Abstract
Endovascular aortic repair (EVAR) is often followed by aneurysm recurrence. Alginate oligosaccharide (AOS) has potential antitumor properties as a natural product while the related mechanisms remain unclear. Toll-like receptor (TLR) signaling is associated with inflammatory activity of aneurysm and may be affected by miR-29b. Thus, inhibitory function of AOS on aneurysms was explored by measuring the important molecules in TLR4 signaling. After EVAR, a total of 248 aortic aneurysm patients were recruited and randomly assigned into two groups: AOS group (AG, oral administration 10-mg AOS daily) and control group (CG, placebo daily). The size of residual aneurysms, aneurysm recurrence, and side effects were investigated. Aneurysm recurrence was determined by Kaplan–Meier analysis. After 2 years, eight and two patients died in the CG and AG, respectively. The sizes of residual aneurysms were significantly larger in the CG than in the AG (P<0.05). The incidence of aneurysm recurrence was also significantly higher in the CG than in the AG (P<0.05). AOS treatment reduced the levels of miR-29b, TLR4, mitogen-activated protein kinase (MAPK), nuclear factor kappa B (NF-kappa B), interleukin 1 (IL-1) beta, and interleukin 6 (IL-6). Overexpression and silence of miR-29b increased and reduced the level of TLR4, phospho-p65 NF-kappa B, phospho-p38 MAPK, IL-1 beta, and IL-6. Spearman’s rank correlation analysis shows that the level of miR-29b is positively related to the levels of TLR4, NF-kappa B, IL-1 beta, and IL-6 (P<0.05). Thus, AOS represses aneurysm recurrence by indirectly affecting TLR signaling via miR-29b.
Introduction
Aortic aneurysm (AA) is a major and emerging threat to public health.Citation1 Endovascular aortic repair (EVAR) has been developed as a minimally invasive therapy. Although there are some advantages for EVAR, the technique still has some shortcomings: the residual aneurysm often regrows following EVAR and is an important risk factor for the surgery failure;Citation2 High-rate aneurysm recurrence has been widely reported in EVAR.Citation3 Stent-assisted coil embolization for large or giant aneurysms has high recurrence rates, which may be associated with metal coverage rates of the stents used in these procedures.Citation4 More than 17% patients had aneurysm recurrence and 13% patients had residual aneurysms after 2-year segmental artery coil embolization.Citation5
In most cases, medical therapy is often considered to control the disease before and after aneurysm therapy.Citation6 EVAR infection is usually followed by the technique and will result in a high risk of aneurysm recurrence.Citation7 Persistent endoleak is also a main side effect after EVAR.Citation8 All the adverse effects significantly affect life quality of AA patients. Most research shows that oxidative stressCitation9–Citation12 and inflammationCitation13–Citation16 are associated with the risks of various cancers. Marine algae have been found to be with various bioactive compounds, such as alginate oligosaccharide (AOS). AOS has been reported to have antioxidantCitation17,Citation18 and anti-inflammatory properties.Citation19 AOS has been found to have antitumor activities.Citation20 Furthermore, AOS has few side effects as a kind of natural product. AOS may offer novel anticancer agents for various cancers.Citation21
However, the effects of AOS on aneurysm recurrence and regrowth, and related molecular mechanisms remain unclear. Toll-like receptor (TLR) is an important protein in various innate immunity and inflammatory activities. Previous study demonstrates that the silence of TLR4 signaling pathway inhibits the progression of abdominal aortic aneurysm (AAA).Citation22 miRNA, as a small noncoding RNA, is involved with various biological activities, including cell development,Citation23,Citation24 homeostasis,Citation25 immune,Citation26 and inflammatory responses.Citation27,Citation28 miRNAs have been reported to be important biomarkers for vascular diseases, while they have been considered as potential targets for exploiting therapeutic approaches for the disease.Citation29 Therefore, miRNA is an important regulator for controlling immune and inflammatory responses to tumor progression.Citation30 Present evidence shows therapeutic manipulation of miR-29b, which holds a great promise for controlling AAA development.Citation30 Therefore, we aim to explore the functional role of AOS in AAs’ regression by exploring its effects on TLR4 signaling and miR-29b. To understand safety and efficacy of AOS, the size of residual aneurysms, aneurysm recurrence, and the side effects were also investigated.
Methods
AOS prepared from alginate sodium
The AOS with α-L-guluronate units and β-D-mannuronate units was bought from Qingdao Qingya Chemical Co., Ltd (Qingdao, China). AOS was prepared from alginate sodium according to an earlier report using alginate lyase,Citation31 which depolymerizes alginate sodium. Briefly, 1 kg sodium alginate was depolymerized in 100 L tap water with 10 mg alginate lyase at 39°C for 2 h. The lyase was denatured at 100°C for 10 min. The amounts of unsaturated saccharides were measured at 234 nm in a UV-2100PC UV-VIS spectrophotometer (Shimadzu, Kyoto, Japan). The degrees of polymers (DP) were further determined by electrospray ionization mass spectrometry (ESI-MS). The hydrolysate was transferred to a carbograph column to remove salt, and then concentrated, dried, and resolved in 1 mL methanol. Two-microliter supernatant was injected into a LTQ XL mass spectrometer (ThermoFinnigan, Austin, TX, USA). AOS was detected in a positive-ion mode: ion source, 5 kV; capillary temperature, 280°C–310°C; tube lens, 260 V; sheath gas, 35 arbitrary units. The mass spectrometer was set over the range m/z 500–1,500.
Participants
All the procedures were approved by the Ethical Committee of the Second People’s Hospital of Yunnan Province and written informed consent was obtained from each patient for this study. The study was performed according to the Declaration of Helsinki.Citation32 A total of 397 patients, experiencing severe chest and back pain, attended the Second People’s Hospital of Yunnan Province during the period from January 2013 to December 2014.
