore size, 32–63 µm, standard grade). Analytical thin-layer chromatography was undertaken using glass plates pre-coated with 0.25 mm 230–400 mesh silica gel impregnated with a fluorescent indicator (254 nm). Thin-layer chromatography plates were visualized by exposure to ultraviolet light. Organic solutions were concentrated on rotary evaporators at ~20 Torr at 25°C–35°C. Nuclear magnetic resonance (NMR) spectra are recorded in parts per million from internal tetramethylsilane on the δ scale.
Cell culture and cell growth inhibition assay
The human ovarian cancer SKOV3 cells (purchased from Sigma-Aldrich Co., St Louis, MO, USA) were seeded in 96-well flat-bottomed cell culture plates at a density of 1×103 cells/well and grown overnight.Citation17 Then, 5 mg compound stock in 100% dimethylsulfoxide (DMSO) was diluted in medium at concentrations of 0, 1, 2.5, 5, 7.5, and 10 µg/mL separately, filtered, serially added into a 96-well plate, and continuously incubated for 24 h. Viable cells were quantified by a CCK-8 assay in which the absorbance of the samples was measured at 450 nm.Citation18 IC50 was calculated according to the cell growth curves. Experiments were repeated at least three times.
Apoptosis assay
Apoptotic nuclear changes were assessed by Hoechst staining, followed by fluorescent microscopic examination.Citation19 SKOV3 cells were seeded in dish slides. After incubation with testing agents (5 µg/mL compounds and 1% DMSO; 5 µg/mL cisplatin [DDP] as positive control; phosphate buffered-saline [PBS] as negative control) for 24 h, cells were stained with 20 mg/mL Hoechst at 37°C for 20 min, washed three times with PBS, and fixed with 4% formaldehyde for 15 min at room temperature. Morphological changes of nuclei were observed under a fluorescent microscope. The percentage of apoptotic cells was calculated for each field and averaged for the treatment group. The cytotoxicity of cisplatin was also evaluated under the same experimental conditions for comparison. In each slide, at least 120 cells were assessed in three contiguous fields.
SKOV-3 cells were treated with different concentrations of each compound (5 µg/mL of compounds and 1% DMSO; 10 µg/mL of compounds and 1% DMSO) or PBS for 24 h. Cells were washed and resuspended in binding buffer at a concentration of 1×106 cells/mL; 100 µL of the solution (1×105 cells) was transferred to a 5 mL culture tube. Then, 5 µL of recombinant human annexin V-FITC conjugate (ANNEXINV01, Invitrogen) and 5 µL of propidium iodide solution (P4864, Sigma-Aldrich) were added, and cells were gently vortexed and incubated for 15 min at room temperature (25°C) in the dark. Thereafter, 400 µL binding buffer was added to each tube. Finally, the harvested cells were analyzed by flow cytometry.
Tumor xenograft study
This study was approved by the Animal Ethics Committee of Fudan University (approval number: 20140823), and all animals were maintained and used in accordance with the guidelines of the Institutional Animal Care and Use Committee of Fudan University. SKOV3 cells were suspended in PBS, and then implanted subcutaneously into the right oxter of 40 female nude mice (age 6–8 weeks).Citation20 Tumor volumes were evaluated using the formula V = 0.5 × a × b2, where a and b are the largest and smallest perpendicular tumor diameters, respectively. Six mice with the appropriate mean tumor volume were assigned randomly to each of the four groups. The mean tumor volume for all four groups was 189±3 mm3 at the initiation of treatment (day 0). Mice (six per group) were treated with compound B01002, C26001, and DDP by intraperitoneal injection at a dose of 4 mg/kg every other day, with PBS as the control. Tumor volumes and mice body weights were measured every other day. Percent tumor growth inhibition (TGI) was calculated using the formula % TGI = 100 * (1 − tumor volumedose/tumor volumePBS). At the end of the experiment, the mice were sacrificed and the tumor tissues were harvested and weighed; the liver, spleen, small bowel, kidneys, and lungs were excised; and blood samples were collected.
Hematological and biochemical study
At the end of the experimental period, blood samples were collected from all animals from the retro-orbital venous plexus for safety evaluation. The effect of these compounds on blood parameters in mice was determined in the collected blood samples. Serum aspartate aminotransferase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), blood urea nitrogen (BUN), and creatinine (Cr) levels were determined using enzyme-linked immunosorbent assay kits (Shanghai Institute of Biological Products Co.).
