?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
Cisplatin plus gemcitabine (GEM) is a standard regimen for the first-line treatment of advanced non-small cell lung cancer. The aim of this study was to prepare biocompatible and biodegradable polymeric prodrugs and construct nanoparticles (NPs) with layer-by-layer (LbL) technique.
Methods
Platinum (Pt) (IV) complex with a carboxyl group was conjugated to the amino group of chitosan (CH), resulting in a CH-Pt conjugation with positive charge. GEM with amino group was conjugated to the carboxyl group of hyaluronic acid (HA), resulting in a HA-GEM conjugation with negative charge. Novel LbL NPs consisting of the CH-Pt core and the HA-GEM layer, named as HA-GEM/CH-Pt NPs, were constructed. The physicochemical properties of the HA-GEM/CH-Pt NPs were investigated. In vitro cytotoxicity against human non-small lung cancer cells (NCl-H460 cells) was investigated, and in vivo antitumor efficiency was evaluated on mice bearing NCl-H460 cells xenografts.
Results
HA-GEM/CH-Pt NPs have a size of about 187 nm, a zeta potential value of −21 mV and high drug encapsulation efficiency of 90%. The drug release of HA-GEM/CH-Pt NPs exhibited a sustained behavior. HA-GEM/CH-Pt NPs could significantly enhance in vitro cytotoxicity and in vivo antitumor effect against lung cancer animal model compared to the single-drug-loaded NPs and free drug solutions.
Conclusion
The results demonstrated that the HA-GEM/CH-Pt NPs might be a promising system for the synergetic treatment of lung carcinoma.
Introduction
Lung cancer remains the leading cause of cancer-associated deaths worldwide, and the non-small cell lung cancer (NSCLC) constitutes about 85% of lung cancer cases.Citation1,Citation2 Currently, the main clinical therapeutic regimens for NSCLC still include surgery, radiotherapy and chemotherapy. Because the majority of patients are diagnosed at later or metastatic stages, chemotherapy or palliative treatment is the main therapeutic schedule.Citation3,Citation4 Combination chemotherapy is effective in the treatment of cancer for its benefits, which include maximized therapeutic efficacy, reduced side effects and overcome drug resistance.Citation5 Based on a large number of clinical trials, treatment with platinum-based combination chemotherapy is considered the first-line therapy for patients with advanced NSCLC and superior to the single-agent treatment in terms of overall survival.Citation6,Citation7
Cisplatin (CIS) plus gemcitabine (GEM) is a standard regimen for the first-line treatment of advanced NSCLC.Citation8,Citation9 CIS has been approved by the FDA for the treatment of epithelial malignancies such as lung, ovarian and bladder cancer. However, its poor hydrophilic and hydrophobic property, severe toxicity such as renal toxicity, neurotoxicity and drug resistance have hindered its clinical application.Citation10,Citation11 Intensive efforts have been implemented to improve its toxicity and drug resistance, which include prodrugs especially platinum (IV) (Pt) prodrugs and nanocarrier-based formulations.Citation12,Citation13 Compared to CIS, Pt (IV) shows better pharmacokinetics and decreased side effects. GEM is an efficacious antitumor agent, which has a wide spectrum of antitumor activity including NSCLC, pancreatic cancer, bladder cancer, breast cancer and so on. Because of its short plasma half-life and hydrophilic nature, researchers have devoted to finding novel prodrug or nanotechnology and improving its in vivo pharmacokinetics and pharmco-dynamics.Citation14 However, it is difficult to deliver both CIS and GEM with drastically different physical chemistry in the same nanoparticles (NPs).