The diagnosis of thoracic aortic aneurysm (TAA) and dissection was confirmed from clinical diagnostic criteria issued by a previous report.Citation33 One of the following including criteria were considered: the diameter of aneurysm was more than 5 cm; the diameter of aneurysm was 4–5 cm and increased in size by 0.5 cm in less than half a year; the diameter of aneurysm was twofold the diameter of the normal infrarenal aorta; sudden onset of severe chest pain that had a tearing or ripping quality (classic symptom); anterior chest pain, associated with anterior arch or aortic root dissection; neck or jaw pain, with aortic arch involvement and extension into the great vessels; tearing or ripping intrascapular pain, involving the descending aorta; cerebrovascular accident symptoms, hemianesthesia, hemiparesis, and hemiplegia. The following excluding criteria were used: a family history of AA and dissection; malignancy; psychological disorders.
A computed tomography (CT) scan was performed and the selected patients were revealed with TAA and/or dissection. Finally, 248 patients were selected for the present study and received modified EVAR. The Zenith TX2 TAA Endovascular Graft with Pro-Form is a two-piece cylindrical endovascular graft with proximal and distal materials (COOK Medical Inc., Bloomington, IN, USA). The proximal parts can be either nontapered or tapered. The stent grafts are made of full-thickness woven polyester fabric sewn to steel Cook-Z stents with polyester and monofilament polypropylene. The Zenith TX2 TAA endovascular graft with ProForm is fully stented to provide stability and the expansible force to open the lumen of the graft during deployment. All endovascular grafts with Pro-Form are two cylindrical endovascular grafts with proximal and distal materials. The proximal parts can be either nontapered or tapered. The diameter and length of each stent-graft was identified preoperatively with use of multislice CT or diagnostic imaging via a catheter with a marker. The graft diameter was >10% than normal size associated with the diameter of the nondiseased segments of aorta under the aneurysm. The length of graft was equal to the distance from the transection site to the nondiseased size of the descending aorta below the aneurysm.
After surgery, all the patients were randomly and evenly assigned into two groups: AOS group (AG), 124 AA patients received 10 mg AOS daily and control group (CG), 124 AA patients received a 10 mg placebo daily.
End points
Outcome criteria were established according to a previous report for thoracic EVAR.Citation34 The end points included early morbidities (stroke and paraplegia), respiratory failure, acute kidney injury, need for dialysis, aneurysm-related death, and reoperation. The proximal attachment zones were determined based on the proximal attachment site of the proximal edge of the covered graft; <2 cm of the left subclavian artery (without covering it) is defined as zone 3; it is defined as zone 4 if the proximal extent of the endograft is >2 cm to the left subclavian artery and ended within the proximal half of the TAA (T6 level is near the midpoint of TAA).Citation34 Aneurysm-related death would be measured at any time. Respiratory failure was determined if mechanical ventilation was more than 1 day and reintubation and tracheostomy would be needed.
Clinical follow-up
Detailed data were collected using the index admission when surgery was conducted. After discharge, all the patients were followed up at 1, 3, 6, and 12 months and yearly at outpatient clinics. In addition, a CT angiogram was performed once a year for 2 years.
The measurement of AA size
AA size was measured by using an ultrasound scanning system (Xuzhou Forward Medical Instrument Co., Limited, Xuzhou, China). The ultrasound was sensitive and specific. Maximum transverse and anterior-posterior external diameters were measured in infrarenal aorta, which is perpendicular to aortic axis.
Enzyme-linked immunosorbent assay analysis
Five-milliliter blood was obtained by vein puncture. Serum was separated by using the centrifugation at 1,500 g, 4°C for 10 min. The concentration of tumor necrosis factor (TNF)-alpha was measured by using a human TNF-alpha enzyme-linked immunosorbent assay (ELISA) kit (Thermo Fisher Scientific, Inc. Corporate, Waltham, MA, USA). The concentration of TLR4 was measured by human TLR4 ELISA Kit from RayBiotech (Norcross, GA, USA). The levels of interleukin 1 (IL-1) beta and IL-6 were measured by the ELISA kits from R&D Systems (Minneapolis, MN, USA). All the concentrations were calculated according to standard calibrating curves.
The effects of AOS on the viability of endothelial cells
Endothelial cells (ECs), isolated from human aneurysm, were purchased from Shanghai Cell Bank (Shanghai, China) and cultured in DMEM at 37°C under a 5% CO2 environment. The cell concentrations were adjusted to 1×105/cell in a 96-cell plate. Cell viability of ECs was determined using Trypan blue (Solarbio, Beijing, China) and [3H]thymidine (Amersham biosciences, Piscataway, NJ, USA, specific activity 5 Ci/mmol) uptake after the cells were cultured with different concentrations of AOS (0, 1, 10, 100, and 1,000 ng/mL) for 3 days. The Trypan blue-positive cells were measured with a hemocytometer under a microscope. For the [3H] thymidine absorption, cells were counted after exposure to different concentrations of AOS, and 200 μL cell suspension of 1.0×105 cells/mL for each condition were added to each well of a 96-well plate. After 24-h culture, cells were labeled with one Ci/well of [3H]thymidine for 4 h, then collected with an automated sample harvester and measured with a liquid scintillation counter (Beckman Coulter, Brea, CA, USA).
Cell co-infection
Antagomirs and mimics of miR-29b were synthesized by Sangon Biotech (Shanghai) Co. Ltd (Shanghai, China). The cell lines were co-infected with the antagomirs and mimics via Lipofectamine 3000 (Life Technologies, Inc., Carlsbad, CA, USA). After 3-day co-infection, the mutant cells were picked by using 200 μg/mL G-418. TLR4, nuclear factor kappa-B (NF-kappa B), IL-1 beta, and IL-6 were measured by ELISA in the cell lines. Meanwhile, cell viability was also measured using Trypan blue and [3H]thymidine uptake.