TUNEL staining and immunofluorescence analysis
TUNEL assay was conducted using the In Situ Cell Death Detection Kit (Roche) according to the kit protocol. Briefly, the dissected tumors were fixed in 4% paraformaldehyde. Then, TUNEL staining was conducted in fixed tumor sections (5 µM) that were counterstained with 4,6-diamidino-2-phenylindole (DAPI). The numbers of TUNEL-positive cells and the total cells in tissue sections were counted.Citation21
Immunohistochemical assessment of Ki-67
Moreover, 5 µm-thick sections were cut from the tissue blocks, and then dewaxed and rehydrated. MIB1 antibody (Abcam) at a dilution of 1:100 was used for Ki-67 immunostaining.Citation22 Harris hematoxylin was used to counterstain Ki-67–immunostained sections. All sections were assessed blindly and independently by two observers. Preceding this, the percentage of positively staining cells was recorded and used as a quality control measure in our statistical analysis.
Western blot analysis
The resected tumors were washed with cold PBS, and prepared by homogenization in RIPA buffer (1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecylsulfate in PBS) supplemented with protease inhibitor cocktail. Then, 30 mg protein was resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with the respective antibodies.Citation23
Gene expression analysis
We isolated RNA with the RNeasy Lipid Tissue kit (QIAGEN) and DNase digestion, synthesized cDNA with the High-capacity cDNA Reverse Transcription kit, and conducted quantitative real-time PCR (RT-PCR) with goTaq qPCR Master Mix in a Bio-Rad CDX96 Real-time PCR system. We calculated relative gene expression levels by the ΔΔCt method using cyclophilin A as the internal control.Citation24
Statistical analysis
All data were presented as mean ± standard error. For relative gene expression, the mean value of the control group was defined as 100%. Two-tailed unpaired Student’s t-test and ANOVA were used for statistical evaluation of the data.Citation24 The Sigma stat statistical analysis program was used for data analysis. P<0.05 was considered significant.
Results
Isoquinoline derivatives were synthesized to inhibit IAP
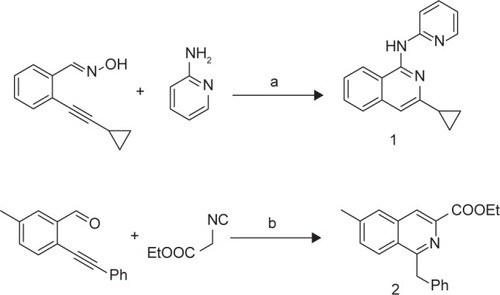
The synthetic routes for isoquinolines 1-2 are shown in . Isoquinoline 1 (B01002) was prepared by a silver triflate-catalyzed reaction of 2-alkynylbenzaldoxime with amine. Isoquinoline 2 (C26001) was synthesized by a silver triflate-catalyzed reaction of 2-alkynylbenzaldehyde with 2-isocyanoacetate.
Isoquinoline derivatives inhibit proliferation and promote apoptosis
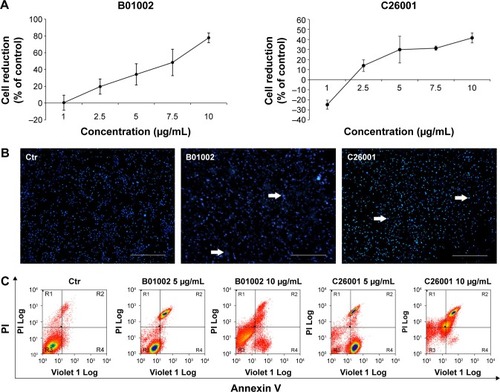
Molecular structures of isoquinoline derivatives are shown in Supplementary materials. Firstly, we tested the antiproliferative activity of each compound in vitro on human SKOV3 ovarian cancer cells and, as shown in , CCK-8 assays revealed that two compounds – denoted as B01002 and C26001 – inhibited SKOV3 cell proliferation in a concentration-dependent manner with an IC50 of 7.65 and 11.68 µg/mL, respectively. The screened results of all 533 compounds are shown in Supplementary materials.
Figure 1 B01002 and C26001 inhibited SKOV3 cell proliferation and promoted apoptosis in vitro. SKOV3 cells were treated with B01002 and C26001 for 24 h. (A) Cell viability inhibition curve of B01002 and C26001 by CCK-8 assay. (B) Representative images of Hoechst staining of cells treated by B01002 and C26001. Arrows: apoptotic nuclear changes. Scale bar: 100 µm. (C) Apoptosis of cells treated by different concentrations of B01002 and C26001 evaluated by flow cytometry analysis using V-FITC and PI staining.
