Abstract
In continuation of our endeavor with respect to the development of potent and effective isatin-based anticancer agents, we adopted the molecular hybridization approach to design and synthesize four different sets of isatin-quinazoline (6a–f and 7a–e)/phthalazine (8a–f)/quinoxaline (9a–f) hybrids. The antiproliferative activity of the target hybrids was assessed towards HT-29 (colon), ZR-75 (breast) and A-549 (lung) human cancer cell lines. Hybrids 8b–d emerged as the most active antiproliferative congener in this study. Compound 8c induced apoptosis via increasing caspase 3/7 activity by about 5-fold in the A-549 human cancer cell line. In addition, it exhibited an increase in the G1 phase and a decrease in the S and G2/M phases in the cell cycle effect assay. Furthermore, it displayed an inhibitory concentration 50% value of 9.5 µM against multidrug-resistant NCI-H69AR lung cancer cell line. The hybrid 8c was also subjected to in vitro metabolic investigations through its incubation with rat liver microsomes and analysis of the resulting metabolites with the aid of liquid chromatography-mass spectrometry.
Introduction
In the current medical era, molecular hybridization approach has stood out as a valuable and important structural modification tool useful for the discovery and development of better therapies for diverse human diseases, mostly for cancer.Citation1 The growing endeavors to discover hybrid drugs resulting from the combination of two or more haptophoric moieties of different bioactive substances have brought a new hope for the treatment of multifactorial disorders in recent years. Moreover, hybrid drugs can potentially overcome most of the pharmacokinetic drawbacks encountered by conventional anticancer drugs as well as provide combination therapies in a single multifunctional therapeutic agent at the target molecule conferring a more powerful, selective and safer drug compared to conventional classic treatments.Citation2–Citation4
Isatin (1H-indole-2,3-dione) is an endogenous compound found in many organisms, which was first isolated in 1988.Citation5 As a privileged scaffold, isatin has emerged as an attractive and promising nucleus in the development of novel anticancer agents.Citation6 Sunitinib (I) (Sutent™, ), granted the US Food and Drug Administration (FDA) approval in 2006, is an isatin-based orally active multi-targeted tyrosine kinase inhibitor used for the management of imatinib-resistant gastrointestinal stromal tumors and metastatic renal-cell carcinoma.Citation7–Citation9 By 2014, Nintedanib (II, Vargatef™, ), an orally available triple angiokinase inhibitor, was approved in the US for the treatment of idiopathic pulmonary fibrosis. One year later, the European Medicines Agency approved Nintedanib (II) as a second-line treatment in combination with docetaxel for non-small cell lung cancer of adenocarcinoma.Citation10,Citation11 Also, semaxanib and orantinib are other examples for isatin-based anticancer agents that are being used in clinical trials and possess multiple tyrosine kinase receptor inhibitory activities.Citation12
Over the last decade, several studies suggested the significance of developing isatin-based hybrids as promising anticancer agents;Citation13 among them, isatin-chromene VII,Citation14 isat-in-pyridine VIII,Citation15 bis-isatin IX,Citation16,Citation17 isatin-benzoxazole X,Citation18 isatin-benzimidazole XI,Citation19 isatin-benzothiazole,Citation20 isatin-thi-azolidine/thiazolidinone,Citation21–Citation25 isatin-4-piperazinylquinolineCitation26 and isatin-pyrazolineCitation27 hybrids were reported ().
Figure 2 Structures of some reported isatin-based hybrids with promising anticancer activity and structures of the target hybrids 6a–f, 7a–e, 8a–f and 9a–f.
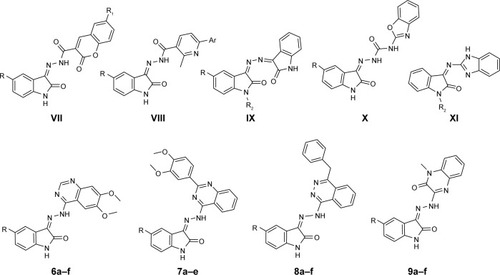
On the other hand, quinazolines constitute the cornerstone for a number of tyrosine kinase inhibitors such as the reversible EGFR-inhibitor. Erlotinib (III, Tarceva™, ), as well as the dual VEGFR-2-EGFR inhibitor Vandetanib (IV, Caprelsa™, ), is indicated for the treatment of symptomatic or progressive medullary thyroid cancer in patients with unresectable locally advanced or metastatic disease.Citation28,Citation29 Also, phthalazine is another attractive scaffold forming the backbone of certain promising antitumor lead candidates. Among them, Vatalanib (V, PTK787, ) is an orally active VEGFR-1 and VEGFR-2 inhibitor undergoing phase III clinical trials for the treatment of colorectal cancer.Citation30,Citation31 Olaparib (VI, Lynparza, ), the first approved phthalazine-based anticancer drug, is an oral small molecule poly (ADP-ribose) polymerase inhibitor that was approved in 2014 for the treatment of BRCA-mutated ovarian cancer.Citation32 Recently, much attention has been paid to anticancer drug discovery based on the quinoxaline nucleus as an important heterocyclic one that exhibited interesting biological activities.Citation33 It has been documented that several isatin-quinazoline and isatin-phthalazine hybrids displayed promising anticancer activities.Citation34,Citation35
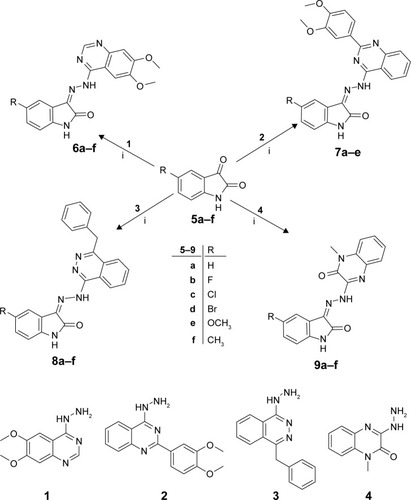
In the light of the aforementioned findings and in continuation of our endeavor with respect to the development of potent and effective isatin-based anticancer agents,Citation35,Citation36 we adopted the molecular hybridization approach to design and synthesize four different sets of isatin-quinazolines (6a–f and 7a–e)/phthalazines (8a–f)/quinoxaline (9a–f) hybrids (). All the synthesized hybrids (6a–f, 7a–e, 8a–f and 9a–f) were in vitro evaluated for their antiproliferative activity against three human cancer cell lines, namely human colon cancer HT-29 cell line, breast cancer ZR-75 cell line and lung cancer A-549 cell line. Moreover, the most active congeners were further assessed for their apoptosis induction potential using human cancer A-549 cell line, via evaluation of their effects on the expression of caspase 3/7 as well as on the cell cycle progression, to obtain mechanistic insights into their anticancer activity. Furthermore, their antiproliferative activity against multidrug-resistant lung cancer NCI-H69AR cell line was evaluated. Finally, the most active candidates were subjected to in vitro metabolic investigations through their incubation with rat liver microsomes (RLMs) and analysis of the resulting metabolites with the aid of liquid chromatography-mass spectrometry (LC-MS).