Nanocarriers based on the conjugation of a polymer with a drug have attracted considerable attention in the field of cancer treatment due to their passive or active tumor-targeted efficiency, enhanced drug bioavailability and balanced pharmacokinetics for hydrophilic and hydrophobic drugs.Citation15–Citation17 Among the various polymeric nanocarriers, layer-by-layer (LbL) NPs are another research hot topic. The systems are designed by the electrostatic attraction of oppositely charged polyelectrolytes. Recent work has demonstrated the potential for LbL NPs to promote in vivo pharmacokinetics, the ability to control release of loading agents and the capability to enhance molecular-targeting capabilities.Citation18,Citation19 These commonly applied polyelectrolytes contain hyaluronic acid (HA),Citation20 chitosan (CH),Citation20 poly(L-lysine),Citation21 poly(glutamic acid),Citation21 poly(acrylic acid)Citation22 and so on.Citation23
In the present study, we designed a novel LbL NP platform equipped with active targeting ligand HA as a carrier for CIS and GEM delivery. As a prodrug of CIS, Pt (IV) complex with a carboxyl group was conjugated to the amino group of CH, resulting in a CH-Pt conjugation with positive charge. GEM with amino group was conjugated to the carboxyl group of HA, resulting in a HA-GEM conjugation with negative charge. The key strategies here were to use biocompatible and biodegradable polymeric prodrug (CH-Pt and HA-GEM) with the LbL technique. The LbL NPs consisted of the CH-Pt core and the HA-GEM layer, named as HA-GEM/CH-Pt NPs. We evaluated their physiochemical properties such as particle size and zeta potential, in vitro release kinetics. The antitumor efficacy was also evaluated both in vitro and in vivo on a CD44-overexpressed lung cancer cell line and mice tumor model, respectively.
Materials and methods
Materials and animals
CIS, GEM, dicyclohexylcarbodiimide (DCC), dimethylaminopyridine (DMAP), Dulbecco’s Modified Eagle’s Medium (DMEM) and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St Louis, MO, USA). All chemicals and reagents used were of analytical grade or higher. NCl-H460 human non-small lung cancer cells (NCl-H460 cells) were obtained from the American Type Culture Collection (Rockville, MD, USA). BALB/c nude mice (4–6 weeks old, 18–22 g weight) were purchased from Shanghai Slack Laboratory Animal Co., Ltd. (Shanghai, People’s Republic of China). Experiments were performed according to the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No 8023, revised 1978); all the animal studies were approved by the Medical Ethics Committee of Shanghai Xuhui District Central Hospital (No FDU-XHH-1002017001011302).
Synthesis of CH-Pt
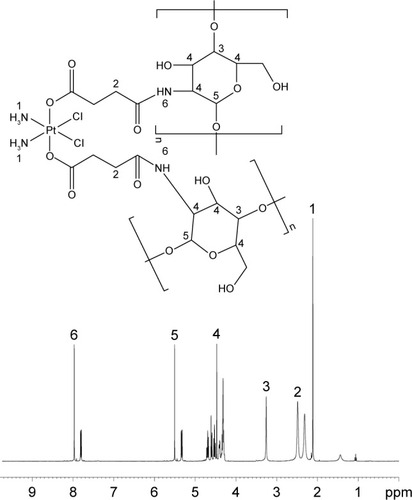
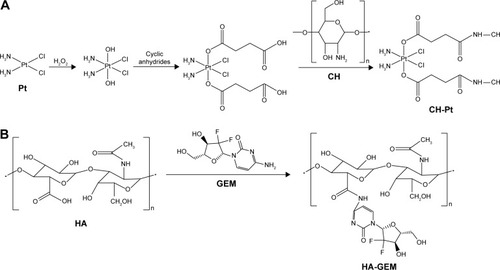
CH-Pt was synthesized by linking the carboxyl groups of Pt (IV) complex with the amino groups of CH ().Citation24 Briefly, CIS (100 mg) was treated with hydrogen peroxide to obtain dihydroxyl Pt (IV) derivative. Dihydroxyl Pt (IV) derivative (200 mg), DCC (20 mg) and DMAP (15 mg) were dissolved in dichloromethane (10 mL). CH (500 mg) was then added into the aforementioned organic solution and stirred at 600 rpm at room temperature for 24 h in the dark. The product was purified by repeated filtration. CH-Pt was dried under the vacuum to remove the solvent.
Figure 1 Synthesis of CH-Pt and HA-GEM. CH-Pt was synthesized by linking the carboxyl groups of Pt (IV) complex with the amino groups of CH (A); HA-GEM was synthesized by linking the carboxyl groups of HA with the amino groups of GEM (B).