Spectroscopic data for B01002 are as follows: 1H NMR (400 MHz, CDCl3) δ 8.57 (d, J=8.4 Hz, 1H) (Ar-H, the eighth position of isoquinoline), 8.28 (dd, J=1.2, 5.2 Hz, 1H) (Ar-H, the sixth position of pyridinyl), 8.08 (s, 1H) (ArNHAr’), 7.94 (d, J=8.4 Hz, 1H) (Ar-H, the fifth position of isoquinoline), 7.71 (t, J=8.0 Hz, 1H) (Ar-H, the fourth position of pyridinyl), 7.65 (d, J=8.0 Hz, 1H) (Ar-H, the third position of pyridinyl), 7.58 (t, J=8.0 Hz, 1H) (Ar-H, the sixth position of isoquinoline), 7.44 (t, J=7.6 Hz, 1H) (Ar-H, the seventh position of isoquinoline), 7.10 (s, 1H) (Ar-H, the fourth position of isoquinoline), 6.93 (t, J=6.0 Hz, 1H) (Ar-H, the fifth position of pyridinyl), 2.06–2.11 (m, 1H) (CH, cyclopropyl), 1.11–1.18 (m, 2H), (CH2, cyclopropyl), 0.97–0.99 (m, 2H) (CH2, cyclopropyl); 13C NMR (100 MHz, CDCl3) δ 154.00, 153.80, 150.75, 147.92, 147.55, 138.04, 130.20, 126.84, 125.66, 121.57, 117.56, 117.26, 113.13, 110.62, 17.40 (CH, cyclopropyl), 9.01 (CH2, cyclopropyl); IR (cm−1): 3446.23, 3048.23, 2927.25, 2852.63; HRMS (ESI) calculated for C17H15N3: 262.1344 (M + H+), found: 262.1396.
Spectroscopic data for C26001 are as follows: 1H NMR (400 MHz, CDCl3) δ 8.38 (s, 1H) (Ar-H, the fourth position of isoquinoline), 8.00 (d, J=8.4 Hz, 1H) (Ar-H, the eighth position of isoquinoline), 7.67 (s, 1H) (Ar-H, the fifth position of isoquinoline), 7.40 (d, J=8.4 Hz, 1H) (Ar-H, the seventh position of isoquinoline), 7.22–7.12 (m, 5H) (Ar-H, phenyl), 4.73 (s, 2H) (CH2, Ar-CH2-Ph), 4.53–4.49 (m, 2H) (CH2, -COOCH2CH3), 2.49 (s, 3H) (CH3, Ar-CH3), 1.47 (t, J=6.8 Hz, 3H) (CH3, -COOCH2CH3); 13C NMR (100 MHz, CDCl3) δ 166.2 (carbonyl or the first position of isoquinoline), 160.4 (carbonyl or the first position of isoquinoline), 141.0, 139.3, 136.6, 131.7, 128.6, 128.5, 127.7, 127.0, 126.8, 126.3, 126.0, 125.0, 61.7 (-0-CH2-), 42.5 (Ar-CH2-Ph), 21.8 (CH3, Ar-CH3), 14.5 (CH3, -CH2-CH3); HRMS (ESI) calculated for C20H19NO2: 306.1494 (M + H+), found: 306.1525.
To gain insights into the underlying mechanisms, apoptotic nuclear changes were assessed by Hoechst staining. demonstrated that B01002 and C26001, both at a concentration of 5 µg/mL significantly induced apoptosis of SKOV3 cells. Then, a flow cytometric analysis with annexin V–FITC and PI staining was further performed. The level of apoptotic cells in SKOV3 was assessed by the percentage of annexin V-positive/PI-negative cells present after exposure of SKOV3 cells to different concentrations (5 or 10 µg/mL) of B01002 and C26001 for 24 h, as illustrated in . At the concentration of 5 µg/mL, B01002 and C26001 induced apoptosis, as indicated by a 21% and 9% increase in apoptotic cells (annexin V-positive/PI-negative), respectively. At a concentration of 10 µg/mL, B01002 and C26001 promoted cell death via both necrosis and apoptosis. For B01002, 46% of cells were necrotic (annexin V-positive/PI-positive), and 5% of cells were apoptotic, whereas for C26001, 20% cells were necrotic and 46% were apoptotic. As a comparison, 4% cells were apoptotic in the control group.
