Abstract
Metformin is the most commonly prescribed drug for type 2 diabetes mellitus. In recent years, in addition to glucose lowering, several studies have presented evidence suggesting some potential role for metformin, such as antitumor effect, antiaging effect, cardiovascular protective effect, neuroprotective effect or an optional treatment for polycystic ovary syndrome. This paper will critically review the role of metformin to provide reference for doctors and researchers.
Introduction
Metformin has become one of the most widely used drugs in the treatment of type 2 diabetes mellitus (T2DM) since its approval in the United Kingdom in 1958 and in the United States in 1995, with doses ranging from 500 to 2,500 mg/day.Citation1 It is the first-line therapy for patients with T2DM according to the American Diabetes Association/European Association for Study of Diabetes guidelines.Citation2 Metformin works by decreasing intestinal glucose absorption, improving peripheral glucose uptake, lowering fasting plasma insulin levels and increasing insulin sensitivity, which result in a reduction of blood glucose concentrations without causing overt hypoglycemia.Citation3 Additionally, metformin can inhibit gluconeogenesis with the activation of AMP-activated protein kinase (AMPK).Citation4 AMPK is an important player in the regulation of energy metabolism, which plays a key role in diabetes and related metabolic diseases. It is demonstrated that AMPK is required for maintaining glucose homeostasis.Citation5 Metformin has few adverse side effects, the most common adverse side effects being gastrointestinal symptoms (incidence rate 20%–30%), including nausea and vomiting,Citation6 and the most serious adverse effects being lactic acidosis (incidence rate 1/30,000), mainly in diabetic patients with liver and kidney dysfunction.Citation7
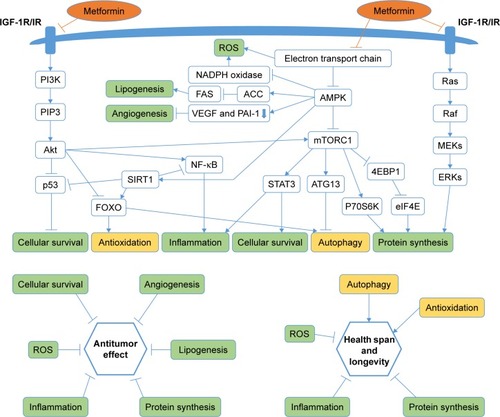
Since metformin’s worldwide spread for over 50 years, numerous studies concerning other potential indications have emerged, which showed that metformin can also be used as an anticancer agent,Citation8 an antiaging agent,Citation9 a cardiovascular protective agent,Citation10 a neuroprotective agentCitation11 or an optional drug for polycystic ovary syndrome (PCOS).Citation12 In this review, we summarized the currently potential indications and possible mechanism of metformin, and expect many more studies for further verification.
Antitumor effect of metformin
Metformin was first discovered as an antitumor agent on hamsters in 2001. In this experiment, there were two groups of high-fat (HF)-fed hamsters. One group received metformin in drinking water for life (HF + Met group), and the other group served as the control group (HF group). All hamsters were treated with N-nitrosobis-(2-oxopropyl) amine, a pancreatic carcinogen, and after 42 weeks, 50% of the hamsters in the high-fat group developed malignant lesions; however, none was found in the HF + Met group (P<0.05).Citation13 A large case-control study in Scotland first showed that metformin reduced the risk of cancer in patients with T2DM (odds ratio [OR] 0.77, 95% CI 0.64–0.92 for any metformin exposure versus no metformin exposure).Citation14 A representative population prospective cohort study of 800,000 individuals showed that metformin reduced the incidences of several gastroenterological cancers in treated diabetes (total 0.12 (0.08–0.19), colorectal 0.36 (0.13–0.98), liver 0.06 (0.02–0.16), pancreas 0.15 (0.03–0.79)), the dose of metformin is shown in .Citation15 In addition to the reduction of cancer incidence,Citation16,Citation17 metformin intake was also associated with a decrease of cancer mortality. Landman et al showed that metformin was associated with lower cancer mortality (hazard ratio [HR] 0.43 [0.23–0.80]) and that the effect was dose dependent ().Citation18 A recent meta-analysis concluded that metformin reduced cancer incidence and mortality in patients with diabetes, with overall cancer incidence reduced by 31% and cancer mortality reduced by 34%.Citation8 Furthermore, a meta-analysisCitation19 suggested that metformin had the greatest benefits as an adjuvant agent in colorectal and prostate cancer treatment, particularly in those receiving radiotherapy. However, the dose of metformin needs to be further explored. So far, several epidemiologic studies have reported the antitumor effect of metformin in different tumors, such as ovarian,Citation20,Citation21 breast,Citation22,Citation23 prostateCitation24 and colorectal.Citation25
Table 1 Summary of effective dose of metformin in studies