Synthesis of HA-GEM
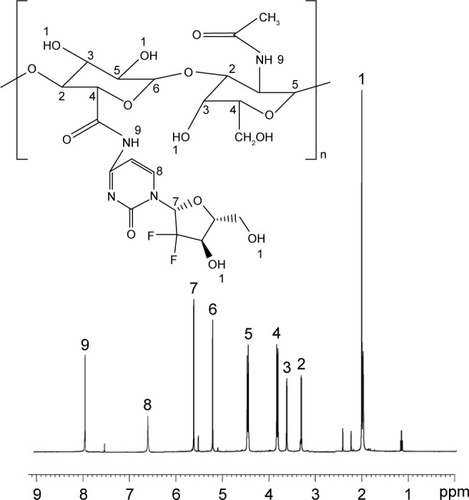
HA-GEM was synthesized by linking the carboxyl groups of HA with the amino groups of GEM ().Citation25 Briefly, HA (100 mg) and GEM (25 mg) were dissolved in dimethyl sulfoxide (DMSO; 10 mL) under nitrogen gas. DCC (15 mg) and DMAP (10 mg) were then sequentially added into the DMSO solution. The reaction mixture was stirred at 600 rpm at room temperature for 24 h in the dark. The reaction was quenched using the excess cold methanol, and the product was purified by repeated filtration. HA-GEM was dried under vacuum to remove the solvent. CH-Pt and HA-GEM were characterized by 1H-NMR spectroscopy and FT-IR spectroscopy.
Preparation of HA-GEM/CH-Pt NPs
CH-Pt-loaded NPs without HA-GEM (CH-Pt NPs) were prepared by solvent diffusion technique.Citation26 Briefly, CH-Pt (200 mg) was firstly dissolved in acetic acid solution (10 mL). The solution was added drop-by-drop into the Milli-Q water (40 mL) stirred at 600 rpm at room temperature. CH-Pt NPs were collected by centrifugation at 15,000 rpm for 10 min, resuspended in Milli-Q water and stored at 2°C–8°C.
HA-GEM/CH-Pt NPs were prepared by self-assembly technique. Briefly, HA-GEM (200 mg) was dissolved in Milli-Q water (10 mL), added drop-by-drop into the CH-Pt NP suspension and stirred at 600 rpm at room temperature. HA-GEM/CH-Pt NPs were collected by centrifugation at 15,000 rpm for 10 min, resuspended in Milli-Q water and stored at 2°C–8°C.
HA-coated CH-Pt NPs without GEM were prepared by the same method using HA instead of HA-GEM, named HA/CH-Pt NPs.
HA-GEM-coated CH NPs without Pt were prepared by the same method using CH instead of CH-Pt, named HA-GEM/CH NPs.
Blank NPs without Pt and GEM were prepared by the same method using HA instead of HA-GEM, and CH instead of CH-Pt, named blank HA/CH NPs.
Particle morphology, particle size and zeta potential
The morphology of HA-GEM/CH-Pt NPs was analyzed using transmission electron microscopy (TEM).Citation27 An aliquot of the NP suspensions was placed onto a clean copper grid and stained using 2% phosphotungstic acid for 2 min. The stained samples were then imaged using a Hitachi H-7000 transmission electron microscope (Hitachi, Tokyo, Japan). Particle size, polydispersity (PDI) and zeta potential of different samples were determined with Zeta Sizer Nano ZS apparatus (Malvern, Southborough, MA, USA).
Drug encapsulation and loading efficiency
The drug encapsulation efficiency (EE) and drug loading efficiency (DL) were measured after the drugs were removed from the NPs by ultrafiltration (Millipore, Boston, MA, USA).Citation28 The amount of Pt in NPs was measured by using the HITACHI P-4010 inductively coupled plasma mass spectrometry (Hitachi Ltd, Kyoto, Japan).Citation29 The amount of GEM in NPs was determined by HPLC (LC-20A; Shimadzu, Kyoto, Japan).Citation25 Chromatographic separations were carried out using the Inertsil® ODS-3V (250×4.6 mm). The mobile phase was methanol and water (90:10, v/v); the detection wavelength was 248 nm. The EE and DL were calculated as follows:
Serum stability of NPs
The stability of NPs in serum was evaluated by incubating the NPs with 10% (v/v) fetal bovine serum (FBS) at 37°C for 72 h.Citation30 Samples (100 μL) were diluted in Milli-Q water at predetermined time points. The variation in size, PDI and EE was measured to determine the stability of the particles.