In vivo dosage experiment proved the safety of isoquinoline derivatives
B01002 and C26001 were selected for in vivo assays. The compounds B01002 and C26001 were injected into six animals, and DDP was administered as positive control, with PBS as negative control. Daily observation of clinical signs, physical examination, body weight, and food consumption with no abnormalities suggested good tolerance. A general observation of these organs treated with B01002 and C26001 showed no abnormal changes, whereas there were some lesions in the liver of the mice treated with DDP. Histopathological studies were carried out subsequently, and were also consistent. Abnormal findings were observed in the organs of the mice treated with DDP; no lesions were found in the organs of mice treated with B01002 or C26001. Blood samples were tested for blood count (RBC, WBC, hemoglobin, and platelets), and hepatorenal function (AST, ALT, ALP, BUN, and Cr). Supplementary materials show that the counts of blood components in mice treated with these two compounds were not altered significantly compared with the control. WBCs were slightly reduced by C26001, by 12%. Supplementary materials also show mean AST, ALT, ALP, BUN, and Cr values in animals treated with B01002 and C26001 were largely unchanged.
Isoquinoline derivatives decreased tumor size in a xenograft mouse model
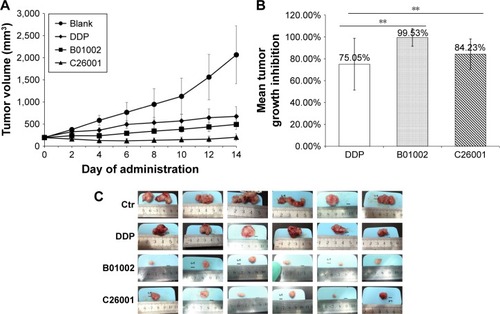
The in vivo antitumor activity of B01002 and C26001 was determined in nude mice bearing ovarian xenografted tumors. The two compounds, as well as DDP and PBS, were slowly injected intraperitoneally at the dosages and the time intervals described earlier (). Both compounds induced tumor growth delay for a longer period of time when compared with PBS controls. Furthermore, B01002 and C26001 revealed a clear tumor reduction of 99.53% and 84.23%, respectively, compared with PBS controls (). In summary, these preliminary data demonstrate the potential of B01002 and C26001 for the treatment of ovarian cancer.
Figure 2 B01002 and C26001 inhibited tumor growth on SKOV3 xenograft mouse model. (A) Tumor growth curves of different treatment groups of PBS, DDP, B01002, and C26001. (B) Mean tumor growth inhibition (TGI) was calculated at the end of the treatment. (C) Pictures of resected tumors of four groups. **P<0.05.
Isoquinoline derivatives inhibited tumor cell proliferation mainly in an apoptotic way
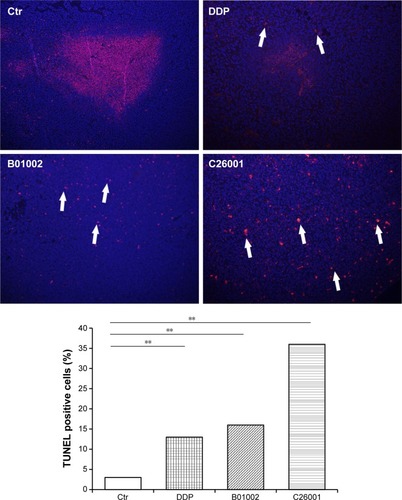
To study the antiproliferative effects of B01002 and C26001 on xenografted tumors, we immunostained the resected tumors with a Ki-67 antibody and immunoblotted the resected tumor with a proliferating cell nuclear antigen (PCNA) antibody, respectively. The proliferation index was determined according to the Ki-67 expression level. We found that tumors treated with B01002 and C26001 had a significantly lower percentage of Ki-67 positivity, compared with tumors treated with PBS (33.2% and 68.5%, respectively; ). To further verify these findings, we examined the expression level of a typical marker of cell proliferation, PCNA, which is abundant in SKOV3 cell lines. shows that PCNA (a typical marker of cell proliferation) was significantly decreased after treatment with C26001 (13.1%). However, PCNA was not decreased in tumors treated with B01002. Additionally, quantitative real-time PCR was conducted to assess mRNA levels of PCNA in resected tumors. Treatment with C26001 resulted in an evident rise (33%) in the mRNA levels of PCNA (). These results suggest that the antiproliferative potential of C26001 is associated with the elevation of mRNA levels of PCNA. Moreover, B01002 and C26001 caused tumor cell apoptosis (16.5% and 37.2%, respectively) in vitro as revealed by TUNEL assay (), suggesting that these two compounds may predominantly kill cells via the apoptosis pathway.