Materials and methods
General
Melting points of the synthesized compounds were measured with a Stuart melting point apparatus (Staffordshire, UK) and were uncorrected. Infrared (IR) spectra were recorded as KBr disks using FT-IR Spectrum BX apparatus (Perkin Elmer, Shelton, CT, USA). Mass spectra were recorded using Agilent Quadrupole 6120 LC-MS with electrospray ionization (ESI) source (Agilent Technologies, Palo Alto, CA, USA). NMR spectra were recorded on a Bruker NMR spectrometer (Bruker Biospin, Billerica, MA, USA). 1H spectra were run at 500 or 700 MHz, and 13C spectra were run at 125 or 175 MHz in deuterated dimethyl sulfoxide (DMSO-d6). Chemical shifts are expressed in δ values (ppm) using the solvent peak as internal standard. All coupling constant (J) values are given in hertz. The abbreviations used are as follows: s, singlet; d, doublet; m, multiplet. Elemental analyses were carried out at Microanalytical Centre, Cairo University, Egypt. High-resolution mass spectrometry (HRMS) measurements were performed on an LTQ-Orbitrap XL coupled to matrix-assisted laser desorption ionization (MALDI). Reaction courses and product mixtures were routinely monitored by thin layer chromatography on silica-gel precoated F254 Merck plates (Merck Millipore, Billerica, MA, USA). Unless otherwise noted, all solvents and reagents were commercially available and used without further purification. Compounds 1,Citation37 2,Citation35 3,Citation38 and 4Citation39 were prepared according to the reported method. All cell lines were purchased from the American Type Culture Collection (ATCC) as follows: HT-29: (ATCC® HTB-38™); ZR75: (ATCC® CRL-1500™); A549: (ATCC® CRM-CCL-185™); IEC-6: (ATCC® CRL-1592™); 3T3-Swiss albino (ATCC® CCL-92™); MCF 10A (ATCC® CRL-10317™); H69AR (ATCC® CRL-11351™).
Chemistry
General procedure for preparation of the target hybrids 6a–f, 7a–e, 8a–f and 9a–f
The appropriate indoline-2,3-dione derivative 5a–f (1 mmol) was added to a suspension of each hydrazinyl intermediate 1–4 (1 mmol) in ethanol (10 mL) and a catalytic amount of glacial acetic acid. The reaction mixture was refluxed for 1 h. The precipitate formed was collected by filtration while hot, washed with hot ethanol, dried and re-crystallized from DMF/ethanol to furnish the desired hybrids.
3-(2-(6,7-Dimethoxyquinazolin-4-yl)hydrazono) indolin-2-one (6a) – orange powder (yield 75%), m.p. 297°C–299°C; IR (KBr, ν cm−1): 3,411 (NH) and 1,699 (C=O); 1H NMR (DMSO-d6) δ ppm: 3.96 (s, 3H, OCH3), 4.00 (s, 3H, OCH3), 6.87–6.89 (m, 2H, Ar-H), 7.17 (t, 1H, Ar-H, J =8.9 Hz), 7.21 (s, 1H, Ar-H), 7.74 (s, 1H, Ar-H), 8.06 (s, 1H, Ar-H), 8.25 (d, 1H, Ar-H, J =8.8 Hz), 10.61 (s, 1H, NH), 12.36 (s, 1H, NH); 13C NMR (DMSO-d6) δ ppm: 55.8 (OCH3), 56.58 (OCH3), 104.2, 108.6, 111.1, 111.2, 112.9, 114.4, 114.5, 117.4, 118.9, 139.6, 144.3, 149.6, 155.4, 157.2, 158.5, 166.4 (C=O); MS (ESI) m/z: 350.0 [M+H]+; Anal. calcd. for C18H15N5O3 (349.12): C, 61.89; H, 4.33; N, 20.05; found C, 62.13; H, 4.28; N, 20.11; HRMS (MALDI) calcd. for C18H15N5O3: 350.1253, found: 350.1224 [M+H]+.
3-(2-(6,7-Dimethoxyquinazolin-4-yl)hydrazono)-5-fluoroindolin-2-one (6b) – orange powder (yield 73%), m.p. >300°C; IR (KBr, ν cm−1): 3,420 (NH) and 1,700 (C=O); 1H NMR (DMSO-d6) δ ppm: 4.01 (s, 3H, OCH3), 4.03 (s, 3H, OCH3), 7.00–7.39 (m, 3H, Ar-H), 7.73 (s, 1H, Ar-H), 8.07 (s, 1H, Ar-H), 8.72 (s, 1H, Ar-H), 11.33 (s, 1H, NH), 13.65 (s, 1H, NH); 13C NMR (DMSO-d6) δ ppm: 56.0, 56.5, 104.4, 108.5, 110.4 (Citation3JF-C =9.0 Hz), 111.9, (Citation2JF-C =27.0 Hz), 113.0 (Citation2JF-C =25.5 Hz), 118.4 (Citation2JF-C =8.0 Hz), 120.4, 122.3, 128.0, 131.5, 133.4, 144.4, 149.5, 153.8 (Citation1JF-C =238.0 Hz), 155.5, 166.2; MS (ESI) m/z: 368.0 [M+H]+; Anal. calcd. for C18H14FN5O3 (367.11): C, 58.85; H, 3.84; N, 19.07; found C, 59.09; H, 3.77; N, 19.13; HRMS (MALDI) calcd. for C18H14FN5O3: 368.1159, found: 368.1151 [M+H]+.
5-Chloro-3-(2-(6,7-dimethoxyquinazolin-4-yl)hydra-zono)indolin-2-one (6c) – orange powder (yield 80%), m.p. >300°C; IR (KBr, ν cm−1): 3,421 (NH) and 1,706 (C=O); 1H NMR (DMSO-d6) δ ppm: 3.96 (s, 3H, OCH3), 4.00 (s, 3H, OCH3), 6.90 (d, 1H, Ar-H, J =8.3 Hz), 7.21 (s, 1H, Ar-H), 7.36 (d, 1H, Ar-H, J =8.3 Hz), 7.72 (s, 1H, Ar-H), 8.08 (s, 1H, Ar-H), 8.57 (s, 1H, Ar-H), 10.71 (s, 1H, NH), 12.45 (s, 1H, NH); 13C NMR (DMSO-d6) δ ppm: 55.7 (OCH3), 56.6 (OCH3), 104.0, 108.5, 111.78, 112.9, 119.7, 125.7, 127.4, 130.5, 141.9, 142.5, 144.0, 149.5, 152.4, 153.7, 155.4, 166.0 (C=O); MS (ESI) m/z: 384.0 [M+H]+; Anal. calcd. for C18H14ClN5O3 (383.08): C, 56.33; H, 3.68; N, 18.25; found C, 56.21; H, 3.72; N, 18.17; HRMS (MALDI) calcd. for C18H14ClN5O3: 384.0863, found: 384.0853 [M+H]+.
5-Bromo-3-(2-(6,7-dimethoxyquinazolin-4-yl)hydrazono)indolin-2-one (6d) – orange powder (yield 85%), m.p. >300°C; IR (KBr, ν cm−1): 3,420 (NH) and 1,717 (C=O); 1H NMR (DMSO-d6) δ ppm: 3.96 (s, 3H, OCH3), 4.01 (s, 3H, OCH3), 6.86 (d, 1H, Ar-H, J =8.3 Hz), 7.21 (s, 1H, Ar-H), 7.48 (d, 1H, Ar-H, J =8.3 Hz), 7.72 (s, 1H, Ar-H), 8.08 (s, 1H, Ar-H), 8.73 (s, 1H, Ar-H), 10.73 (s, 1H, NH), 12.45 (s, 1H, NH); 13C NMR (DMSO-d6) δ ppm: 55.9 (OCH3), 56.6 (OCH3), 104.0, 108.4, 112.3, 112.9, 113.5, 120.2, 130.3, 133.3, 142.3, 142.9, 143.6, 144.3, 149.6, 155.5, 155.8, 165.9 (C=O); MS (ESI) m/z: 428.0 [M+H]+; Anal. calcd. for C18H14BrN5O3 (427.03): C, 50.48; H, 3.30; N, 16.35; found C, 50.61; H, 3.26; N, 16.28; HRMS (MALDI) calcd. for C18H14BrN5O3: 428.0358, found: 428.0353 [M+H]+.