Real-time polymerase chain reaction quantification of miR-29b
Total cellular RNA was isolated using an RNA Isolation Kit (Tiangen, Beijing, China), RNeasy Mini kit (QIA-GEN, Valencia, CA, USA). cDNA was synthesized from total RNA by reverse transcription using TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). The levels of miR-29b were measured by using real-time polymerase chain reaction (Bio-Rad Lab., Hercules, CA, USA). MiR-29b, forward primer: 5′-GGCTTCAGGAAGCTGGTTT-3′; reverse primer: 5′-GTGCAGGGTCCGAGGT-3′. U6, forward primer: 5′-TTGGTGCTCGCTTCGGCA-3′; reverse primer: 5′-GTGCAGGGTCCGAGGT-3′. U6 snRNA was used as an internal control.
Western blot
TLR4 can regulate TLR inflammatory response via phospho-p65 NF-kappa B p65.Citation35 Mitogen-activated protein kinase (MAPK) as regulatory signal mediators plays a pivotal role in organism immunity.Citation36 To understand the effects of miR-29b on TLR4 signaling, phosphorylated MAPK and NF-kappa B as second messengers in TLR4 signalingCitation37 were measured by Western blot. The antihuman antibodies of TLR4 (ab17942), Phospho-p65 anti-NF-kappa B (ab86299), phospho-p38 mitogen-activated protein kinase (MAPK, ab31828), IL-1 beta (ab2105), IL-6 (ab6672), β-actin antibody (ab8227), and secondary antibody Goat Anti-Rabbit IgG H&L (HRP, ab6721) were purchased from Abcam (Beijing, China). Proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA). The membranes were blocked with 5% skim milk in the buffer (10 mM Tris [pH 7.5] and 50 mM NaCl) and incubated with primary antibodies overnight at 4°C. The membrane was further treated with secondary antibodies (1:3,000). Immunoreactive bands were visualized by enhanced chemiluminescence system (Amersham, Arlington Heights, IL, USA).
Statistical analysis
All data were presented as mean ± SD or frequency and percentage. The variables were compared by the t-test, χ2, or Fisher’s exact test. Early outcomes and late outcomes were compared using univariate statistics. Overall survival and being free from aneurysm-related death and reintervention were measured using the Kaplan–Meier and the log-rank test. Spearman’s rank correlation coefficient was calculated to confirm the strength of correlation between relative levels of miR-29b and the levels of TLR4, TNF-alpha, IL-1 beta, and IL-6. Statistical calculation was conducted via SPSS 20 (SPSS Inc., Chicago, IL, USA). There were significantly statistical differences if P-values were <0.05.
Results
Characterization of AOS
Four main components (DP3, DP4, DP5, and DP6) were isolated from alginate sodium after enzyme digestion. The isolated fractions of AOS were further confirmed by ESI-MS under the conditions that produced mass spectra with [M + K]+. The predicted masses DP3 (C18H23O19Na3), DP4 (C24H30O25Na4), DP5 (C30H37O31Na5), and DP6 (C36H44O37Na6) were 612, 810, 1,008, and 1,206 Da, respectively ().
Figure 1 Electrospray ionization mass spectrometry analysis of the DP of digested AOS from alginate sodium with produced mass spectra as [M + K]+.
Abbreviations: AOS, alginate oligosaccharide; DP, degree of polymerization.
![Figure 1 Electrospray ionization mass spectrometry analysis of the DP of digested AOS from alginate sodium with produced mass spectra as [M + K]+.](/cms/asset/e7f62361-7f0b-486c-8108-05effb042a6f/dddt_a_140206_f0001_b.jpg)
The baseline characters of the patients with TAA and dissection
After selection, a total of 248 patients with TAA received the treatment with modified EVAR. After 2-year therapies of AOS and placebo, eight patients died in the CG and two patients died in the AG. Thus, 116 patients and 122 patients finished the experiments of CG and AG, respectively (). The baseline characters of all patients are listed in . The number of men was more than women. Most patients had concomitant diseases, such as cerebrovascular, ischemic heart, chronic obstructive pulmonary, and renal dysfunction. The ages (65.8±14.9 years in AG group vs 62.1±15.3 years in AG, P=0.48) and renal insufficiency (33.1% in CG vs 30.6% in AG; P=0.68) were similar in both groups. The incidences of dissection aneurysm, the proportion of aortic rupture, the maximal diameters of the aneurysms, and all other parameters were also similar between two groups (P>0.05) ().
Table 1 Baseline characteristics of aortic aneurysm patients
Figure 2 Present study flowchart.
Abbreviations: AA, aortic aneurysm; CG, control group; AOS, alginate oligosaccharide; AG, AOS group; TLR4, toll-like receptor 4; NF-kappa B, nuclear factor kappa B.
Primary outcomes of EVAR
CT of the thoracic aorta showed that it could be observed easily before operation. Deployment of multilayer stent was successfully established in the thoracic aorta after operation. Three-dimensional reconstruction based on a series of angiography images showed TAA bulging in the T2–T5 segment. The images showed the surgery was successful and no endoleak was found. Comparatively, emergency CT showed AAA bulging before surgery. Finally, graft implantation and artificial vessel were successfully placed in AAs.
End points
The average follow-up duration was 24±5 months (see ). Overall, survival was 93.5% and 98.4% in the CG and AG, respectively (P=0.05) (). More patients underwent reintervention in the CG than in the AG (P<0.05). Comparatively, the Kaplan–Meier analysis also revealed that being free from reintervention was significantly higher in the CG than in the AG during the same period (log rank test, P=0.04). There were 16 patients in the CG and 6 patients in the AG with aortic reintervention, including endoleak and retrograde dissection in the 2-year follow-up.
Table 2 Late outcomes (n=124 in each group before repair)
The covered stent grafts were delivered successfully, and complete thrombosis of the aneurysm or entry closure with stable thrombosis was observed in all patients. According to Kaplan–Meier analysis, the patient survival rates were 93.5% in the CG and 98.4% in the AG at 2-year postoperation. During the 2-year follow-up, 10 patients died. Among those, three patients died of aortic reintervention and seven patients died of endoleak and retrograde dissection.