However, some studies also considered that there was no significant effect of metformin on cancer risk, survival time and mortality risk of cancer in T2DM patients, such as lung cancer,Citation26 breast cancerCitation27 and prostate cancer.Citation28 A recent study also reported that metformin has no protective association with survival in colorectal cancer patients with T2DM (HR 1.06, 95% CI 0.80–1.40).Citation29
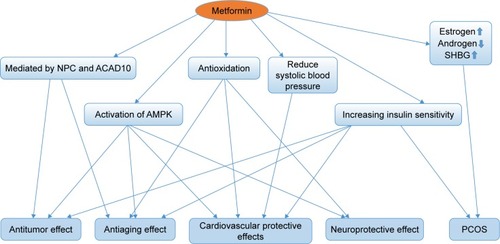
Therefore, whether metformin has antitumor effect or not has attracted much attention. Accumulating evidence showed metformin’s role in attenuating tumorigenesis. First, Wu et alCitation30 uncovered that metformin’s antitumor properties rely on two elements of a single genetic pathway – the nuclear pore complex (NPC), which allows the passage of molecules into and out of the nucleus, and an enzyme called acyl-CoA dehydrogenase family member-10 (ACAD10) (). Basically, metformin’s suppression of mitochondrial respiratory capacity reduces cellular energy, restricting transit of the RagA-RagCGTPase heterodimer through the NPC. This shuts off an important cellular growth molecule called mTORC1, and the inactivation of mTORC1 subsequently inhibits growth and extends the lifespan of Caenorhabditis elegans through transcriptional induction of ACAD10. Moreover, in human melanoma and pancreatic cancer cells, the investigators confirmed that application of biguanides restricted nuclear pore transit and induced ACAD10 expression. After all, the experiments showed that metformin can no longer block the growth of cancer cells, if we force the nuclear pore to remain open, or if we permanently close the ACAD10. This pathway provides a unified mechanism by which metformin can kill cancer cells and extend lifespan, and in specific environments, the nuclear pore and ACAD10 may be manipulated for the prevention or even treatment of certain cancers. Second, metformin can significantly reduce the risk factors of tumor in patients with T2DM, including glucose, insulin and insulin-like growth factor 1 (IGF-1). In order to create a fuel-rich environment for cancer progression, cancer cells usually uptake high levels of glucose.Citation31 Metformin, as a glucose-lowering agent, can cut off supplies for cancer cells and inhibit tumor growth.Citation32 Insulin and IGF-1 may act as potential growth factors capable of stimulating cell survival and mitogenesis, protecting cells from apoptosis to promote cancer development and progression.Citation31,Citation33,Citation34 This effect is mediated by the insulin receptor and the insulin-like growth factor 1 receptor (IGF-1R), which are expressed on many cancer cells,Citation35 through Ras/Raf/MEK/ERK signaling and PI3K/Akt/mTORC1 signaling.Citation36–Citation38 Moreover, hyperinsulinemia has been shown to increase free or bioactive IGF-1 levels by the downregulation of insulin-like growth factor binding protein, resulting in the activation of IGF-1R.Citation39 Metformin treatment can reduce the levels of insulin and IGF-1, thereby reducing the growth of cell.Citation40 Third, activating the AMPK signaling pathway is also an important anticancer mechanism of metformin. The activated AMPK leads to energy preservation processes for cell survival at the expense of growth and proliferation. It can phosphorylate tuberous sclerosis complex 1 and 2, leading to the suppression of mTORC1 activation by inhibiting Ras homolog enrichment in brain (an mTORC1 activator).Citation41–Citation43 This inhibition of mTORC1 ultimately decreases protein synthesis and cell growth.Citation36,Citation44 Metformin can also inhibit mTORC1 by suppressing Rag GTPasesCitation45 and upregulating the expression of REDD1 in a p53-dependent manner.Citation46 Meanwhile, metformin has other AMPK-mediated actions that may be implicated in cancer, such as reduced lipogenesis,Citation47,Citation48 decreased angiogenesis,Citation49 inhibition of the synthesis of proinflammatory cytokinesCitation50 and increase in the number of CD8(+) tumor-infiltrating lymphocytes.Citation51 Metformin’s multiple tumor-relevant actions are depicted in and . However, most of the evidence for the treatment of metformin has been derived from retrospective cohort studies and case–control studies, instead of rigorous prospective or randomized controlled trials. There is still a lack of clinical evidence for metformin’s antitumor activity in non-diabetic patients. Therefore, more clinical trials are needed to evaluate the role of metformin in different tumors.
Figure 1 Model of the ancient pathway by which metformin extends lifespan and inhibits growth.
Abbreviations: NPC, nuclear pore complex; ACAD10, acyl-CoA dehydrogenase family member-10.
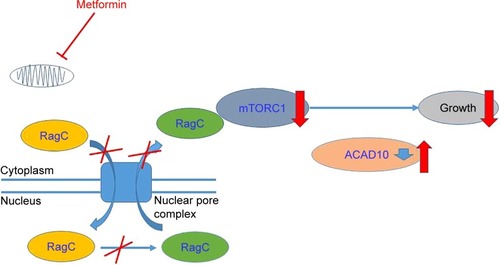
Figure 2 Metformin targets multiple pathways of oncogenesis and aging.
Abbreviations: IGF-1R, insulin-like growth factor 1 receptor; IR, insulin receptor; ROS, reactive oxygen species; AMPK, AMP-activated protein kinase.