3-(2-(6,7-Dimethoxyquinazolin-4-yl)hydrazono)-5-methoxyindolin-2-one (6e) – red powder (yield 72%), m.p. >300°C; IR (KBr, ν cm−1): 3,413 (NH) and 1,699 (C=O); 1H NMR (DMSO-d6) δ ppm: 3.81 (s, 3H, OCH3), 3.93 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 6.80 (d, 1H, Ar-H, J =8.4 Hz), 6.90 (d, 1H, Ar-H, J =8.4 Hz), 7.19 (s, 1H, Ar-H), 7.76 (s, 1H, Ar-H), 8.06 (s, 1H, Ar-H), 8.71 (s, 1H, Ar-H), 10.40 (s, 1H, NH), 12.23 (s, 1H, NH); 13C NMR (DMSO-d6) δ ppm: 55.6 (OCH3), 55.7 (OCH3), 56.5 (OCH3), 104.2, 108.6, 111.1, 112.5, 113.0, 118.0, 118.7, 137.2, 143.2, 143.7, 144.1, 149.4, 154.1, 154.9, 155.2, 166.4 (C=O); MS (ESI) m/z: 380.0 [M+H]+; Anal. calcd. for C19H17N5O4 (379.13): C, 60.15; H, 4.52; N, 18.46; found C, 59.91; H, 4.58; N, 18.59; HRMS (MALDI) calcd. for C19H17N5O4: 380.1359, found: 380.1345 [M+H]+.
3-(2-(6,7-Dimethoxyquinazolin-4-yl)hydrazono)-5-methylindolin-2-one (6f) – orange powder (yield 75%), m.p. >300°C; IR (KBr, ν cm−1): 3,421 (NH) and 1,700 (C=O); 1H NMR (DMSO-d6) δ ppm: 2.31 (s, 3H, CH3), 3.95 (s, 3H, OCH3), 4.01 (s, 3H, OCH3), 6.77 (d, 1H, Ar-H, J =7.5 Hz), 7.12 (d, 1H, Ar-H, J =7.0 Hz), 7.18 (s, 1H, Ar-H), 7.77 (s, 1H, Ar-H), 8.02 (s, 1H, Ar-H), 8.69 (s, 1H, Ar-H), 10.48 (s, 1H, NH), 12.32 (s, 1H, NH); 13C NMR (DMSO-d6) δ ppm: 21.3 (CH3), 55.7 (OCH3), 56.5 (OCH3), 104.2, 108.6, 110.1, 113.1, 118.6, 128.9, 130.8, 131.8, 141.2, 143.8, 144.8, 149.4, 153.3, 155.1, 156.3, 166.4 (C=O); MS (ESI) m/z: 364.0 [M+H]+; Anal. calcd. for C19H17N5O3 (363.13): C, 62.80; H, 4.72; N, 19.27; found C, 63.03; H, 4.66; N, 19.15; HRMS (MALDI) calcd. for C19H17N5O3: 364.1409, found: 364.1422 [M+H]+.
3-(2-(2-(3,4-Dimethoxyphenyl)quinazolin-4-yl) hydrazono)indolin-2-one (7a).Citation35
3-(2-(2-(3,4-Dimethoxyphenyl)quinazolin-4-yl) hydrazono)-5-fluoroindolin-2-one (7b).Citation35
5-Chloro-3-(2-(2-(3,4-dimethoxyphenyl)quinazolin-4-yl) hydrazono)indolin-2-one (7c).Citation35
5-Bromo-3-(2-(2-(3,4-dimethoxyphenyl)quinazolin-4-yl) hydrazono)indolin-2-one (7d) – red powder (yield 80%), m.p. 295°C–297°C; IR (KBr, ν cm−1): 3,421 (NH) and 1,718 (C=O); 1H NMR (DMSO-d6) δ ppm: 3.87 (s, 3H, OCH3), 3.92 (s, 3H, OCH3), 6.96 (d, 1H, Ar-H, J =8.0 Hz), 7.15 (d, 1H, Ar-H, J =8.0 Hz), 7.55 (d, 1H, Ar-H, J =8.5 Hz), 7.73 (t, 1H, Ar-H, J =7.0 Hz), 7.85 (s, 1H, Ar-H), 7.94–7.97 (m, 2H, Ar-H), 8.12 (s, 1H, Ar-H), 8.18 (d, 1H, Ar-H, J =8.5 Hz), 8.52–8.53 (m, 1H, Ar-H), 11.49 (s, 1H, NH), 13.81 (s, 1H, NH); 13C NMR (DMSO-d6) δ ppm: 56.2 (OCH3), 56.3 (OCH3), 110.7, 110.9, 112.1, 118.1, 120.6, 122.4, 125.1, 125.3, 127.7, 128.0, 128.4, 132.3, 132.5, 134.9, 143.3, 146.0, 147.4, 149.3, 152.5, 166.2 (C=O); MS (ESI) m/z: 504 [M+H]+; Anal. calcd. for C24H18BrN5O3 (503.06): C, 57.16; H, 3.60; N, 13.89; found C, 57.29; H, 3.63; N, 13.79; HRMS (MALDI) calcd. for C24H18BrN5O3: 504.0671, found: 504.0653 [M+H]+.
3-(2-(2-(3,4-Dimethoxyphenyl)quinazolin-4-yl) hydrazono)-5-methoxyindolin-2-one (7e) – orange powder (yield 79%), m.p. 263°C–265°C; IR (KBr, ν cm−1): 3,412 (NH) and 1,718 (C=O); 1H NMR (DMSO-d6) δ ppm: 3.76 (s, 3H, OCH3), 3.89 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 6.83 (d, 1H, Ar-H, J =8.5 Hz), 6.96 (d, 1H, Ar-H, J =8.5 Hz), 7.14 (s, 1H, Ar-H), 7.19 (d, 1H, Ar-H, J =9.0 Hz), 7.81 (t, 1H, Ar-H, J =7.5 Hz), 7.96 (s, 1H, Ar-H), 8.06–8.09 (m, 4H, Ar-H), 11.31 (s, 1H, NH), 13.08 (s, 1H, NH); 13C NMR (DMSO-d6) δ ppm: 55.8 (OCH3), 56.2 (OCH3), 56.3 (OCH3), 110.0, 110.8, 111.5, 113.3, 114.4, 117.6, 120.9, 123.1, 124.8, 125.8, 127.1, 127.9, 129.1, 131.8, 133.0, 135.2, 142.5, 144.7, 148.2, 149.1, 154.8, 166.3 (C=O); MS (ESI) m/z: 456 [M+H]+; Anal. calcd. for C25H21N5O4 (455.16): C, 65.93; H, 4.65; N, 15.38; found C, 66.17; H, 4.59; N, 15.52; HRMS (MALDI) calcd. for C25H21N5O4: 456.1672, found: 456.1662 [M+H]+.
3-(2-(4-Benzylphthalazin-1-yl)hydrazono)indolin-2-one (8a) – orange powder (yield 75%), m.p. 259°C–261°C; IR (KBr, ν cm−1): 3,412 (NH) and 1,700 (C=O); 1H NMR (DMSO-d6) δ ppm: 4.36 (s, 2H, CH2), 6.89 (d, 1H, Ar-H, J =7.5 Hz), 7.06 (t, 1H, Ar-H, J =7.5 Hz), 7.19 (t, 1H, Ar-H, J =7.5 Hz), 7.29–7.36 (m, 5H, Ar-H), 7.86–7.98 (m, 3H, Ar-H), 8.45 (d, 1H, Ar-H, J =7.5 Hz), 8.63 (d, 1H, Ar-H, J =7.5 Hz), 10.63 (s, 1H, NH), 12.89 (s, 1H, NH); 13C NMR (DMSO-d6) δ ppm: 38.1 (CH2), 111.6, 115.6, 118.3, 120.3, 122.3, 126.2, 127.0, 127.6, 127.8, 128.9, 129.1, 131.4, 132.9, 134.0, 138.6, 143.3, 144.0, 148.4, 156.5, 166.7; MS (ESI) m/z: 380.0 [M+H]+; Anal. calcd. for C23H17N5O (379.14): C, 72.81; H, 4.52; N, 18.46; found C, 73.01; H, 4.48; N, 18.55; HRMS (MALDI) calcd. for C23H17N5O: 380.1511, found: 380.1525 [M+H]+.