Side effects
EVAR-related adverse effects were pain (back or chest), fainting, persistent cough, rapid heartbeat, dizziness, sudden weakness, ischemia of intestines, pulse-less legs, cold arms or legs, and so on (). Among these adverse effects, back and chest pain rate, and the rate of persistent cough and wound infection were higher in the AG than in the CG (P<0.05). Endovascular therapy has excellent short-term effect and stable mid-to-long-term result. AOS can be used as a therapy option such as in an emergency situation or a rescue method by reducing the side effects caused by EVAR.
Table 3 The side effects between CG and AG, n (%)
AOS reduces AA size
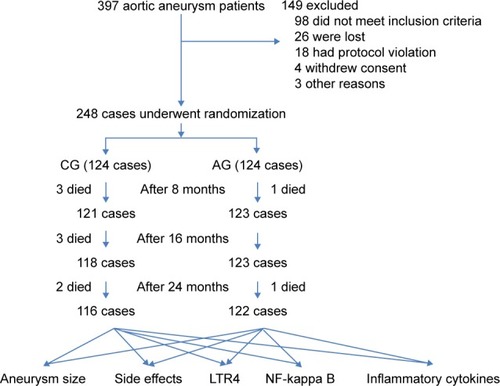
EVAR surgery reduced the AA size after 2-year follow-up in comparison with the size before surgery in the CG and the AG (). Before AOS treatment, there was no significantly statistical difference for AA size between the AG and the CG (P>0.05). Compared with CG, AOS significantly reduced AA size after 2-year repair (P<0.01) (). The results suggest that long-term AOS consumption will further control the growth of residual aneurysms.
Figure 3 Scatter dot plot of the AA size variances in different groups.
Abbreviations: AA, aortic aneurysm; AG, AOS group; AOS, alginate oligosaccharide; CG, control group; EVAR, endovascular aortic repair.
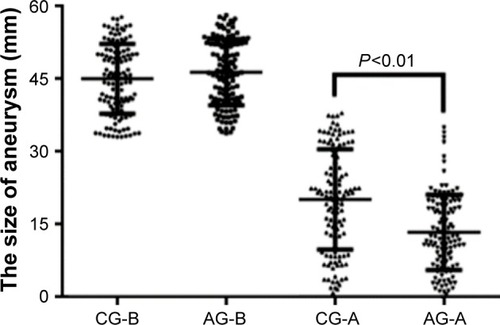
AOS reduces the levels of miR-29b in AA patients
Before AOS treatment, there was no significantly statistical difference for relative level of miR-29b between the AG and the CG (P>0.05). After long-term consumption of AOS, it significantly reduced the relative level of miR-29b (P<0.05) (). The results suggest that long-term AOS consumption will reduce the relative level of miR-29b.
Figure 4 Relative levels of miR-29b in different groups.
Abbreviations: AA, aortic aneurysm; AG, AOS group; AOS, alginate oligosaccharide; CG, control group; EVAR, endovascular aortic repair.
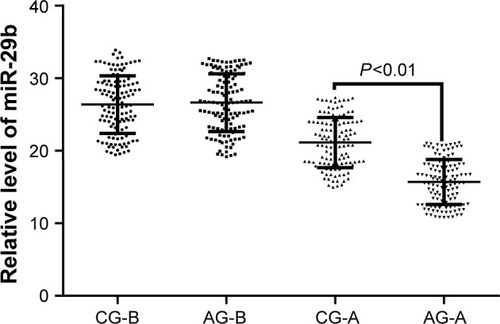
AOS decreases the levels of TLR4, NF-kappa B, IL-1 beta, and IL-6
Before EVAR and AOS treatment, there was no significantly statistical difference for serum levels of TLR4 (), NF-kappa B (), and IL-1 beta () (P>0.05). After 2-year follow-up, there was a greater reduction in the serum levels of TLR4 (), NF-kappa B (), and IL-1 beta () in the AG when compared with the CG (P<0.05). Before EVAR and AOS treatment, serum levels of IL-6 were lower in the CG than the AG () (P<0.05). After 2-year follow-up, there was a greater reduction in the serum levels of IL-6 in the AG () when compared with the CG (P<0.05). The results suggest that long-term AOS consumption decreases the levels of TLR4, NF-kappa B, IL-1 beta, and IL-6.
Figure 5 The effects of AOS on the levels of TLR4, NF-kappa B, IL-1 beta, and IL-6 in AA patients.
Abbreviations: AA, aortic aneurysm; AG, AOS group; AOS, alginate oligosaccharide; CG, control group; IL-6, interleukin 6; TLR, toll-like receptor; NF-kappa B, nuclear factor kappa B.
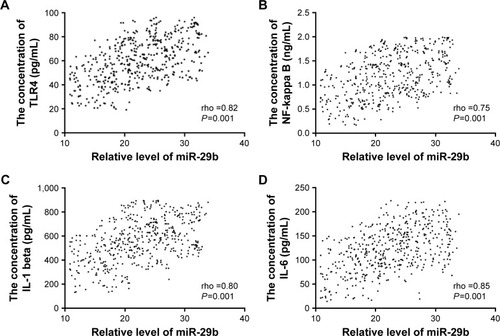
The levels of miR-29b are positively associated with the serum levels of TLR4, NF-kappa B, IL-1 beta, and IL-6
shows that the increase in miR-29b also increases the levels of TLR4, NF-kappa B, IL-1 beta, and IL-6. Spearman’s rank correlation test demonstrates that the levels of miR-29b are positively associated with the serum levels of TLR4 (), NF-kappa B (), IL-1 beta (), and IL-6 () (P<0.05) because the rho values are 0.82, 0.75, 0.80, and 0.85, respectively.
Figure 6 The relationship between the level of miR-29b and the levels of TLR4, NF-kappa B, IL-1 beta, and IL-6.
Abbreviations: IL-6, interleukin 6; NF-kappa B, nuclear factor kappa B; TLR, toll-like receptor.