Figure 3 B01002 and C26001 inhibited tumor proliferation in vivo. (A) Left, representative Ki-67 staining images of tumors resected from tumor-bearing mice treated with B01002, C26001, DDP, or PBS, magnification ×100; Right, the ratio of positive Ki-67 nuclear staining to total number of nuclei was measured for each field, and the cumulative results are summarized as bar graphs. (B) Changes in PCNA expression of xenografted tumors following 14-day treatment. (C) mRNA levels of PCNA in different groups determined by qRT-PCR. *P>0.05; **P<0.05.
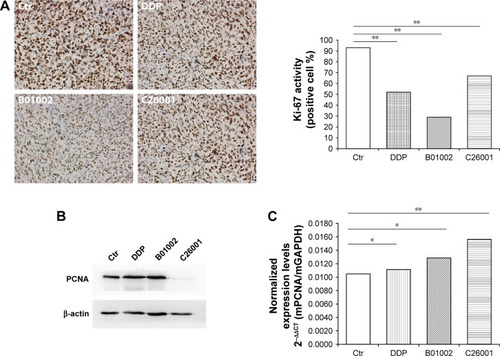
Figure 4 B01002 and C26001 induced apoptosis in vivo. TUNEL detection of apoptotic cells of tumors resected from tumor-bearing mice from different groups (red). Nuclei are stained with DAPI (blue). Top, representative image of DAPI and TUNEL staining. Arrows, cells exhibiting typical apoptotic morphology. Magnification ×100. Bottom, percentages of TUNEL-positive cells determined by cell counting. **P<0.05.
Isoquinoline derivatives inhibited IAP at the protein level and showed antagonistic effect through the caspase–IAP pathway
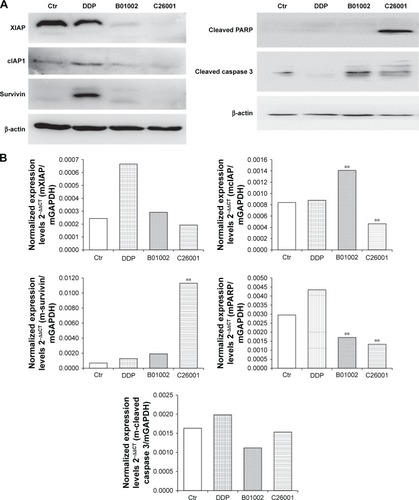
Levels of IAP including XIAP, cIAP, and survivin in resected tumors were measured using Western blotting assay, and the results were shown in . XIAP, cIAP-1, and survivin were all reduced in tumors treated with B01002 and C26001, compared with tumors treated with DDP or PBS. mRNA levels of XIAP, cIAP-1, and survivin were examined by quantitative RT-PCR (). For mRNA levels of XIAP, no significant differences were found between each group. However, an increased mRNA level of cIAP-1 in the B01002 group and an increased mRNA level of survivin in C26001 group were found.
Figure 5 B01002 and C26001 inhibited inhibitor of apoptosis proteins (IAP). (A) Western blot of IAPs and apoptosis-related proteins; β-actin was used as loading control. (B) mRNA levels of IAP and apoptosis-related proteins by qRT-PCR. **P<0.05 compared with the control group.
Scheme 1 Syntheses of isoquinoline 1 and 2.
Cleaved PARP and cleaved caspase-3 were also tested by Western blotting assay (). Cleaved PARP was elevated in cells treated by C26001, whereas both cleaved PARP and cleaved caspase-3 were elevated in cells treated by B01002, indicating that both of the compounds activated apoptosis through the caspase–IAP pathway. However, it is notable that the protein level of cleaved caspase-3 was correlated with that of XIAP (Pearson’s correlation 0.911, P<0.05). mRNA levels of PARP in the B01002 and C26001 group were decreased as compared with the control group. However, there were no significant differences in mRNA levels of caspase-3 between groups; instead, mRNA levels of all treatment groups were decreased as compared with the control group.