3-(2-(4-Benzylphthalazin-1-yl)hydrazono)-5-fluor-oindolin-2-one (8b) – orange powder (yield 79%), m.p. 275°C–277°C; IR (KBr, ν cm−1): 3,411 (NH) and 1,705 (C=O); 1H NMR (DMSO-d6) δ ppm: 4.39 (s, 2H, CH2), 6.88–7.36 (m, 7H, Ar-H), 7.90–8.01 (m, 3H, Ar-H), 8.19 (d, 1H, Ar-H, J =8.5 Hz), 8.61 (d, 1H, Ar-H, J =7.0 Hz), 10.62 (s, 1H, NH), 12.93 (s, 1H, NH); 13C NMR (DMSO-d6) δ ppm: 38.1 (CH2), 111.1 (Citation3JF-C =8.8 Hz), 114.0 (Citation2JF-C =24.5 Hz), 117.3 (Citation2JF-C =25.0 Hz), 118.7 (Citation3JF-C =8.8 Hz), 125.9, 126.2, 126.6, 127.0, 127.6, 128.9, 129.1, 133.1, 134.1, 138.6, 139.4, 143.3, 148.9, 152.8, 157.3 (Citation1JF-C =234.5 Hz), 166.5; MS (ESI) m/z: 398.0 [M+H]+; Anal. calcd. for C23H16FN5O (397.13): C, 69.51; H, 4.06; N, 17.62; found C, 69.32; H, 4.13; N, 17.51; HRMS (MALDI) calcd. for C23H16FN5O: 398.1417, found: 398.1405 [M+H]+.
3-(2-(4-Benzylphthalazin-1-yl)hydrazono)-5-chlor-oindolin-2-one (8c) – orange powder (yield 80%), m.p. 295°C–297°C; IR (KBr, ν cm−1): 3,410 (NH) and 1,700 (C=O); 1H NMR (DMSO-d6) δ ppm: 4.39 (s, 2H, CH2), 6.91 (d, 1H, Ar-H, J =8.5 Hz), 7.21 (t, 1H, Ar-H, J =7.5 Hz), 7.30–7.36 (m, 5H, Ar-H), 7.90–8.02 (m, 3H, Ar-H), 8.42 (s, 1H, Ar-H), 8.56 (d, 1H, Ar-H, J =7.0 Hz), 10.73 (s, 1H, NH), 12.96 (s, 1H, NH); 13C NMR (DMSO-d6) δ ppm: 38.1 (CH2), 111.8, 119.5, 125.7, 126.3, 126.6, 126.7, 127.0, 127.7, 128.9, 129.0, 129.1, 130.5, 133.1, 134.2, 138.6, 141.8, 142.7, 149.0, 152.8, 166.2 (C=O); MS (ESI) m/z: 414.0 [M+H]+; Anal. calcd. for C23H16ClN5O (413.10): C, 66.75; H, 3.90; N, 16.92; found C, 66.97; H, 3.83; N, 17.05; HRMS (MALDI) calcd. for C23H16ClN5O: 414.1122, found: 414.1146 [M+H]+.
3-(2-(4-Benzylphthalazin-1-yl)hydrazono)-5-bro-moindolin-2-one (8d) – orange powder (yield 86%), m.p. 298°C–299°C; IR (KBr, ν cm−1): 3,412 (NH) and 1,716 (C=O); 1H NMR (DMSO-d6) δ ppm: 4.39 (s, 2H, CH2), 6.86 (d, 1H, Ar-H, J =8.5 Hz), 7.21 (t, 1H, Ar-H, J =7.5 Hz), 7.30–7.36 (m, 3H, Ar-H), 7.47 (d, 1H, Ar-H, J =8.5 Hz), 7.90–8.02 (m, 3H, Ar-H), 8.20 (s, 1H, Ar-H), 8.53–8.56 (m, 2H, Ar-H), 10.74 (s, 1H, NH), 12.69 (s, 1H, NH); 13C NMR (DMSO-d6) δ ppm: 38.1 (CH2), 112.3, 113.5, 120.0, 126.4, 126.6, 127.0, 127.7, 128.8, 128.9, 129.1, 129.5, 133.0, 133.3, 134.2, 138.5, 142.1, 142.6, 149.0, 152.8, 166.0 (C=O); MS (ESI) m/z: 458.0 [M+H]+; Anal. calcd. for C23H16BrN5O (457.05): C, 60.28; H, 3.52; N, 15.28; found C, 60.46; H, 3.47; N, 15.40; HRMS (MALDI) calcd. for C23H16BrN5O: 458.0617, found: 458.0605 [M+H]+.
3-(2-(4-Benzylphthalazin-1-yl)hydrazono)-5-methoxy-indolin-2-one (8e) – red powder (yield 74%), m.p. >300°C; IR (KBr, ν cm−1): 3,420 (NH) and 1,707 (C=O); 1H NMR (DMSO-d6) δ ppm: 3.79 (s, 3H, OCH3), 4.37 (s, 2H, CH2), 6.80 (d, 1H, Ar-H, J =8.5 Hz), 6.91 (d, 1H, Ar-H, J =8.5 Hz), 7.19 (t, 1H, Ar-H, J =7.5 Hz), 7.29–7.36 (m, 4H, Ar-H), 7.87–7.99 (m, 3H, Ar-H), 8.08 (s, 1H, Ar-H), 8.56 (d, 1H, Ar-H, J =8.0 Hz), 10.44 (s, 1H, NH), 12.88 (s, 1H, NH); 13C NMR (DMSO-d6) δ ppm: 38.1 (CH2), 55.8 (OCH3), 110.7, 112.1, 113.5, 116.9, 120.1, 123.7, 126.4, 127.1, 127.6, 128.8, 129.0, 129.2, 131.7, 133.8, 138.5, 141.7, 144.2, 146.2, 149.9, 154.5, 166.5 (C=O); MS (ESI) m/z: 410.0 [M+H]+; Anal. calcd. for C24H19N5O2 (409.15): C, 70.40; H, 4.68; N, 17.10; found C, 70.64; H, 4.63; N, 16.98; HRMS (MALDI) calcd. for C24H19N5O2: 410.1617, found: 410.1644 [M+H]+.
3-(2-(4-Benzylphthalazin-1-yl)hydrazono)-5-methylindolin-2-one (8f) – orange powder (yield 71%), m.p. 253°C–255°C; IR (KBr, ν cm−1): 3,350 (NH) and 1,697 (C=O); 1H NMR (DMSO-d6) δ ppm: 2.34 (s, 3H, CH3), 4.36 (s, 2H, CH2), 6.78 (d, 1H, Ar-H, J =7.5 Hz), 7.12 (d, 1H, Ar-H, J =8.0 Hz), 7.21 (t, 1H, Ar-H, J =7.5 Hz), 7.29–7.36 (m, 4H, Ar-H), 7.88–7.97 (m, 3H, Ar-H), 8.26 (s, 1H, Ar-H), 8.60 (d, 1H, Ar-H, J =7.5 Hz), 10.52 (s, 1H, NH), 12.84 (s, 1H, NH); 13C NMR (DMSO-d6) δ ppm: 21.4 (CH3), 38.1 (CH2), 110.1, 118.5, 125.8, 126.1, 126.8, 127.0, 127.5, 128.3, 128.9, 129.1, 130.8, 131.7, 132.8, 133.8, 138.6, 141.2, 144.6, 148.2, 152.1, 166.6 (C=O); MS (ESI) m/z: 394.0 [M+H]+; Anal. calcd. for C24H19N5O (393.16): C, 73.27; H, 4.87; N, 17.80; found C, 73.39; H, 4.91; N, 17.72; HRMS (MALDI) calcd. for C24H19N5O: 394.1668, found: 394.1646 [M+H]+.