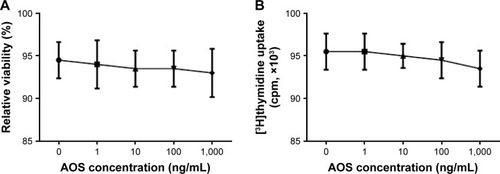
Effects of AOS toxicity on ECs
To avoid the effects of AOS on ECs because of its toxicity, the concentration of AOS was measured. Trypan blue analysis showed that exposure of the ECs to AOS, in the presence of more than 100 ng/mL, resulted in loss of cell viability (; P<0.05). The viability of cells was still more than 95% after 72-h culture when the concentration of AOS was 1,000 ng/mL. The toxicity of AOS was also evaluated by measuring [3H]thymidine incorporation into DNA after exposure to different concentrations of AOS. As shown in , there is no significant reduction in [3H]thymidine uptake that was measured when the concentration was more than 1,000 ng/mL (P<0.05). Based on these results, administration of 10 mg daily will not cause toxicity to ECs.
Figure 7 Effects of AOS on the viability of ECs.
Abbreviations: AOS, alginate oligosaccharide; ECs, endothelial cells.
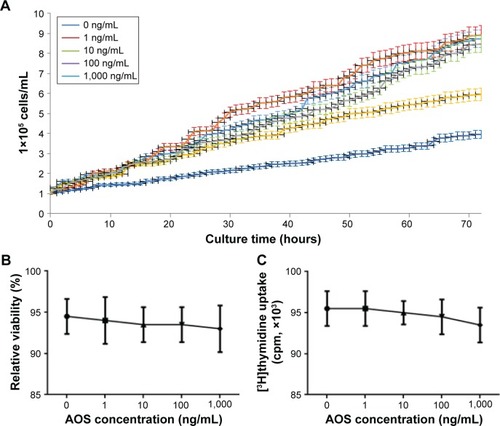
AOS inhibits the growth of ECs but not their viabilities
ECs were isolated from human aneurysm. shows that AOS inhibits the growth of ECs when its concentration is more than 100 ng/mL. Based on the results, 10 mg/day was used for all patients (the weight of each patient was <100 kg). Cell viability analysis shows that AOS cannot affect cell viability by using Trypan Blue () and [3H]thymidine incorporation () even when the concentration is 1,000 ng/mL.
Figure 8 The effects of AOS on the growth of ECs.
Abbreviations: AOS, alginate oligosaccharide; ECs, endothelial cells.
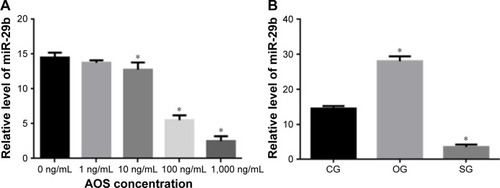
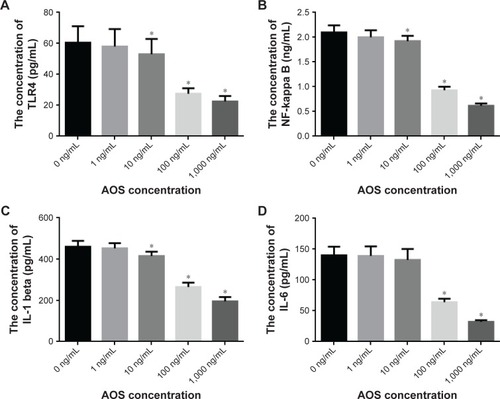
AOS treatment reduces relative levels of miR-29b
showed that AOS could not affect the relative levels of miR-29b when the concentration was <10 ng/mL. AOS reduced the relative levels of miR-29b when the concentration was more than 10 ng/mL and reached the lowest level when the concentration was 1,000 ng/mL. showed that the relative levels of miR-29b reached the lowest level when the microRNA was blocked, and reached the highest level when the microRNA was overexpressed.
Figure 9 Relative levels of miR-29b in different groups.
Abbreviations: AOS, alginate oligosaccharide; CG, control group; SG, miR-29b was silenced by antagomirs; OG, miR-29b was overexpressed.
AOS treatment reduces the levels of TLR4, NF-kappa B, IL-1 beta, and IL-6 in ECs
showed that AOS could not affect the levels of TLR4 when the concentration was <10 ng/mL. AOS reduced the levels of TLR4 when the concentration was more than 10 ng/mL and reached the lowest level when the concentration was 1,000 ng/mL. showed that AOS could not affect the levels of NF-kappa B when the concentration was <10 ng/mL. AOS reduced the levels of NF-kappa B when the concentration was more than 10 ng/mL and reached the lowest level when the concentration was 1,000 ng/mL. showed that AOS could not affect the levels of IL-1 beta when the concentration was <10 ng/mL. AOS reduced the levels of IL-1 beta when the concentration was more than 10 ng/mL and reached the lowest level when the concentration was 1,000 ng/mL. showed that AOS could not affect the levels of IL-6 when the concentration was <100 ng/mL. AOS reduced the levels of IL-6 when the concentration was more than 100 ng/mL and reached the lowest level when the concentration was 1,000 ng/mL. All the results suggest that AOS treatment reduces the levels of TLR4, NF-kappa B, IL-1 beta, and IL-6 in ECs. The results can be compared with the levels obtained from the serum in patients.
Figure 10 The effects of AOS on the levels of TLR4, NF-kappa B, IL-1 beta, and IL-6 in ECs.
Abbreviations: AOS, alginate oligosaccharide; CG, control group; ECs, endothelial cells; IL-6, interleukin 6; NF-kappa B, nuclear factor kappa B; TLR, toll-like receptor.