Discussion
In this study, we synthesized 533 new isoquinoline derivatives in the hope of inhibiting IAP and killing ovarian cancer. The CCK-8 assay showed that two of the isoquinoline derivatives could inhibit tumor cell proliferation. Hoechst staining, as well as fluorescence-activated cell sorting (FACS), results displayed that the two isoquinoline derivatives induced tumor cell apoptosis but not necrosis. Moreover, both of these new compounds were proved capable of decreasing tumor growth and have good safety profiles in the xenograft mouse model. Further analysis of the resected tumors revealed that the two compounds downregulated several IAP, including XIAP, cIAP-1, and survivin, thus promoting caspase-induced apoptosis, which may be one of the mechanisms of their antitumor activity.
As the incidence of ovarian cancer keeps rising and chemoresistance becomes increasingly common in the clinic, exploring new chemotherapeutic agents has become an important and urgent task, especially for patients with locally advanced, platinum-resistant ovarian cancer.Citation25 Modern drug discovery often involves screening small molecules for their ability to bind to a preselected protein target. Drug discovery can also involve screening small molecules for their ability to modulate a biological pathway in cells or organisms, without regard for any particular protein target.Citation26–Citation28 In this study, we synthesized a collection of 533 molecules with an isoquinoline skeleton, aiming at perturbing the caspase–IAP pathway to induce apoptosis and kill cancer cells.
IAP block programmed cell death and are expressed at high levels in various human cancers. Moreover, IAP have been implied in the regulation of additional signaling cascades, such as mitogen-activated protein kinase (MAPK) pathway,Citation29 TGFb signaling,Citation30,Citation31 as well as innate and adaptive immunity signaling pathwaysCitation32,Citation33 – making them attractive targets for cancer drug development.Citation34 Therefore, many efforts have been made over the past decade to develop strategies to neutralize IAP, including antisense oligonucleotides and small-molecule inhibitors.Citation35,Citation36 Smac is a mitochondrial protein that is released into the cytosol upon the induction of programmed cell death and promotes apoptosis by neutralizing IAP.Citation37 On this basis, a variety of small-molecule inhibitors have been developed that mimic the binding domain of the native Smac protein to IAP. So far, various Smac mimetics (monovalent or bivalent) are proved to inhibit Smac tetrapeptide binding to recombinant IAP, rescue IAP-bound caspase-3 activity, and show antiproliferative activities against malignant human cancer cells.Citation38,Citation39 Monovalent Smac mimetics are less potent than their corresponding bivalent Smac mimetics.Citation40 However, because bivalent Smac mimetics have molecular weights exceeding 1,000, such compounds are expected to have very low oral bioavailability and will have to be administered intravenously – a potential disadvantage if the drug must be administered frequently.Citation41 Therefore, smaller molecules with higher efficacy are still needed.
Isoquinoline derivatives have been reported to show high affinity for the BIR2 domain and demonstrated potent IAP inhibitory activities in biochemical and cellular assays.Citation42 Therefore, we screened the 533 isoquinoline derivatives synthesized earlier to obtain compounds with effective antitumor activity on ovarian cancer.
Firstly, we tested their cytotoxicity in vitro using the SKOV3 ovarian cancer cell line, and found that both B01002 and C26001 could inhibit tumor cell proliferation effectively (IC50 =7.65 and 11.68 µg/mL, respectively). Moreover, we found that both compounds could induce apoptosis of tumor cell, which seems the main mechanism of the toxicity, as a very small percentage of necrotic cells was observed. To evaluate the translational potential of these new agents, we tested their in vivo efficacy and safety using a xenograft mouse model. Consistent with the in vitro results, our study showed that both compounds decreased the size of xenografted tumors. By Ki-67 staining and PCNA level, we showed that both compounds inhibited the tumor proliferation in vivo. By measuring body weight, food consumption, observing clinical signs, physical examination, blood routine testing, and biochemical tests, as well as necropsy of major organs, the mice showed good tolerance in various dosages to both compounds, indicating their clinical translational potential in future applications.
Our data also revealed that these two new isoquinoline derivatives can downregulate XIAP, cIAP-1, and survivin at the protein level, but not at the mRNA level, suggesting that they work on the translational or post-translational process. As IAP block apoptosis by neutralizing caspases, it is not surprising that our new isoquinoline derivatives exert a pro-apoptosis effect, as also evidenced by TUNEL staining. The fact that both compounds upregulated the cleaved caspase-3 and partly upregulated cleaved PARP at the protein level further verified that the two compounds inhibited the tumor cells through the IAP–caspases pathway.