1-Methyl-3-(2-(2-oxoindolin-3-ylidene)hydrazinyl) quinoxalin-2(1H)-one (9a) – orange powder (yield 80%), m.p. >300°C; IR (KBr, ν cm−1): 3,347 (NH) and 1,711 (C=O); 1H NMR (DMSO-d6) δ ppm: 3.71 (s, 3H, CH3), 6.95 (d, 1H, Ar-H, J =7.5 Hz), 7.10 (t, 1H, Ar-H, J =7.5 Hz), 7.33–7.39 (m, 2H, Ar-H), 7.46 (t, 1H, Ar-H, J =7.5 Hz), 7.54 (d, 1H, Ar-H, J =8.0 Hz), 7.64 (d, 1H, Ar-H, J =7.5 Hz), 7.71 (d, 1H, Ar-H, J =7.5 Hz), 10.72 (s, 1H, NH), 11.24 (s, 1H, NH); 13C NMR (DMSO-d6) δ ppm: 29.8 (CH3), 111.5, 115.3, 120.1, 121.0, 122.9, 124.7, 126.6, 131.5, 132.6, 137.8, 140.5, 142.0, 143.2, 150.5, 162.3, 168.3; MS (ESI) m/z: 320.0 [M+H]+; Anal. calcd. for C17H13N5O2 (319.11): C, 63.94; H, 4.10; N, 21.93; found C, 64.17; H, 4.07; N, 21.84; HRMS (MALDI) calcd. for C17H13N5O2: 320.1148, found: 320.1133 [M+H]+.
3-(2-(5-Fluoro-2-oxoindolin-3-ylidene)hydrazinyl)-1-methylquinoxalin-2(1H)-one (9b) – red powder (yield 75%), m.p. >300°C; IR (KBr, ν cm−1): 3,412 (NH) and 1,701 (C=O); 1H NMR (DMSO-d6) δ ppm: 3.71 (s, 3H, CH3), 6.94–6.97 (m, 1H, Ar-H), 7.18 (t, 1H, Ar-H, J =7.0 Hz), 7.37 (t, 1H, Ar-H, J =7.5 Hz), 7.43–7.50 (m, 2H, Ar-H), 7.55 (d, 1H, Ar-H, J =8.0 Hz), 7.71 (d, 1H, Ar-H, J =7.5 Hz), 11.26 (s, 1H, NH), 13.70 (s, 1H, NH); 13C NMR (DMSO-d6) δ ppm: 29.7 (CH3), 111.4 (Citation3JF-C =9.0 Hz), 115.2 (Citation2JF-C =28.8 Hz), 118.5 (Citation2JF-C =28.00 Hz), 119.0 (Citation3JF-C =9.0 Hz), 121.9, 123.9, 125.7, 128.2, 131.5, 138.3, 140.4, 147.1, 150.8, 156.8 (Citation1JF-C =239.5 Hz), 163.5, 165.9; MS (ESI) m/z: 338.0 [M+H]+; Anal. calcd. for C17H12FN5O2 (337.10): C, 60.53; H, 3.59; N, 20.76; found C, 60.74; H, 3.62; N, 20.67; HRMS (MALDI) calcd. for C17H12FN5O2: 338.1053, found: 338.1041 [M+H]+.
3-(2-(5-Chloro-2-oxoindolin-3-ylidene)hydrazinyl)-1-methylquinoxalin-2(1H)-one (9c) – orange powder (yield 81%), m.p. >300°C; IR (KBr, ν cm−1): 3,411 (NH) and 1,698 (C=O); 1H NMR (DMSO-d6) δ ppm: 3.71 (s, 3H, CH3), 6.97 (d, 1H, Ar-H, J =8.5 Hz), 7.38–7.41 (m, 2H, Ar-H), 7.50 (t, 1H, Ar-H, J =7.5 Hz), 7.56 (d, 1H, Ar-H, J =8.5 Hz), 7.62 (s, 1H, Ar-H), 7.72 (d, 1H, Ar-H, J =8.0 Hz), 11.37 (s, 1H, NH), 13.65 (s, 1H, NH), 13C NMR (DMSO-d6) δ ppm: 29.8 (CH3), 112.1, 115.4, 117.5, 120.3, 122.8, 124.7, 126.1, 128.3, 130.7, 132.1, 134.7, 140.9, 142.7, 150.9, 163.3, 165.5; MS (ESI) m/z: 354.0 [M+H]+; Anal. calcd. for C17H12ClN5O2 (353.07): C, 57.72; H, 3.42; N, 19.80; found C, 57.88; H, 3.38; N, 19.91; HRMS (MALDI) calcd. for C17H12ClN5O2: 354.0758, found: 354.0757 [M+H]+.
3-(2-(5-Bromo-2-oxoindolin-3-ylidene)hydrazinyl)-1-methylquinoxalin-2(1H)-one (9d) – orange powder (yield 84%), m.p. >300°C; IR (KBr, ν cm−1): 3,411 (NH) and 1,708 (C=O); 1H NMR (DMSO-d6) δ ppm: 3.72 (s, 3H, CH3), 6.93 (d, 1H, Ar-H, J =8.5 Hz), 7.38 (t, 1H, Ar-H, J =7.5 Hz), 7.48–7.58 (m, 3H, Ar-H), 7.73–7.74 (m, 2H, Ar-H), 11.44 (s, 1H, NH), 13.64 (s, 1H, NH), 13C NMR (DMSO-d6) δ ppm: 29.8 (CH3), 112.6, 115.4, 117.1, 119.7, 122.8, 124.0, 127.6, 128.3, 131.2, 133.4, 141.2, 143.1, 147.8, 150.8, 163.1, 165.4; MS (ESI) m/z: 398.0 [M+H]+; Anal. calcd. for C17H12BrN5O2 (397.02): C, 51.27; H, 3.04; N, 17.59; found C, 51.09; H, 3.07; N, 17.70; HRMS (MALDI) calcd. for C17H12BrN5O2: 398.0253, found: 398.0243 [M+H]+.
3-(2-(5-Methoxy-2-oxoindolin-3-ylidene)hydrazinyl)-1-methylquinoxalin-2(1H)-one (9e) – red powder (yield 78%), m.p. >300°C; IR (KBr, ν cm−1): 3,413 (NH) and 1,707 (C=O); 1H NMR (DMSO-d6) δ ppm: 3.69 (s, 3H, CH3), 3.79 (s, 3H, OCH3), 6.80–6.94 (m, 2H, Ar-H), 7.14–7.51 (m, 4H, Ar-H), 7.69 (d, 1H, Ar-H, J =8.0 Hz), 10.53 (s, 1H, NH), 13.70 (s, 1H, NH); 13C NMR (DMSO-d6) δ ppm: 38.1 (CH3), 55.8 (OCH3), 110.8, 113.6, 116.5, 118.9, 125.5, 126.8, 127.6, 128.9, 129.1, 132.9, 133.9, 137.0, 138.6, 148.4, 154.9, 166.5; MS (ESI) m/z: 350.0 [M+H]+; Anal. calcd. for C18H15N5O3 (349.12): C, 61.89; H, 4.33; N, 20.05; found C, 62.11; H, 4.29; N, 19.94; HRMS (MALDI) calcd. for C18H15N5O3: 350.1253, found: 350.1239 [M+H]+.
1-Methyl-3-(2-(5-methyl-2-oxoindolin-3-ylidene) hydrazinyl)quinoxalin-2(1H)-one (9f) – orange powder (yield 77%), m.p. >300°C; IR (KBr, ν cm−1): 3,411 (NH) and 1,700 (C=O); 1H NMR (DMSO-d6) δ ppm: 3.70 (s, 3H, CH3), 2.32 (s, 3H, CH3), 6.82 (d, 1H, Ar-H, J =7.5 Hz), 7.14–7.16 (m, 2H, Ar-H), 7.36 (d, 1H, Ar-H, J =7.0 Hz), 7.44 (s, 1H, Ar-H), 7.52 (d, 1H, Ar-H, J =7.5 Hz), 7.68 (d, 1H, Ar-H, J =7.0 Hz), 11.11 (s, 1H, NH), 13.63 (s, 1H, NH); 13C NMR (DMSO-d6) δ ppm: 21.0 (CH3), 29.8 (CH3), 111.3, 115.4, 118.1, 121.4, 124.6, 127.2, 131.8, 132.5, 134.6, 137.4, 140.7, 146.3, 152.4, 157.8, 162.5, 164.1; MS (ESI) m/z: 334.0 [M+H]+; Anal. calcd. for C18H15N5O2 (333.12): C, 64.86; H, 4.54; N, 21.01; found C, 64.69; H, 4.61; N, 21.12; HRMS (MALDI) calcd. for C18H15N5O2: 334.1304, found: 350. 334.1322 [M+H]+.