Antiaging effect of metformin
Aging is a complex process, which is associated with accumulation of damage, loss of function and increased vulnerability to disease, ultimately leading to death. Human aging and age-related diseases are becoming one of the greatest challenges and financial burdens faced by developed and developing countries.Citation52 A growing body of evidence showed that metformin could delay aging and increase lifespan in vivo, specifically in nematodes and mice. Cabreiro et al found that metformin at 25, 50 and 100 mM concentration increased mean lifespan by 18%, 36% and 3%, respectively, in C. elegans.Citation53 Martin-Montalvo et al showed that metformin increased lifespan by 4%–6% in different mouse breeds, and the long-term treatment with metformin (0.1% metformin in diet) extended lifespan, while a higher dose (1% metformin) was toxic ().Citation54 A recent study determined that mean lifespan would be increased by 14% and maximum lifespan by 1 month if treatment with metformin is started early in life, but at older age, this effect would be declined.Citation55 Additionally, there are also many studies that focus on whether antiaging effects of metformin can be demonstrated in patients with T2DM. In the United Kingdom Prospective Diabetes Study (UKPDS), the use of metformin decreased the risk of cardiovascular disease,Citation56 cancer incidence and overall mortality,Citation57 compared with other antidiabetic drugs. Furthermore, a large retrospective observational study including over 180,000 subjects showed that patients with T2DM initiated with metformin monotherapy had longer survival than did matched, non-diabetic controls; however, in this study they did not investigate for a dose-response association.Citation9 On the contrary, Slack et alCitation58 found that metformin can activate AMPK and reduce lipid stores, but cannot extend lifespan in Drosophila (the final concentrations are 1, 2.5, 5, 10, 25, 50 and 100 nM). One possible reason is that the dose of metformin in the study is toxic. Similarly, a study confirmed that diet supplementation with 0.1% metformin led to a 5.83% extension of mean lifespan of C57BL/6 mice, while a higher concentration of metformin (1%) was toxic and significantly shortened mean lifespan by 14.4%.Citation54
Interventions that target aging-related pathways are capable of extending lifespan dramatically, especially health span, a period of life during which an individual is fully functional and free of chronic illness.Citation59 These include intermittent or prolonged fasting, mild caloric restriction combined with a low glycemic index diet and protein restriction, inhibition of the GH/IGF-I axis, inhibition of TOR–S6K signaling, activation of sirtuins or AMPK and chronic metformin use. Metformin’s multiple aging-relevant actions are also depicted in and . On one hand, as previously described, NPC and ACAD10 mediate biguanide-induced growth inhibition and lifespan extension.Citation30 On the other hand, specifically for aging, metformin can not only decrease insulin and IGF-1 levels,Citation60 reduce the endogenous production of reactive oxygen species (ROS)Citation61,Citation62 and active AMPK,Citation62–Citation65 and inhibit mTOR,Citation66,Citation67 but also influence metabolic and cellular processes such as inflammationCitation68 and autophagy.Citation69 The United States intends to carry out a big clinical trial about the antiaging effect of metformin enrolling 3,000 non-diabetics aged 70–80 years at roughly 15 centers. The follow-up will last for 5–7 years, and the situation of disease suffering and death after metformin treatment will be emphatically studied. The result of the clinical trial aimed to prove that metformin has a positive impact on human lifespan in non-diabetics and healthy people.
Cardiovascular protective effects of metformin
Diabetic patients mainly die of cardiovascular complications,Citation70 including macrovascular complications (such as stroke, coronary artery disease [CAD] and myocardial infarction) and microvascular complications (such as kidney disease, retinal injury and peripheral nerve disease), of which approximately 70% of all diabetic patients die of heart and brain macrovascular diseases. A number of clinical studies have shown that metformin has cardiovascular protective effects and reduces the incidence and mortality of cardiovascular events. In 1998, UKPDS, a randomized, prospective, multicenter trial, was the first trial to determine that metformin could significantly reduce the risk of all-cause mortality and acute myocardial infarction in overweight patients with T2DM; the dose of metformin is shown in .Citation56 In addition, a 10-year post-interventional follow-up of the UKPDS survivor cohort further examined that metformin treatment had a long-term benefit on cardiovascular risk in overweight patients. Compared with sulfonylurea and insulin treatment, metformin treatment can effectively reduce the risk of myocardial infarction and death.Citation71 Similarly, Roumie et al also showed that compared with sulfonylurea therapy, metformin treatment was associated with a decreased hazard of cardiovascular disease events or death in T2DM.Citation72 Moreover, data from the Reduction of Atherothrombosis for Continued Health Registry indicated that the use of metformin as a means of secondary prevention was associated with a 24% reduction in all-cause mortality after 2-year follow-up among patients with atherothrombosis. Thus, metformin has cardiovascular protective effects independent of glucose-lowering effects.Citation73 Furthermore, in a multicenter, randomized, double-blind, placebo-controlled clinical trial, Hong et al found that among type 2 diabetic patients with CAD, compared with glipizide, metformin treatment for 3 years (mean daily dose was 1.4±0.2 g; ) substantially reduced major cardiovascular events in a median follow-up of 5 years, which indicated a potential benefit of metformin treatment on cardiovascular outcomes in high-risk patients.Citation10 After all, metformin is the only antidiabetic drug to be recommended by the 2013 AACE guidelines for cardiovascular benefit.