In addition, it is worth noting that isoquinoline-skeleton compounds are reported to participate in several signaling ways associated with tumor proliferation, invasion, metastasis, and angiogenesis, indicating that the antitumor effect of isoquinoline derivatives is not only limited to downregulating IAP.Citation43 We hope to explore more biological functions of these new compounds to fully elaborate their antitumor mechanisms.
In conclusion, we have synthesized two new isoquinoline derivatives that significantly inhibit ovarian tumor growth and promote tumor apoptosis, both in vivo and in vitro, partly by downregulating the IAP. Combined with their good safety profile, this makes it worthwhile to evaluate their efficacies in a clinical setting in the future.
Acknowledgments
This study was supported by Science and Technology Commission of Shanghai Municipality (124119a2900, 114119a2100 to L Yao), and New Hundred Talents Program of Health and Family Planning Commission of Shanghai (13B122 to L Yao).
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
- HanahanDWeinbergRAHallmarks of cancer: the next generationCell20111445 646 67421376230
- FuldaSVucicDTargeting IAP proteins for therapeutic intervention in cancerNat Rev Drug Discov2012112 109 12422293567
- GillCDowlingCO’NeillAJWatsonRWEffects of cIAP-1, cIAP-2 and XIAP triple knockdown on prostate cancer cell susceptibility to apoptosis, cell survival and proliferationMol Cancer20098 3919549337
- LaCasseECMahoneyDJCheungHHPlenchetteSBairdSKornelukRGIAP-targeted therapies for cancerOncogene20082748 6252 627518931692
- SongZYaoXWuMDirect interaction between survivin and Smac/DIABLO is essential for the anti-apoptotic activity of survivin during taxol-induced apoptosisJ Biol Chem200327825 23130 2314012660240
- FuldaSRegulation of cell migration, invasion and metastasis by IAP proteins and their antagonistsOncogene2014336 671 67623474760
- FuldaSInhibitor of apoptosis (IAP) proteins as therapeutic targets for radiosensitization of human cancersCancer Treat Rev2012386 760 76622342104
- WenXLinZQLiuBWeiYQCaspase-mediated programmed cell death pathways as potential therapeutic targets in cancerCell Prolif2012453 217 22422429822
- VucicDFairbrotherWJThe inhibitor of apoptosis proteins as therapeutic targets in cancerClin Cancer Res20071320 5995 600017947460
- YangXXingHGaoQRegulation of HtrA2/Omi by X-linked inhibitor of apoptosis protein in chemoresistance in human ovarian cancer cellsGynecol Oncol2005972 413 42115863139
- MehrotraSLanguinoLRRaskettCMMercurioAMDohiTAltieriDCIAP regulation of metastasisCancer Cell2010171 53 6420129247
- González-LópezMWelshKFinlayDDesign, synthesis and evaluation of monovalent Smac mimetics that bind to the BIR2 domain of the anti-apoptotic protein XIAPBioorg Med Chem Lett20112114 4332 433621680182
- BoulahjarROuachAMatteoCNovel tetrahydropyrido[1,2-a] isoindolone derivatives (valmerins): potent cyclin-dependent kinase/glycogen synthase kinase 3 inhibitors with antiproliferative activities and antitumor effects in human tumor xenograftsJ Med Chem20125522 9589 960623083119
- MorrisGFMathewsMBRegulation of proliferating cell nuclear antigen during the cell cycleJ Biol Chem198926423 13856 138642569465
- ZhengDChenZLiuJWuJAn efficient route to 1-aminoisoquinolines via AgOTf-catalyzed reaction of 2-alkynylbenzaldoxime with amineOrg Biomol Chem2011913 4763 476521617812
- ZhengDLiSWuJAn unexpected silver triflate catalyzed reaction of 2-alkynylbenzaldehyde with 2-isocyanoacetateOrg Lett20121411 2655 265722616758
- FaniSKamalidehghanBLoKMHashimNMChowKMAhmadipourFSynthesis, structural characterization, and anticancer activity of a monobenzyltin compound against MCF-7 breast cancer cellsDrug Des Devel Ther20159 6191 6201
- YaoZSunBHongQPACE4 regulates apoptosis in human prostate cancer cells via endoplasmic reticulum stress and mitochondrial signaling pathwaysDrug Des Devel Ther20159 5911 5923