Pharmacological evaluation
The details of the experimental protocols are provided in Supplementary materials.
Metabolic investigations
The study protocol was approved by the Research Ethics Committee at College of Pharmacy, King Saud University. Animals were maintained according to the guidelines of Animal Care Center, College of Pharmacy, King Saud University, and approved by the Local Animal Care and Use Committee of King Saud University. The details of the experimental protocolsCitation40–Citation42 are provided in Supplementary materials.
Results and discussion
Chemistry
The synthetic pathway employed to prepare the target isatin derivatives is outlined in . The target compounds 6a–f, 7a–e, 8a–f and 9a–f were obtained by the reaction of the appropriate indoline-2,3-diones 5a–f with the hydrazinyl intermediates 1–4 in refluxed ethanol in the presence of a catalytic amount of glacial acetic acid with 70%–86% yields ().
IR spectra of the target compounds 6a–f, 7a–e, 8a–f and 9a–f showed absorption bands due to the NH groups in the region 3,347–3,421 cm−1, in addition to carbonyl bands in the region 1,697–1,718 cm−1. Their 1H NMR spectra showed two singlet signals attributable to NH protons of the isatin and the hydrazine function (=N–NH–) in the region δ 10.40–11.44 and 11.24–13.81 ppm. Also, the methoxy (–OCH3) protons of compounds 6a–f appeared as singlet signals around δ 4.00 ppm, while the methoxy protons of derivatives 7a–e appeared around δ3.80 ppm in the 1H NMR spectra. Furthermore, the (–CH2) protons of benzylic moiety of 8a–f appeared as a singlet signal in the range δ 4.36–4.37 ppm, while in case of 9a–f the signals of the aliphatic protons (N–CH3) were observed as singlets near to δ 3.70 ppm.
Pharmacological evaluation
Antiproliferative activity
A total of 23 compounds were analyzed for cancer cell growth inhibitory activity. These studies were carried out using cells derived from human lung, colon and breast tumors (A-549, HT-29 and ZR-75 cells, respectively). This initial assessment of activity tested each compound in quadruplicate at a single concentration of 30 µM, if solubility permitted. As indicated in , compounds 8b–d are the most potent congeners, inhibiting growth of all three cell lines with average growth inhibition values of 93.8, 96.5 and 96.4%, respectively, at a test concentration of 30 µM. The rest of the compounds showed an average growth inhibition values from 6.1%–81.2% at the tested concentration levels.
Table 1 Antiproliferative (cell growth inhibitory activity at 30 µM concentration) activity of the target compounds 6a–f, 7a–e, 8a–f and 9a–f against HT-29, ZR-75 and A-549 cell lines
The most active promising compounds 8b–d in the preliminary antiproliferative screening were subjected to quantitative inhibitory concentration 50% (IC50) determination for their cell growth inhibitory activity towards A-549, HT-29 and ZR-75 cancer cell lines and the results are presented in .
Table 2 IC50 of antiproliferative activity of the selected compounds 8b–d and sunitinib against HT-29, ZR-75 and A-549 cell lines
Compound 8c bearing 4-benzylphthalazine moiety exhibited the best average IC50 value of 5.53 µM, as compared with the positive control, sunitinib, which showed an average IC50 =8.11 µM. Therefore, compound 8c was subjected to deeper pharmacological investigations in order to gain insight into its pharmacological profile.
Apoptosis and caspase 3/7 activity
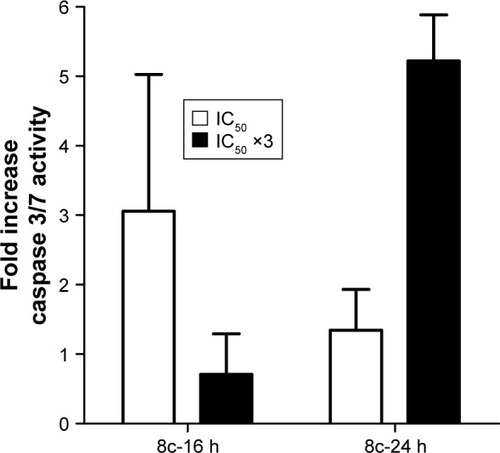
Compound 8c was analyzed for apoptosis-inducing activity in cancer cells. These studies were carried out using cells derived from human lung (A-549). This further assessment of activity tested compounds in quadruplicate at concentrations equivalent to IC50 value to inhibit growth and a concentration 3-fold above the IC50 concentrations over a time course ranging from 2–48 h. As indicated in , compound 8c at 5 µM increased caspase activity by 3-fold after 16 h of treatment and to over 5-fold after 24 h of treatment at a concentration of 15 µM.
Cell cycle effects
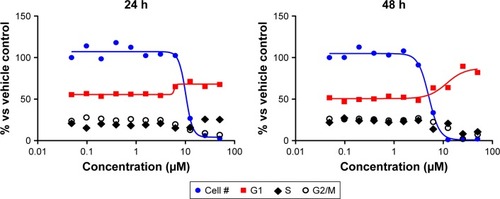
Compound 8c was analyzed for effects on various aspects of the cell cycle progression in human cancer cells. These studies were carried out using cells derived from lung adenocarcinoma (A-549). This follow-up assessment of activity tested compounds using immunofluorescent imaging of phosphorylated Rb protein and total DNA content of each cell to assess phase of cell cycle. The ability of test compounds to affect cell cycle distribution and Rb phosphorylation was tested over a range of concentrations less than 100 nM to 50 µM. As shown in , compound 8c produced dose-dependent effects on the tested parameters; however, compound 7d displayed no effects on the tested parameters (not shown). Compound 8c caused a significant reduction in the total cell number after 24 h of treatment with IC50 value =10.19 µM and with IC50 value =5.11 µM after 48 h ().
Table 3 IC50 for reductions in the total cell number and cell cycle effects of compound 8c and sunitinib
In addition, compound 8c caused an increase in the percentage of cells in the G1 phase of the cell cycle with corresponding decrease in S and G2/M phases. This suggests that part of the compound effects on growth may be attributable to the decreased rate of progression through the cell cycle and corresponding decrease in proliferation. By contrast, sunitinib caused a reduction in the percentage of cells in G1, with corresponding increases in S or G2/M phases. Arrest in G2 may represent a checkpoint blockade, whereas mitotic arrest may, in some cases, lead to mitotic catastrophe and subsequent programmed death of cells with multiple or aberrant nuclei.
As with other cell cycle parameters, levels of phosphorylated Rb protein were substantially reduced in a dose-dependent manner by the control and the test compound 8c. After 24 h of treatment, the IC50 value was lower than the IC50 value for reductions in the cell number caused by compound 8c (). This may support the hypothesis that inhibition of cyclin-dependent kinases by isatin compounds plays a role in their growth inhibitory activity. However, the correlation is less apparent at the 48-h time point. Furthermore, compound 8c was analyzed for effects on total cellular levels of phosphorylated tyrosine residues in human cancer cells. These studies were carried out using cells derived from lung adenocarcinoma (A-549) and immunofluorescent imaging. The ability of the test compounds to affect acute serum stimulation of tyrosine phosphorylation was tested over a range of concentrations less than 100 to 50 µM. Compound 8c had no significant effect on P-Tyr labeling.