Metformin may exert beneficial effects to prevent cardiovascular disease. The risk factors of cardiovascular disease include dyslipidemia, obesity, hypertension, insulin resistance and so on. First, metformin may improve lipometabolism and reduce the level of LDL cholesterol by activation of AMPK.Citation74 Second, metformin was associated with weight loss or less weight gain,Citation75–Citation77 the mechanism of which is thought to be the decreased perceived hunger resulting in diminished food intake.Citation78 Third, a recent meta-analysis suggested that metformin could effectively lower systolic blood pressure in non-diabetic patients;Citation79 possible mechanisms of blood pressure lowering by metformin include reduction of insulin resistance and plasma insulin, adrenergic receptor deactivation, reduction of intracytoplasmic calcium, inhibition of sympathetic drive especially in conditions of high dietary salt intake and increase of glomerular filtration rate and sodium excretion.Citation80 In addition, metformin can alleviate oxidative stress and inflammatory response as well as improve endothelial cell function.Citation81,Citation82
The neuroprotective effect of metformin
Clinical studies concerning whether metformin could improve cognitive function and reduce the incidence of dementia in patients with T2DM are inconsistent. A Singapore Longitudinal Aging Study by Ng et al found that long-term treatment (>6 years) with metformin among T2DM patients was significantly associated with lowest risk of cognitive impairment in both cross-sectional analysis (OR 0.30, 95% CI 0.11–0.80) and in longitudinal analysis (OR 0.27, 95% CI 0.12–0.60).Citation83 Herath et al also showed that metformin has better protective effect on domain of verbal learning, working memory and executive function, compared to other diabetic treatments.Citation84 A small clinical trial by Guo et al found that the treatment with metformin for 24 weeks significantly improved cognitive performance and reduced depressive symptoms in T2DM patients with depression; the dose of metformin is shown in .Citation85 Similarly, significant improvements can be found in subjects without treated diabetes with mild cognitive impairment after 12 months of metformin treatment (1,000 mg twice a day, ).Citation86 In addition, Cheng et al reported that T2DM patients with metformin have lower risk of dementia than those with other diabetes medications.Citation11 Furthermore, it is reported that compared with no metformin use, 1 year, 2 years, 2–4 years, and >4 years of metformin exposure among elderly veterans with diabetes increased 7% (P=0.61) and decreased 29% (P=0.08), 41% (P=0.0026) and 84% (P<0.0001) risk of neurodegenerative diseases (ND), including Alzheimer, Huntington, Parkinson and dementia among elder adults, which concluded that the long-term metformin treatment has protective effect on the incidence of ND (American Diabetes Association, 2016).Citation114 However, some studies have different results. A case–control study from the United Kingdom found that long-term use of metformin was associated with a slightly higher risk of AD (OR 1.71, 95% CI 1.12–2.60).Citation87 A recent Australian study suggested that T2DM patients treated with metformin had increased risk for impaired cognitive performance (OR 2.23, 95% CI 1.05–4.75), but metformin users who were taking vitamin B12 and calcium may have alleviated metformin-induced vitamin B12 deficiency and improved cognitive outcomes (OR 0.41, 95% CI 0.91–0.92).Citation88 Therefore, a larger trial seems warranted to evaluate the efficacy of metformin in neuroprotective effect.
Studies about the effect of metformin mostly focus on the Aβ production and tau level. Metformin may decrease tau phosphorylation and total tau level,Citation89,Citation90 but its effect on Aβ production is still inconsistent.Citation91,Citation92 Besides, it is reported that AMPK play an important role in various ND;Citation93 the activation of AMPK via an AMPK activator (metformin) may be neuroprotective, via the enhancement of angiogenesis, neurogenesis and induction of autophagy.Citation93–Citation96 Metformin can also prevent brain mitochondrial dysfunction, decrease oxidative stress, increase brain-derived neurotrophic factor levels, ameliorate cognitive impairment and improve neurological deficits.Citation97–Citation100
About PCOS
PCOS is an endocrine and metabolic disorder found among women of reproductive age, which is characterized by hyperandrogenism, ovulatory dysfunction, altered LH/FSH ratio (>2/3:1), oligomenorrhea/amenorrhea and polycystic ovaries.Citation101,Citation102 Approximately 50%–70% of PCOS patients suffer from insulin resistance and resulting hyperinsulinemia.Citation103–Citation105 Patients with PCOS are predisposed to many complications such as cardiovascular and cerebrovascular diseases, hypertension, metabolic syndrome and T2DM.Citation106–Citation109 Metformin has been used for PCOS treatment since 1994,Citation110 by which most of the metabolic abnormalities of PCOS can be reversed.Citation111 Metformin dose ranged from 850 to 1,700 mg in different studies (). The mechanism is thought to be mediated through increased insulin sensitivity, increased ovarian secretion of estrogen, decreased ovarian production of androgen and augmentation of the production of sex hormone binding globulin.Citation111,Citation112 A recent meta-analysis by Tang et al demonstrated that metformin can reduce testosterone and insulin in PCOS women.Citation113
Conclusion
Metformin is the most commonly prescribed therapy for patients with T2DM. It has a good safety profile and is associated with low cost. With further exploration of the clinical effect and possible mechanism of metformin, its indications have been extended to antitumor effect, antiaging effect, cardiovascular protective effects, neuroprotective effects and an optional treatment for PCOS; the linkage of these effects is shown in . Furthermore, many questions such as whether these potential indications of metformin can be observed in non-diabetics and whether genetic factors have an influence on the effect of metformin need to be clarified by substantial basic experiments and clinical trials.