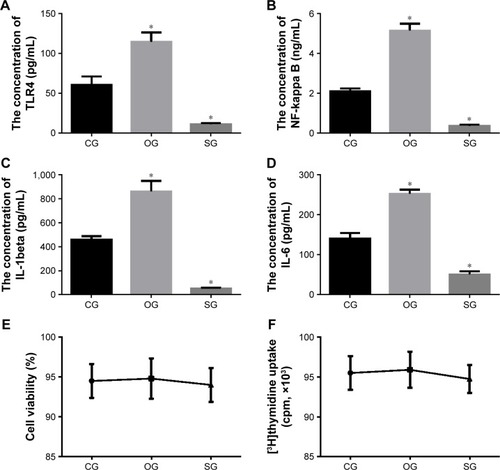
MiR-29b level is positively associated with the levels of TLR4, NF-kappa B, IL-1 beta, and IL-6 in ECs
MiR-29b mimics and antagomirs were successfully transfected with ECs and the estimated transfection rate was between 60% and 80%. showed that the overexpression of miR-29b increased the level of TLR4 and the silence of miR-29b reduced the level of TLR4. showed that the overexpression of miR-29b increased the level of NF-kappa B and the silence of miR-29b reduced the level of NF-kappa B. showed that the overexpression of miR-29b increased the level of IL-1 beta and the silence of miR-29b reduced the level of IL-1 beta. showed that the overexpression of miR-29b increased the level of IL-6 and the silence of miR-29b reduced the level of IL-6. Therefore, miR-29b level is positively associated with the levels of TLR4, NF-kappa B, IL-1 beta, and IL-6 in ECs, suggesting that the change of miR-29b will affect the levels of TLR4, NF-kappa B, IL-1 beta, and IL-6.
Figure 11 The effects of miR-29b on ECs.
Abbreviations: CG, control group; ECs, endothelial cells; IL-6, interleukin 6; NF-kappa B, nuclear factor kappa B; OG, miR-29b was overexpressed; SG, miR-29b was silenced by antagomirs; TLR, toll-like receptor.
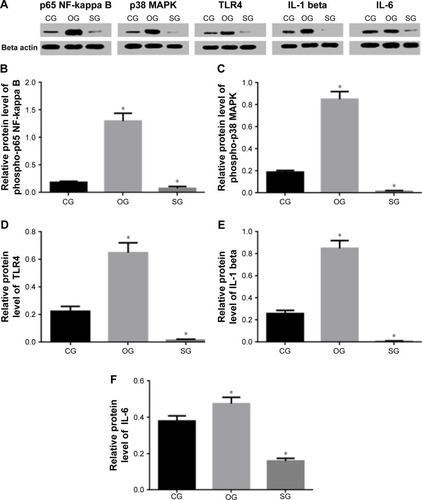
Western blot
To measure the effects of miR-29b on the changes of phospho-p65 NF-kappa B, and phospho-p38 MAPK, Western blot analysis was performed. Western blot analysis shows that miR-29b overexpression increases the levels of phospho-p65 NF-kappa B (), phospho-p38 MAPK (), TLR4 (), IL-1 beta (), and IL-6 () when compared with the level of CGs. In contrast, miR-29b inhibitor reduces the levels of phospho-p65 NF-kappa B (), phospho-p38 MAPK (), TLR4 (), IL-1 beta (), and IL-6 () when compared with the level of CGs. All the results suggest that miR-29b can affect the activity of TLR4 signaling.
Figure 12 (A–F) Western blot analysis of the effects of miR-29b on the expression of TLR4 signaling molecules.
Abbreviations: CG, control group; IL-6, interleukin 6; NF-kappa B, nuclear factor kappa B; OG, miR-29b was overexpressed; SG, miR-29b was silenced by antagomirs; TLR, toll-like receptor.
Discussion
This was a retrospective research, and the efficacy and suitability of AOS were assessed in AA therapy. Based on 248 patients who underwent EVAR, there was no significant difference for early mortality and neurologic complications between the CG and AG. According to late outcomes, the overall survival rate was higher in the AG than in the CG (P<0.05). The results suggest that AOS contributes to AA repair. The reintervention rate and continued presence of complications were higher in the CG than in the AG (P<0.05). There were significant differences for being free from aneurysm-related death after two-year therapy between the groups (P<0.05).
AA therapy is still a challenge although a less invasive method can be commercially available for treating AA. Since introduction of EVAR, it is a safe and feasible alternative to conventional open repair. However, the surgery still has some side effects. Respiratory failure is a major complication, which affects postoperative morbidity and mortality and results in prolonged hospitalization. In addition to thoracotomy, cardiopulmonary bypass and hypothermia inducing an inflammatory reaction also attribute to postoperative respiratory failure.Citation38 Because of their less invasive methods, respiratory failure was relatively rare in EVAR. In the present study, more patients developed respiratory failure in the CG than in the AG (P<0.05). AOS reduced these side effects significantly (P<0.05).
EVAR has been quickly developed because of its improved perioperative mortality and morbidity. However, high reintervention is still linked with EVAR.Citation39 Here, a significantly lower rate of recurrent AA was associated with AOS treatment (16 patients in CG and 6 patients in AG). At cell levels, AOS is demonstrated to reduce the levels of miR-29b (), TLR4, NF-kappa B, IL-1 beta, and IL-6 (), while the overexpression and silence of miR-29b will increase and reduce the levels of TLR4, phospho-p65 NF-kappa B, phospho-p38 MAPK, IL-1 beta, and IL-6, respectively (). NF-kappa B contains sites for phosphorylation, which is important for activating TLR4 signaling pathways.Citation40 Phosphorylated MAPK also plays an important role in regulating TLR4 signaling.Citation41 The reasons for reducing recurrent AA cases may be caused by AOS, which reduced the level of serum miR-29b () and resulted in the reduction of TLR4, phospho-p65 NF-kappa B, phospho-p38 MAPK, IL-1 beta, and IL-6 (). On the other hand, there is a strong positive relationship between miR-29b and the levels of serum TLR4, NF-kappa B, IL-1 beta, and IL-6 (). These results suggest that AOS treatment reduced the level of serum TLR4, NF-kappa B, IL-1 beta, and IL-6 by reducing the level of miR-29b.