Selectivity
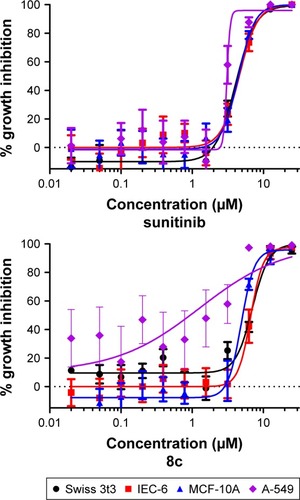
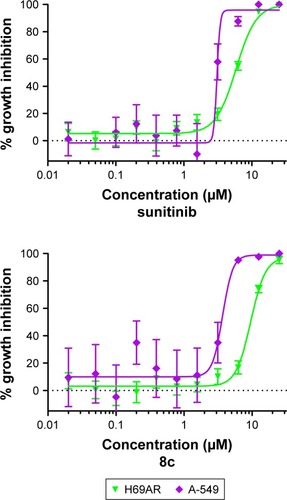
As an indicator of the selectivity for tumor cells, compound 8c was analyzed for its cell growth inhibitory activity in three non-tumorigenic cell lines. IEC-6 cells derived from rat intestine exhibit morphologic and karyotypic features of normal intestinal epithelial cells.Citation43 Cultures derived from human fibrocystic mammary tissue (MCF-10A) are non-tumorigenic and exhibit features of primary cultures of breast tissue including dome formation.Citation44 Fibroblasts derived from embryonic tissue from mice (Swiss 3t3 fibroblasts) are both non-tumorigenic and contact inhibited.Citation45 For comparison, A-549 human non-small cell lung cancer (NSCLC) cell line was included. This assessment of growth inhibitory activity tested compounds in quadruplicate at maximum concentrations of 25 µM, followed by 10 serially diluted concentrations. As demonstrated in and , compound 8c inhibited growth in both normal and tumor cell lines by >50%. Compound 8c not only inhibited NSCLC with IC50 value =1.27 µM but also inhibited non-tumor cells less potently with 4.8-fold selectivity value. For the control compound, sunitinib, there was a modest degree of selectivity (1.4-fold difference between mean IC50 in non-tumor cell lines versus the NSCLC cells).
Table 4 Selectivity for compound 8c and sunitinib toward tumor and non-tumorigenic cell lines
Multidrug-resistant lung cancer cell line
Compound 8c was analyzed for cancer cell growth inhibitory activity in a sensitive NSCLC cell line (A-549) and a multidrug-resistant lung cancer cell line (NCI-H69AR) that expresses the ABCC1 efflux pump protein. This assessment of activity tested compound 8c in quadruplicate at maximum concentrations of 25 µM, followed by 10 serially diluted concentrations. As illustrated in and summarized in , compound 8c inhibited growth in both sensitive and resistant cancer cell lines with IC50 values =1.3 and 9.5 µM, respectively, being 7.5-fold less sensitive toward the resistant NCI-H69AR cell line, indicating that this compound may be subjected to efflux by ABCC1. Sunitinib showed a lesser degree of fold resistance being 1.9-fold less sensitive toward the resistant NCI-H69AR cell line.
Table 5 Cancer cell growth inhibitory activity of compound 8c and sunitinib toward sensitive (A-549) and resistant NCI-H69AR cancer cell lines
Metabolic investigations
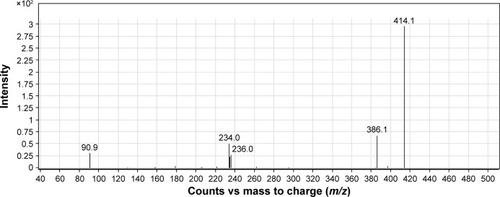
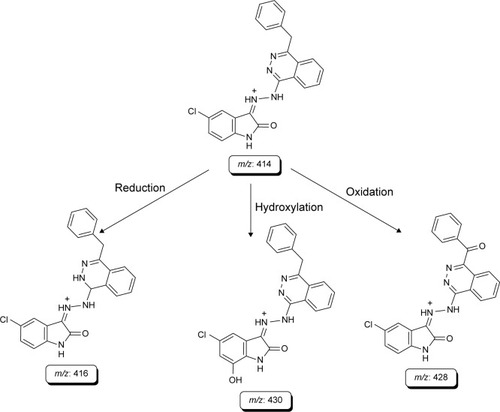
The study of drug metabolism is a core part of the process of drug discovery and development; it has evolved from being a complementary step to that process to becoming crucial to it.Citation46 Nowadays, metabolic profiles of new drugs have to be investigated prior to any clinical use of such drugs. This approach has been prejudiced by data accumulation assuming that poor pharmacokinetics is the main reason for failure of drug substances, in which the metabolic liability of a drug molecule is the primary determinant.Citation47,Citation48 In this study, comparison of the extracted ion chromatograms between incubations with or without RLMs as well as comparison of the product ion mass spectra of the postulated metabolites of 8c allowed the detection of ten metabolites. Such metabolites resulted from the incubation of 7d and 8c with RLMs that involved various metabolic reaction types, namely, demethylation for 7d and isomerization, reduction, hydroxylation and oxidation for 8c ( and ). summarizes the product ions, retention times and metabolic reactions for the in vitro phase I 8c metabolites.
Table 6 In vitro RLMs metabolites of compound 8c
Conclusion
Quinazoline-isatin hybrids 6a–f and 7a–e, phthalazineisatin hybrids 8a–f and 1-methylquinoxaline-isatin hybrids 9a–f were synthesized and characterized with different spectroscopic techniques. The preliminary in vitro antiproliferative activity of the synthesized compounds against various human cancer cell lines revealed that compounds 8b–d were the most active candidates. Therefore, they were subjected to quantitative IC50 determination. Detailed pharmacological investigations were carried out on the most promising compound 8c in order to gain insight into its pharmacological profile. Compound 8c induced apoptosis through increasing caspase 3/7 activity by about 5-fold at 15 µM concentration using human cancer A-549 cell line. In addition, it displayed an increase in the G1 phase and a decrease in the S and G2/M phases in the cell cycle effects assay and it showed IC50 value of 9.5 µM against resistant NCI-H69AR cancer cell lines. In vitro metabolic profiling of compound 8c predicted its possible metabolites. Overall, the current study demonstrated that the new chemical entity 8c might be harnessed for cancer therapy after integration of the required preclinical studies.
Supporting materials
The details of the experimental methods that were adopted for the pharmacological investigations of the prepared compounds, the protocols that were used for metabolic studies and representative NMR (1H and 13C) spectra of the target compounds are provided as Supplementary materials.
Acknowledgments
This project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award Number 11-MED1924-02.
Disclosure
The authors report no conflicts of interest in this work.