Acknowledgments
We are thankful to the National Natural Science Foundation of China for funding (81120108017, 81572951 [Qian Huang] and 81502648 [Jin Cheng]).
Disclosure
The authors report no conflicts of interest in this work.
References
- ScarpelloJHHowlettHCMetformin therapy and clinical usesDiab Vasc Dis Res200853 157 16718777488
- InzucchiSEBergenstalRMBuseJBManagement of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD)Diabetologia2012556 1577 159622526604
- GrzybowskaMBoberJOlszewskaMMetformin – mechanisms of action and use for the treatment of type 2 diabetes mellitusPostepy Hig Med Dosw (Online)201165 277 28521677353
- MatthaeiSGretenHEvidence that metformin ameliorates cellular insulin-resistance by potentiating insulin-induced translocation of glucose transporters to the plasma membraneDiabete Metab1991171 Pt 2 150 1581718789
- ZhangBBZhouGLiCAMPK: an emerging drug target for diabetes and the metabolic syndromeCell Metab200995 407 41619416711
- Diabetes Prevention Program Research GroupLong-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes StudyDiabetes Care2012354 731 73722442396
- SalpeterSGreyberEPasternakGSalpeterERisk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitusCochrane Database Syst Rev20061 CD002967
- GandiniSPuntoniMHeckman-StoddardBMMetformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confoundersCancer Prev Res (Phila)201479 867 88524985407
- BannisterCAHoldenSEJenkins-JonesSCan people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controlsDiabetes Obes Metab20141611 1165 117325041462
- HongJZhangYLaiSSPREAD-DIMCAD InvestigatorsEffects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery diseaseDiabetes Care2013365 1304 131123230096
- ChengCLinCHTsaiYWTsaiCJChouPHLanTHType 2 diabetes and antidiabetic medications in relation to dementia diagnosisJ Gerontol A Biol Sci Med Sci20146910 1299 130524899525
- PatelRShahGEffect of metformin on clinical, metabolic and endocrine outcomes in women with polycystic ovary syndrome: a meta-analysis of randomized controlled trialsCurr Med Res Opin2017 1 13
- SchneiderMBMatsuzakiHHaorahJPrevention of pancreatic cancer induction in hamsters by metforminGastroenterology20011205 1263 127011266389
- EvansJMDonnellyLAEmslie-SmithAMAlessiDRMorrisADMetformin and reduced risk of cancer in diabetic patientsBMJ20053307503 1304 130515849206
- LeeMSHsuCCWahlqvistMLTsaiHNChangYHHuangYCType 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individualsBMC Cancer201111 2021241523
- LibbyGDonnellyLADonnanPTAlessiDRMorrisADEvansJMNew users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetesDiabetes Care2009329 1620 162519564453
- MonamiMColombiCBalziDMetformin and cancer occurrence in insulin-treated type 2 diabetic patientsDiabetes Care2011341 129 13120980415
- LandmanGWKleefstraNvan HaterenKJGroenierKHGansROBiloHJMetformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16Diabetes Care2010332 322 32619918015
- CoyleCCaffertyFHValeCLangleyREMetformin as an adjuvant treatment for cancer: a systematic review and meta-analysisAnn Oncol20162712 2184 219527681864
- BodmerMBeckerCMeierCJickSSMeierCRUse of metformin and the risk of ovarian cancer: a case-control analysisGynecol Oncol20111232 200 20421802715
- TsengCHMetformin reduces ovarian cancer risk in Taiwanese women with type 2 diabetes mellitusDiabetes Metab Res Rev2015316 619 62625820555
- JiralerspongSPallaSLGiordanoSHMetformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancerJ Clin Oncol20092720 3297 330219487376
- CampagnoliCPasanisiPAbbàCEffect of different doses of metformin on serum testosterone and insulin in non-diabetic women with breast cancer: a randomized studyClin Breast Cancer2012123 175 18222607767
- TsengCHMetformin significantly reduces incident prostate cancer risk in Taiwanese men with type 2 diabetes mellitusEur J Cancer20145016 2831 283725201464
- SehdevAShihYCVekhterBBissonnetteMBOlopadeOIPoliteBNMetformin for primary colorectal cancer prevention in patients with diabetes: a case-control study in a US populationCancer20151217 1071 107825424411
- MazzonePJRaiHBeukemannMXuMJainASasidharMThe effect of metformin and thiazolidinedione use on lung cancer in diabeticsBMC Cancer201212 41022978440
- LegaICAustinPCGruneirAGoodwinPJRochonPALipscombeLLAssociation between metformin therapy and mortality after breast cancer: a population-based studyDiabetes Care20133610 3018 302623633525
- MargelDUrbachDLipscombeLLAssociation between metformin use and risk of prostate cancer and its gradeJ Natl Cancer Inst201310515 1123 113123853056
- Mc MenaminÚCMurrayLJHughesCMCardwellCRMetformin use and survival after colorectal cancer: a population-based cohort studyInt J Cancer20161382 369 37926331456