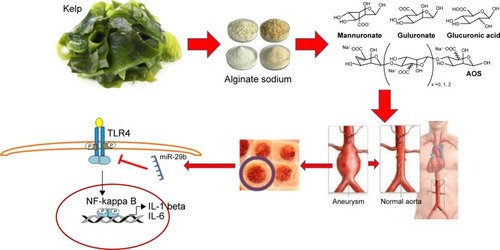
AOS treatment seemed to be associated with the reduction of long-term mortality rates. The midterm follow-up of patients revealed a significant benefit in cumulative survival rates. There were eight deaths in the CG and two deaths in the AG related to aortic disease. All these results provide a potential use of the natural products of marine algae for the therapy of AA patients (). Retrograde dissection and endoleak were significant complications, resulting in the death of patients. These significant complications contributed to an aneurysm-related death rate after EVAR. However, the late aneurysm-related deaths occurred in patients who underwent EVAR in the first 2 years at our hospital. AOS treatment improved EVAR at our institution because aneurysm-related death rates were decreased when compared with the CG. As described above, there are advantages in using the combined technique, including 1) the reduction in the incidences of AAs by AOS; 2) the reduction in infection rates; 3) the reduction in the number of deaths; 4) fewer complications such as bleeding, pneumothorax, and stroke; and 5) the reduction due to AOS in other side effects caused by repair materials.
Figure 13 A cartoon description of AOS from marine algae for the therapy of AA patients. The oligosaccharide has been found to prevent the regrowth of aneurysms by inactivating TLR4, NF-kappa B, IL-1 beta, and IL-6 via the inhibition of miR-29b.
Certainly, there were some limitations for the present work: 1) four main AOS fractions (DP3, DP4, DP5, and DP6) were isolated. We wanted to use one bioactive component for subsequent experiment. However, single component cannot be prepared at a scale suitable for a patient population at the moment. On the other hand, oligosaccharides are complex carbohydrate molecules and the purity will reflect the product quality, so the components were analyzed here. In any way, to confirm the function of specific component, further work is highly demanded in the future; 2) lack of a negative CG, such as open surgery repair; 3) the effects of AOS on most inflammatory cytokines and antioxidant enzymes were not explored here; 4) present findings demonstrate that AOS inhibits the growth of ECs, which is still difficult to be associated with the clinical use of aneurysm therapy. We aimed to control aneurysm growth by inhibiting ECs activation via AOS because the ECs were isolated from aneurysm. To further confirm the result, animal test will be needed in the future work. The epigenetic mechanism of phosphorylation of TLR4 was not studied. In any way, further studies will be required to confirm the therapeutic effects for using AOS. The prospective multicenter data are still needed to confirm the present findings.
Conclusion
Taken together, the present findings demonstrate that AOS represses the growth of residual aneurysms and reduces aneurysm recurrence by indirectly affecting TLR signaling via miR-29b. AOS treatment can be an adjuvant method for AA repair and control its subsequent development. AOS offers an adjuvant method for minimally invasive endovascular repair of AA and dissection. AOS reduced recurrent AA and its side effects, such as wound infection rates. To use AOS better, further work is still needed to be done to confirm the present therapeutic results in the future.
Acknowledgments
The work was supported by Scientific Research Projects from Internal Research Institutions of Medical and Health Units in Yunnan Province (No 2014NS048) and (2017NS137).
Disclosure
The authors report no conflicts of interest in this work.
References
- ButtHZSylviusNSalemMKMicroarray-based gene expression profiling of abdominal aortic aneurysmEur J Vasc Endovasc Surg2016521 47 5527157464
- GrayCGoodmanPO’MalleyMKO’DonohoeMKMcDonnellCOStatins promote residual aneurysm sac regression following endovascular aortic aneurysm repairVasc Endovascular Surg2014482 111 11524347280
- LeclerARaymondJRodriguez-RégentCIntracranial aneurysms: recurrences more than 10 years after endovascular treatment-A prospective cohort study, systematic review, and meta-analysisRadiology20152771 173 18026057784
- GeyikSYavuzKYurttutanNSaatciICekirgeHStent-assisted coiling in endovascular treatment of 500 consecutive cerebral aneurysms with long-term follow-upAm J Neuroradiol20133411 2157 216223886748
- ElmogySAMazroaJAEldawoodyHAFNon-invasive TOF MR angiographic follow up of coiled cerebral aneurysmsEgyptian J Radiol Nuclear Med2012431 33 40
- KooBKShimWHYoonYSEndovascular therapy combined with immunosuppressive treatment for pseudoaneurysms in patients with Behcet’s diseaseJ Endovasc Ther2003101 75 8012751935
- CapocciaLSpezialeFMennaDPreliminary Results from a National Enquiry of Infection in Abdominal Aortic Endovascular Repair (Registry of Infection in EVAR–R.I.EVAR)Ann Vasc Surg201630 198 20426408970
- MursalinRSakamotoINagayamaHImaging-based predictors of persistent type II endoleak after endovascular abdominal aortic aneurysm repairAJR Am J Roentgenol20162066 1335 134027043183
- ParkEJVenkatesanTChoiYWKimYKPinus densiflora stem bark extract induces breast cancer cell death via oxidative stress mediated lysosomal membrane permeabilizationPlanta Med201681S 01 S1 S381
- WangZLiZYeYXieLLiWOxidative stress and liver cancer: etiology and therapeutic targetsOxid Med Cell Longev20162016 789157427957239
- ManiRSAminMALiXInflammation-induced oxidative stress mediates gene fusion formation in prostate cancerCell Reports20161710 2620 263127926866