References
- BérubéGAn overview of molecular hybrids in drug discoveryExpert Opin Drug Discov2016113 281 30526727036
- FortinSBérubéGAdvances in the development of hybrid anticancer drugsExpert Opin Drug Discov201388 1029 104723646979
- Viegas-JuniorCDanuelloAda Silva BolzaniVBarreiroEJFragaCAMolecular hybridization: a useful tool in the design of new drug prototypesCurr Med Chem20071417 1829 185217627520
- MeunierBHybrid molecules with a dual mode of action: dream or reality?Acc Chem Res2008411 69 7717665872
- GloverVHalketJMWatkinsPJClowAGoodwinBLSandlerMIsatin: identity with the purified endogenous monoamine oxidase inhibitor tribulinJ Neurochem1988512 656 6593392550
- VineKLMatesicLLockeJMRansonMSkropetaDCytotoxic and anticancer activities of isatin and its derivatives: a comprehensive review from 2000–2008Anticancer Agents Med Chem200994 397 41419442041
- SunLLiangCShirazianSDiscovery of 5-[5-fluoro-2-oxo-1,2-dihydroindol-(3Z)-ylidenemethyl]-2,4-dimethyl-1H-pyrrole-3-carboxylic acid (2-diethylaminoethyl)amide, a novel tyrosine kinase inhibitor targeting vascular endothelial and platelet-derived growth factor receptor tyrosine kinaseJ Med Chem2003467 1116 111912646019
- PrenenHCoolsJMentensNEfficacy of the kinase inhibitor SU11248 against gastrointestinal stromal tumor mutants refractory to imatinib mesylateClin Cancer Res2006128 2622 262716638875
- MotzerRJMichaelsonMDRedmanBGActivity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinomaJ Clin Oncol2006241 16 2416330672
- McCormackPLNintedanib: first global approvalDrugs2015751 129 13925430078
- RothGJHeckelAColbatzkyFDesign, synthesis, and evaluation of indolinones as triple angiokinase inhibitors and the discovery of a highly specific 6-methoxycarbonyl-substituted indolinone (BIBF 1120)J Med Chem20095214 4466 448019522465
- KrugMHilgerothARecent advances in the development of multi-kinase inhibitorsMini Rev Med Chem2008813 1312 132718991750
- IbrahimHSAbou-SeriSMAbdel-AzizHA3-Hydrazinoindolin-2-one derivatives: chemical classification and investigation of their targets as anticancer agentsEur J Med Chem2016122 366 38127391135
- Abdel-AzizHAElsamanTAl-DhfyanAAttiaMIAl-RashoodKAAl-ObaidARSynthesis and anticancer potential of certain novel 2-oxo-N′-(2-oxoindolin-3-ylidene)-2H-chromene-3-carbohydrazidesEur J Med Chem201370 358 36324177362
- EldehnaWMAltoukhyAMahrousHAbdel-AzizHADesign, synthesis and QSAR study of certain isatin-pyridine hybrids as potential anti-proliferative agentsEur J Med Chem201590 684 69425499988
- HassanTAKadiAAAbdel-AzizHANovel N, N′-hydrazino-bis-isatin derivatives with selective activity against multidrug-resistant cancer cellsUnited States Patent USUS201202528602012
- IbrahimHSAbou-seriSMIsmailNSElaasserMMAlyMHAbdel-AzizHABis-isatin hydrazones with novel linkers: synthesis and biological evaluation as cytotoxic agentsEur J Med Chem2016108 415 42226706352
- GudipatiRAnreddyRNMandaSSynthesis, anticancer and antioxidant activities of some novel N-(benzo[d]oxazol-2-yl)-2-(7-or 5-substituted-2-oxoindolin-3-ylidene)-hydrazinecarboxamide derivativesJ Enzyme Inhib Med Chem2011266 813 81821476831
- TaherATKhalilNAAhmedEMSynthesis of novel isatin-thiazoline and isatin-benzimidazole conjugates as anti-breast cancer agentsArch Pharm Res20113410 1615 162122076761
- SolomonVRHuCLeeHHybrid pharmacophore design and synthesis of isatin–benzothiazole analogs for their anti-breast cancer activityBioorg Med Chem20091721 7585 759219804979
- HavrylyukDKovachNZimenkovskyBVasylenkoOLesykRSynthesis and anticancer activity of isatin-based pyrazolines and thiazolidines conjugatesArch Pharm (Weinheim)20113448 514 52221681810
- RamshidPKJagadeeshanSKrishnanAMathewMNairSAPillaiMRSynthesis and in vitro evaluation of some isatin-thiazolidinone hybrid analogues as anti-proliferative agentsMed Chem201065 306 31221073435
- KaminskyyDKhylukDVasylenkoOZaprutkoLLeskyRA facile synthesis and anticancer activity evaluation of spiro [thiazolidinone-isatin] conjugatesSci Pharm2011794 763 77722145104
- HavrylyukDZimenkovskyBVasylenkoOGzellaALesykRSynthesis of new 4-thiazolidinone-, pyrazoline-, and isatin-based conjugates with promising antitumor activityJ Med Chem20125520 8630 864122992049
- WangSZhaoYZhangGLvYZhangNGongPDesign, synthesis and biological evaluation of novel 4-thiazolidinones containing indolin-2-one moiety as potential antitumor agentEur J Med Chem2011468 3509 351821621880
- SolomonVRHuCLeeHDesign and synthesis of anti-breast cancer agents from 4-piperazinylquinoline: a hybrid pharmacophore approachBioorg Med Chem2010184 1563 157220106668
- SharmaMSharmaSBuddhirajaASaxenaAKNepaliKBediPMSSynthesis and cytotoxicity studies of 3, 5-diaryl N-acetyl pyrazolineisatin hybridsMed Chem Res20142310 4337 4344
- CohenMHJohnsonJRChenYFSridharaRPazdurRFDA drug approval summary: erlotinib (Tarceva) tabletsOncologist2005107 461 46616079312
- ThorntonKKimGMaherVEVandetanib for the treatment of symptomatic or progressive medullary thyroid cancer in patients with unresectable locally advanced or metastatic disease: US Food and Drug Administration drug approval summaryClin Cancer Res20121814 3722 373022665903
- BoldGAltmannKHFreiJNew anilinophthalazines as potent and orally well absorbed inhibitors of the VEGF receptor tyrosine kinases useful as antagonists of tumor-driven angiogenesisJ Med Chem20004312 2310 232310882357
- DumasJDixonJAVEGF receptor kinase inhibitors: phthalazines, anthranilamides and related structuresExpert Opin Ther Pat2005156 647 65820141503
- DeeksEDOlaparib: first global approvalDrugs2015752 231 24025616434
- PinheiroACMendonça NogueiraTCde SouzaMVQuinoxaline nucleus: a promising scaffold in anti-cancer drug discoveryAnticancer Agents Med Chem20161610 1339 135227349448
- WuWYCaoSLMaoBBSynthesis and antiproliferative evaluation of hybrids of indolin-2-one and quinazoline-4-(3H)-one linked via imine bondLett Drug Des Discov2013101 61 66
- FaresMEldehnaWMAbou-SeriSMAbdel-AzizHAAlyMHTolbaMFDesign, synthesis and in vitro antiproliferative activity of novel isatin-quinazoline hybridsArch Pharm (Weinheim)20153482 144 15425664631
- EldehnaWMFaresMCerusoMAmido/ureido substituted benzenesulfonamides-isatin conjugates as low nanomolar/subnanomo-lar inhibitors of the tumor-associated carbonic anhydrase isoform XIIEur J Med Chem2016110 259 26626840366
- HeJWangXZhaoXLiangYHeHFuLSynthesis and antitumor activity of novel quinazoline derivatives containing thiosemicarbazide moietyEur J Med Chem201254 925 93022749192
- El-FekySBayoumyBECyclocondensation of 4-benzyl-1-hydrazinophthalazineJ Prakt Chem19903326 1041 1048
- MakinoKSakataGMorimotoKOchiaiYA facile synthesis of novel tricyclic compounds, tetrazoloquinoxalines and 1, 2, 4-triazoloquinox-alinesHeterocycles1985238 2025 2034
- GillHJTingleMDParkBKN-Hydroxylation of dapsone by multiple enzymes of cytochrome P450: implications for inhibition of haemo-toxicityBr J Clin Pharmacol1995406 531 5388703658
- LowryOHRosebroughNJFarrALRandallRJProtein measurement with the Folin phenol reagentJ Biol Chem19511931 265 27514907713
- BillingsRESex differences in rats in the metabolism of phenytoin to 5-(3, 4-dihydroxyphenyl)-5-phenylhydantoinJ Pharmacol Exp Ther19832253 630 6366864524
- QuaroniAWandsJTrelstadRLIsselbacherKJEpithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteriaJ Cell Biol1979802 248 26588453
- SouleHDMaloneyTMWolmanSRIsolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10Cancer Res19905018 6075 60861975513
- TodaroGJGreenHQuantitative studies of the growth of mouse embryo cells in culture and their development into established linesJ Cell Biol196317 299 31313985244
- KumarGNSurapaneniSRole of drug metabolism in drug discovery and developmentMed Res Rev2001215 397 41111579440
- CaldwellGWCompound optimization in early- and late-phase drug discovery: acceptable pharmacokinetic properties utilizing combined physicochemical, in vitro and in vivo screensCurr Opin Drug Discov Devel200031 30 41
- KennedyTManaging the drug discovery/development interfaceDrug Discov Today1997210 436 444