- WuLZhouBOshiro-RapleyNAn ancient, unified mechanism for metformin growth inhibition in C. elegans and cancerCell20161677 1705 171827984722
- MoralesDRMorrisADMetformin in cancer treatment and preventionAnnu Rev Med201566 17 2925386929
- GallagherEJLeRoithDDiabetes, cancer, and metformin: connections of metabolism and cell proliferationAnn N Y Acad Sci20111243 54 6822211893
- DrazninBMechanism of the mitogenic influence of hyperinsulinemiaDiabetol Metab Syndr201131 1021668983
- DingXZFehsenfeldDMMurphyLOPermertJAdrianTEPhysiological concentrations of insulin augment pancreatic cancer cell proliferation and glucose utilization by activating MAP kinase, PI3 kinase and enhancing GLUT-1 expressionPancreas2000213 310 32011039477
- SachdevDYeeDDisrupting insulin-like growth factor signaling as a potential cancer therapyMol Cancer Ther200761 1 1217237261
- GongJKelekarGShenJShenJKaurSMitaMThe expanding role of metformin in cancer: an update on antitumor mechanisms and clinical developmentTarget Oncol2016114 447 46726864078
- MaJSawaiHMatsuoYIGF-1 mediates PTEN suppression and enhances cell invasion and proliferation via activation of the IGF-1/PI3K/Akt signaling pathway in pancreatic cancer cellsJ Surg Res20101601 90 10119560785
- AlgireCAmreinLBazileMDavidSZakikhaniMPollakMDiet and tumor LKB1 expression interact to determine sensitivity to anti-neoplastic effects of metformin in vivoOncogene20113010 1174 118221102522
- KourelisTVSiegelRDMetformin and cancer: new applications for an old drugMed Oncol2012292 1314 132721301998
- DowlingRJNiraulaSStambolicVGoodwinPJMetformin in cancer: translational challengesJ Mol Endocrinol2012483 R31 R4322355097
- ChiangGGAbrahamRTTargeting the mTOR signaling network in cancerTrends Mol Med20071310 433 44217905659
- YoshidaSHongSSuzukiTRedox regulates mammalian target of rapamycin complex 1 (mTORC1) activity by modulating the TSC1/TSC2-Rheb GTPase pathwayJ Biol Chem201128637 32651 3266021784859
- InokiKZhuTGuanKLTSC2 mediates cellular energy response to control cell growth and survivalCell20031155 577 59014651849
- JalvingMGietemaJALefrandtJDMetformin: taking away the candy for cancer?Eur J Cancer20104613 2369 238020656475
- KalenderASelvarajAKimSYMetformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent mannerCell Metab2010115 390 40120444419
- Ben SahraIRegazzettiCRobertGMetformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1Cancer Res20117113 4366 437221540236
- ZhouGMyersRLiYRole of AMP-activated protein kinase in mechanism of metformin actionJ Clin Invest20011088 1167 117411602624
- Lettieri BarbatoDVeglianteRDesideriECirioloMRManaging lipid metabolism in proliferating cells: new perspective for metformin usage in cancer therapyBiochim Biophys Acta201418452 317 32424569230
- ErsoyCKiyiciSBudakFThe effect of metformin treatment on VEGF and PAI-1 levels in obese type 2 diabetic patientsDiabetes Res Clin Pract2008811 56 6018358555
- HirschHAIliopoulosDStruhlKMetformin inhibits the inflammatory response associated with cellular transformation and cancer stem cell growthProc Natl Acad Sci U S A20131103 972 97723277563
- EikawaSNishidaMMizukamiSYamazakiCNakayamaEUdonoHImmune-mediated antitumor effect by type 2 diabetes drug, metforminProc Natl Acad Sci U S A20151126 1809 181425624476
- ChristensenKDoblhammerGRauRVaupelJWAgeing populations: the challenges aheadLancet20093749696 1196 120819801098
- CabreiroFAuCLeungKYMetformin retards aging in C. elegans by altering microbial folate and methionine metabolismCell20131531 228 23923540700
- Martin-MontalvoAMerckenEMMitchellSJMetformin improves healthspan and lifespan in miceNat Commun20134 219223900241
- AnisimovVNBersteinLMPopovichIGIf started early in life, metformin treatment increases life span and postpones tumors in female SHR miceAging (Albany NY)201132 148 15721386129
- Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) GroupLancet19983529131 854 8659742977
- WuJWBoudreauDMParkYSimondsNIFreedmanANCommonly used diabetes and cardiovascular medications and cancer recurrence and cancer-specific mortality: a review of the literatureExpert Opin Drug Saf2014138 1071 109924999107
- SlackCFoleyAPartridgeLActivation of AMPK by the putative dietary restriction mimetic metformin is insufficient to extend lifespan in DrosophilaPLoS One2012710 e4769923077661
- LongoVDAntebiABartkeAInterventions to slow aging in humans: are we ready?Aging Cell2015144 497 51025902704
- LiuBFanZEdgertonSMYangXLindSEThorADPotent anti-proliferative effects of metformin on trastuzumab-resistant breast cancer cells via inhibition of erbB2/IGF-1 receptor interactionsCell Cycle20111017 2959 296621862872
- BridgesHRJonesAJPollakMNHirstJEffects of metformin and other biguanides on oxidative phosphorylation in mitochondriaBiochem J20144623 475 48725017630