- JinRXiaYChenQDa0324, an inhibitor of nuclear factor-kappaB activation, demonstrates selective antitumor activity on human gastric cancer cellsDrug Des Devel Ther201610 979 995
- JinZNiuHWangXZhangLWangQYangAPreclinical study of CC223 as a potential anti-ovarian cancer agentOncotarget Epub2017510
- JiangSChenXHMGB1 siRNA can reduce damage to retinal cells induced by high glucose in vitro and in vivoDrug Des Devel Ther201711 783 795
- PalaŞAtilganRKuloğluTProtective effects of vitamin C and vitamin E against hysterosalpingography-induced epithelial degeneration and proliferation in rat endometriumDrug Des Devel Ther201610 4079 4089
- PandeySAldose reductase inhibitor fidarestat as a promising drug targeting autophagy in colorectal carcinoma: a pilot studyAsian Pac J Cancer Prev20151612 4981 498526163626
- KadivarAKamalidehghanBAkbari JavarHKarimiBSedghiRNoordinMIAntiproliferation effect of imatinib mesylate on MCF7, T-47D tumorigenic and MCF 10A nontumorigenic breast cell lines via PDGFR-beta, PDGF-BB, c-Kit and SCF genesDrug Des Devel Ther201711 469 481
- RenFShenJShiHHornicekFJKanQDuanZNovel mechanisms and approaches to overcome multidrug resistance in the treatment of ovarian cancerBiochim Biophys Acta201618662 266 27527717733
- HuangRLeungIKProtein-directed dynamic combinatorial chemistry: a guide to protein ligand and inhibitor discoveryMolecules2016217 pii:E910
- NarayanaKKCAswathanarayanJBVittalRREndophytic peptides – a source of therapeutic agentsCurr Protein Pept Sci2017183 284 29027784222
- SchreiberSLTarget-oriented and diversity-oriented organic synthesis in drug discoveryScience20002875460 1964 196910720315
- VarfolomeevEGoncharovTMaeckerHCellular inhibitors of apoptosis are global regulators of NF-kappaB and MAPK activation by members of the TNF family of receptorsSci Signal20125216 ra2222434933
- Birkey ReffeySWurthnerJUParksWTRobertsABDuckettCSX-linked inhibitor of apoptosis protein functions as a cofactor in transforming growth factor-beta signalingJ Biol Chem200127628 26542 2654911356828
- Hofer-WarbinekRSchmidJAStehlikCBinderBRLippJde MartinRActivation of NF-kappa B by XIAP, the X chromosome-linked inhibitor of apoptosis, in endothelial cells involves TAK1J Biol Chem200027529 22064 2206810807933
- BeugSTCheungHHLaCasseECKornelukRGModulation of immune signalling by inhibitors of apoptosisTrends Immunol20123311 535 54522836014
- LopezJMeierPTo fight or die – inhibitor of apoptosis proteins at the crossroad of innate immunity and deathCurr Opin Cell Biol2010226 872 88120888210
- FuldaSPromises and challenges of Smac mimetics as cancer therapeuticsClin Cancer Res20152122 5030 503626567362
- LaCasseECPulling the plug on a cancer cell by eliminating XIAP with AEG35156Cancer Lett20133322 215 22422776562
- StraubCSTargeting IAPs as an approach to anti-cancer therapyCurr Top Med Chem2011113 291 31621320059
- FuldaSMolecular pathways: targeting death receptors and smac mimeticsClin Cancer Res20142015 3915 392024824309
- TalbottRLBorzilleriRMChaudhryCPharmacology of smac mimetics; chemotype differentiation based on physical association with caspase regulators and cellular transportExp Cell Res20153382 251 26026302264
- WangSDesign of small-molecule Smac mimetics as IAP antagonistsCurr Top Microbiol Immunol2011348 89 11321072626
- LiLThomasRMSuzukiHDe BrabanderJKWangXHarranPGA small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell deathScience20043055689 1471 147415353805
- SunHNikolovska-ColeskaZLuJDesign, synthesis, and characterization of a potent, nonpeptide, cell-permeable, bivalent Smac mimetic that concurrently targets both the BIR2 and BIR3 domains in XIAPJ Am Chem Soc200712949 15279 1529417999504
- KimKSZhangLWilliamsDDiscovery of tetrahydroisoquinoline-based bivalent heterodimeric IAP antagonistsBioorg Med Chem Lett20142421 5022 502925278234
- PandeyMKPrasadSTyagiAKTargeting cell survival proteins for cancer cell deathPharmaceuticals (Basel)201691 pii:E11