- YadavDKRaiRKumarNNew arylated benzo[h]quinolines induce anti-cancer activity by oxidative stress-mediated DNA damageSci Rep20166 3812827922047
- SuzukiYOkabayashiKHasegawaHComparison of preoperative inflammation-based prognostic scores in patients with colorectal cancerAnn Surg Epub20161216
- WheelerTMZhaoBSonpavdeGAntigen-specific immunity and tumor inflammation after vaccination with BPX-101, a drug-activated dendritic cell vaccine for metastatic castration-resistant prostate cancer (mCRPC)J Clin Oncol2011297 Suppl 176
- Reyes-GibbyCCSheteSWuXKurzrockRSpitzMInflammation genes and pain severity in lung cancer patientsJ Clin Oncol20092715 Suppl 9618
- OhBButowPMullanBRandomized clinical trial of medical qigong on quality of life, fatigue, side effects, mood, status, and inflammation of cancer patientsJ Clin Oncol20092715 Suppl 9617
- GuoJJMaLLShiHTAlginate oligosaccharide prevents acute doxorubicin cardiotoxicity by suppressing oxidative stress and endoplasmic reticulum-mediated apoptosisMar Drugs20161412 231
- TusiSKKhalajLAshabiGKiaeiMKhodagholiFAlginate oligosaccharide protects against endoplasmic reticulum- and mitochondrial- mediated apoptotic cell death and oxidative stressBiomaterials20113223 5438 545821543116
- ZhouRShiXGaoYCaiNJiangZXuXAnti-inflammatory activity of guluronate oligosaccharides obtained by oxidative degradation from alginate in lipopolysaccharide-activated murine macrophage RAW 264.7 cellsJ Agric Food Chem2015631 160 16825483391
- FujiharaMNagumoTThe effect of the content of D-mannuronic acid and L-guluronic acid blocks in alginates on antitumor activityCarbohydr Res1992224 343 3471591771
- JiJWangLCWuHLuanHMBio-function summary of marine oligosaccharidesInt J Biol201131 74
- LaiCHWangKCLeeFTToll-like receptor 4 is essential in the development of abdominal aortic aneurysmPLoS One2016111 e014656526741694
- ShangAQXieYNWangJPredicative values of serum microRNA-22 and microRNA-126 levels for non-small cell lung cancer development and metastasis: a case-control studyNeoplasma2017643 453 45928253725
- Mahmoodian SaniMRHashemzadeh-ChaleshtoriMSaidijamMJamiMSGhasemi-dehkordiPMicroRNA-183 family in inner ear: hair cell development and deafnessJ Audiol Otol2016203 131 13827942598
- ScottELoyaKMountfordJMilliganGBakerAHMicroRNA regulation of endothelial homeostasis and commitment-implications for vascular regeneration strategies using stem cell therapiesFree Radic Biol Med201364 52 6023665307
- JinCChengLHoxtermannSMicroRNA-155 is a biomarker of T-cell activation and immune dysfunction in HIV-1-infected patientsHIV Med2017185 354 36227981723
- LiJBWangHYYaoYOverexpression of microRNA-138 alleviates human coronary artery endothelial cell injury and inflammatory response by inhibiting the PI3K//Akt/eNOS pathwayJ Cell Mol Med Epub2017331
- ZhangKSongFLuXMicroRNA-322 inhibits inflammatory cytokine expression and promotes cell proliferation in LPS-stimulated murine macrophages by targeting NF-kappa B1 (p50)Biosci Rep2017371 BSR2016023927986864
- KotsinasASigalaFGarbisSDMicroRNAs determining inflammation as novel biomarkers and potential therapeutic targetsCurrent Med Chem20152222 2666 2679
- LochheadRBStrleKKimNDMicroRNA expression shows inflammatory dysregulation and tumor-like proliferative responses in joints of patients with post-infectious Lyme arthritisArthritis Rheumatol2017695 1100 111028076897
- HanWGuJChengYLiuHLiYLiFNovel alginate lyase (Aly5) from a polysaccharide-degrading marine bacterium, flammeovirga sp. strain MY04: effects of module truncation on biochemical characteristics, alginate degradation patterns, and oligosaccharide-yielding propertiesAppl Environ Microbiol2015821 364 37426519393
- StockhausenKThe Declaration of Helsinki: revising ethical research guidelines for the 21st centuryMed J Aust20001726 252 25310860086
- NienaberCAvon KodolitschYNicolasVThe diagnosis of thoracic aortic dissection by noninvasive imaging proceduresN Engl J Med19933281 1 98416265
- FillingerMFGreenbergRKMcKinseyJFChaikofELSociety for Vascular Surgery Ad Hoc Committee on TRS. Reporting standards for thoracic endovascular aortic repair (TEVAR)J Vasc Surg2010524 1022 1033 1033 e101520888533
- Sharif-AskariEVassenLKosanCZinc finger protein Gfi1 controls the endotoxin-mediated Toll-like receptor inflammatory response by antagonizing NF-κB p65Mol Cell Biol20103016 3929 394220547752
- ChangJHParkJYKimSKDependence on p38 MAPK signaling in the up-regulation of TLR2, TLR4 and TLR9 gene expression in Trichomonas vaginalis-treated HeLa cellsImmunology20061182 164 17016771851
- HugginsCPearceSPeriFNeumannFCockerillGPirianovGA novel small molecule TLR4 antagonist (IAXO-102) negatively regulates non-hematopoietic toll like receptor 4 signaling and inhibits aortic aneurysms developmentAtherosclerosis20152422 563 57026318106
- MorimotoKNishimuraKMiyasakaSMaetaHTaniguchiIThe effect of sivelestat sodium hydrate on severe respiratory failure after thoracic aortic surgery with deep hypothermiaAnn Thorac Cardiovas2011174 369 375
- DesaiNDBurtchKMoserWLong-term comparison of thoracic endovascular aortic repair (TEVAR) to open surgery for the treatment of thoracic aortic aneurysmsJ Thorac Cardiovasc Surg20121443 604 609 discussion 609–61122898503
- Mazur-BialyAIPochecEZarawskiMAnti-inflammatory properties of Irisin, mediator of physical activity, are connected with TLR4/MyD88 signaling pathway activationInt J Mol Sci2017184 701
- LiXLianLHBaiTCryptotanshinone inhibits LPS-induced proinflammatory mediators via TLR4 and TAK1 signaling pathwayInt Immunopharmacol20111111 1871 187621835267