- ZhengZChenHLiJSirtuin 1-mediated cellular metabolic memory of high glucose via the LKB1/AMPK/ROS pathway and therapeutic effects of metforminDiabetes2012611 217 22822124463
- LienFBerthierABouchaertEMetformin interferes with bile acid homeostasis through AMPK-FXR crosstalkJ Clin Invest20141243 1037 105124531544
- LuJShiJLiMActivation of AMPK by metformin inhibits TGF-beta-induced collagen production in mouse renal fibroblastsLife Sci2015127 59 6525744403
- DucaFACôtéCDRasmussenBAMetformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in ratsNat Med2015215 506 51125849133
- NairVSreevalsanSBashaRMechanism of metformin-dependent inhibition of mammalian target of rapamycin (mTOR) and Ras activity in pancreatic cancer: role of specificity protein (Sp) transcription factorsJ Biol Chem201428940 27692 2770125143389
- Pérez-RevueltaBIHettichMMCiociaroAMetformin lowers Ser-129 phosphorylated alpha-synuclein levels via mTOR-dependent protein phosphatase 2A activationCell Death Dis20145 e120924810045
- SaishoYMetformin and inflammation: its potential beyond glucose-lowering effectEndocr Metab Immune Disord Drug Targets2015153 196 20525772174
- SongYMLeeYHKimJWMetformin alleviates hepatosteatosis by restoring SIRT1-mediated autophagy induction via an AMP-activated protein kinase-independent pathwayAutophagy2015111 46 5925484077
- BenjaminEJBlahaMJChiuveSEAmerican Heart Association Statistics Committee and Stroke Statistics SubcommitteeHeart disease and stroke statistics-2017 update: a report from the American Heart AssociationCirculation201713510 e146 e60328122885
- HolmanRRPaulSKBethelMAMatthewsDRNeilHA10-Year follow-up of intensive glucose control in type 2 diabetesN Engl J Med200835915 1577 158918784090
- RoumieCLHungAMGreevyRAComparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus: a cohort studyAnn Intern Med20121579 601 61023128859
- RousselRTravertFPasquetBReduction of Atherothrombosis for Continued Health (REACH) Registry InvestigatorsMetformin use and mortality among patients with diabetes and atherothrombosisArch Intern Med201017021 1892 189921098347
- XuTBrandmaierSMessiasACEffects of metformin on metabolite profiles and LDL cholesterol in patients with type 2 diabetesDiabetes Care20153810 1858 186726251408
- SeifarthCSchehlerBSchneiderHJEffectiveness of metformin on weight loss in non-diabetic individuals with obesityExp Clin Endocrinol Diabetes20131211 27 3123147210
- FontbonneADioufIBaccara-DinetMEschwegeECharlesMAEffects of 1-year treatment with metformin on metabolic and cardiovascular risk factors in non-diabetic upper-body obese subjects with mild glucose anomalies: a post-hoc analysis of the BIGPRO1 trialDiabetes Metab2009355 385 39119665415
- MalinSKNightingaleJChoiSEChipkinSRBraunBMetformin modifies the exercise training effects on risk factors for cardiovascular disease in impaired glucose tolerant adultsObesity (Silver Spring)2013211 93 10023505172
- AdeyemoMAMcDuffieJRKozloskyMEffects of metformin on energy intake and satiety in obese childrenDiabetes Obes Metab2015174 363 37025483291
- ZhouLLiuHWenXPengYTianYZhaoLEffects of metformin on blood pressure in nondiabetic patients: a meta-analysis of randomized controlled trialsJ Hypertens2017351 18 2627607453
- ThomopoulosCKatsimagklisGMakrisTMetformin and blood pressure lowering: a questioned associationJ Hypertens2017351 27 2827902624
- WanXHuoYJohnsM5′-AMP-activated protein kinase-activating transcription factor 1 cascade modulates human monocyte-derived macrophages to atheroprotective functions in response to heme or metforminArterioscler Thromb Vasc Biol20133311 2470 248024051143
- IsodaKYoungJLZirlikAMetformin inhibits proinflammatory responses and nuclear factor-kappaB in human vascular wall cellsArterioscler Thromb Vasc Biol2006263 611 61716385087
- NgTPFengLYapKBLeeTSTanCHWinbladBLong-term metformin usage and cognitive function among older adults with diabetesJ Alzheimers Dis2014411 61 6824577463
- HerathPMCherbuinNEramudugollaRAnsteyKJThe effect of diabetes medication on cognitive function: evidence from the PATH through life studyBiomed Res Int20162016 720842927195294
- GuoMMiJJiangQMMetformin may produce antidepressant effects through improvement of cognitive function among depressed patients with diabetes mellitusClin Exp Pharmacol Physiol2014419 650 65624862430
- LuchsingerJAPerezTChangHMetformin in amnestic mild cognitive impairment: results of a pilot randomized placebo controlled clinical trialJ Alzheimers Dis2016512 501 51426890736
- ImfeldPBodmerMJickSSMeierCRMetformin, other antidiabetic drugs, and risk of Alzheimer’s disease: a population-based case-control studyJ Am Geriatr Soc2012605 916 92122458300
- MooreEMManderAGAmesDAIBL InvestigatorsIncreased risk of cognitive impairment in patients with diabetes is associated with metforminDiabetes Care20133610 2981 298724009301
- KicksteinEKraussSThornhillPBiguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signalingProc Natl Acad Sci U S A201010750 21830 2183521098287
- LiJDengJShengWZuoZMetformin attenuates Alzheimer’s disease-like neuropathology in obese, leptin-resistant micePharmacol Biochem Behav20121014 564 57422425595
- PiconePNuzzoDCaruanaLMetformin increases APP expression and processing via oxidative stress, mitochondrial dysfunction and NF-kappaB activation: use of insulin to attenuate metformin’s effectBiochim Biophys Acta201518535 1046 105925667085
- HettichMMMatthesFRyanDPThe anti-diabetic drug metformin reduces BACE1 protein level by interfering with the MID1 complexPLoS One201497 e10242025025689
- PoelsJSpasićMRCallaertsPNorgaKKExpanding roles for AMP-activated protein kinase in neuronal survival and autophagyBioessays2009319 944 95219644919
- JinQChengJLiuYImprovement of functional recovery by chronic metformin treatment is associated with enhanced alternative activation of microglia/macrophages and increased angiogenesis and neurogenesis following experimental strokeBrain Behav Immun201440 131 14224632338
- JiangTYuJTZhuXCAcute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagyBr J Pharmacol201417113 3146 315724611741
- VennaVRLiJHammondMDManciniNSMcCulloughLDChronic metformin treatment improves post-stroke angiogenesis and recovery after experimental strokeEur J Neurosci20143912 2129 213824649970
- PintanaHApaijaiNPratchayasakulWChattipakornNChattipakornSCEffects of metformin on learning and memory behaviors and brain mitochondrial functions in high fat diet induced insulin resistant ratsLife Sci20129111–12 409 41422925597
- ZhaoRRXuXCXuFMetformin protects against seizures, learning and memory impairments and oxidative damage induced by pentylenetetrazole-induced kindling in miceBiochem Biophys Res Commun20144484 414 41724802403
- AlzoubiKHKhabourOFAl-AzzamSITashtoushMHMhaidatNMMetformin eased cognitive impairment induced by chronic l-methionine administration: potential role of oxidative stressCurr Neuropharmacol2014122 186 19224669211
- ChenFDongRRZhongKLAntidiabetic drugs restore abnormal transport of amyloid-beta across the blood-brain barrier and memory impairment in db/db miceNeuropharmacology2016101 123 13626211973
- GuzickDPolycystic ovary syndrome: symptomatology, pathophysiology, and epidemiologyAm J Obstet Gynecol19981796 Pt 2 S89 S939855614
- PasqualiRStener-VictorinEYildizBOPCOS Forum: research in polycystic ovary syndrome today and tomorrowClin Endocrinol (Oxf)2011744 424 43321158892
- CarminaELoboRAUse of fasting blood to assess the prevalence of insulin resistance in women with polycystic ovary syndromeFertil Steril2004823 661 66515374711
- DunaifAInsulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesisEndocr Rev1997186 774 8009408743
- OvalleFAzzizRInsulin resistance, polycystic ovary syndrome, and type 2 diabetes mellitusFertil Steril2002776 1095 110512057712
- MoranLJMissoMLWildRANormanRJImpaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysisHum Reprod Update2010164 347 36320159883
- LimSSDaviesMJNormanRJMoranLJOverweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysisHum Reprod Update2012186 618 63722767467
- de GrootPCDekkersOMRomijnJADiebenSWHelmerhorstFMPCOS, coronary heart disease, stroke and the influence of obesity: a systematic review and meta-analysisHum Reprod Update2011174 495 50021335359
- WildSPierpointTMcKeiguePJacobsHCardiovascular disease in women with polycystic ovary syndrome at long-term follow-up: a retrospective cohort studyClin Endocrinol (Oxf)2000525 595 60010792339
- VelazquezEMMendozaSHamerTSosaFGlueckCJMetformin therapy in polycystic ovary syndrome reduces hyperinsulinemia, insulin resistance, hyperandrogenemia, and systolic blood pressure, while facilitating normal menses and pregnancyMetabolism1994435 647 6548177055
- MoghettiPCastelloRNegriCMetformin effects on clinical features, endocrine and metabolic profiles, and insulin sensitivity in polycystic ovary syndrome: a randomized, double-blind, placebo-controlled 6-month trial, followed by open, long-term clinical evaluationJ Clin Endocrinol Metab2000851 139 14610634377
- GlueckCJWangPFontaineRTracyTSieve-SmithLMetformin to restore normal menses in oligo-amenorrheic teenage girls with polycystic ovary syndrome (PCOS)J Adolesc Health2001293 160 16911524214
- TangTLordJMNormanRJYasminEBalenAHInsulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertilityCochrane Database Syst Rev20125 CD003053
- ShiQLiuSFonsecaVShiL72-OR / 72 - The effect of metformin exposure on neurodegenerative disease among elder adult veterans with diabetes mellitusAbstract presented at: Proceedings of the American Diabetes Association 76th Scientific SessionsJune 10–14; 2016New Orleans, LA