In vitro drug release
In vitro GEM and Pt release from NPs was measured by dialysis technique in phosphate-buffered saline (PBS) (pH 7.4) at 37°C.Citation31 HA-GEM/CH-Pt NPs and other NPs were added into the dialysis tube with a molecular weight cutoff of 3 kDa and dialyzed against 15 mL of PBS in a thermo-controlled shaker with a stirring speed of 100 rpm at 37°C for 72 h. The amount of drugs released was determined by the method mentioned in section “Drug encapsulation and loading efficiency”.
In vitro cytotoxicity and synergistic effects analysis
In vitro cytotoxicity of HA-GEM/CH-Pt NPs was determined using MTT assay.Citation32 Briefly, NCl-H460 cells were seeded in 96-well plates at a density of 5,000 cells per well at 37°C in a 5% CO2 incubator 24 h prior to drug treatment. Subsequently, cells were treated with HA-GEM/CH-Pt NPs, HA-GEM/CH NPs, HA/CH-Pt NPs, CH-Pt NPs, free GEM, free Pt, free GEM and PT combination (free GEM/Pt), blank HA/CH NPs, 0.9% saline control and incubated for 72 h. After treatment, 20 μL MTT (5 mg/mL in PBS) reagent was added to each well and incubated for an additional 4 h at 37°C. The medium was discarded, the formed formazan crystals were dissolved in 200 μL of DMSO and absorbance was read at 570 nm. Cell viability and half-maximal inhibitory concentration (IC50) were then calculated for each sample.
Synergistic effects of the double drug-contained systems were evaluated by combination index (CI) analysis based on the Chou and Talalay’s method.Citation33 CI values for GEM and Pt combinations were calculated according to the following equation: CI = (D)GEM/(Dx)GEM + (D)Pt/(Dx)Pt, where (D)GEM and (D)Pt are the concentrations of GEM and Pt in the combination system at the ICx value; (Dx)GEM and (Dx)P are ICx value of GEM alone and Pt alone. CIx <1 represents synergism and CIx >1 represents antagonism. In this study, CI50 values were applied and the IC50 values were used for calculation.
In vivo tissue distribution analysis
In vivo tissue distribution analysis were conducted on NCl-H460 cells of mouse models.Citation34 NCl-H460 cells (1×107 cells in 200 μL of DMEM) were subcutaneously injected in the right flank regions of mice. When the tumor volumes reached around 100 mm3, the mice were randomly distributed into 9 groups with 8 mice in each group, and intravenously injected with HA-GEM/CH-Pt NPs, HA-GEM/CH NPs, HA/CH-Pt NPs, CH-Pt NPs, free GEM/Pt, free GEM, free Pt, blank HA/CH NPs and 0.9% normal saline (the dosage of GEM and/or Pt is 5 μg per g mice). At 12 and 72 h after intravenous injection, mice were sacrificed and the tumor and other main tissue samples (heart, liver, spleen, lung and kidney) were collected, weighed and homogenized with physiological saline to determine the amount of drugs in each tissue. The amount of drugs was determined by the method mentioned in section “Drug encapsulation and loading efficiency”.
In vivo antitumor effect and systemic toxicity
In vivo antitumor effect and systemic toxicity were conducted on NCl-H460 cells of mouse models as mentioned in section “In vivo tissue distribution analysis”.Citation35 When the tumor volumes reached around 100 mm3, HA-GEM/CH-Pt NPs, HA-GEM/CH NPs, HA/CH-Pt NPs, CH-Pt NPs, free GEM/Pt, free GEM, free Pt, blank HA/CH NPs and 0.9% normal saline were administered intravenously every 3 days until 3 weeks (the dosage of GEM and/or Pt is 5 μg per g mice). The tumor sizes, tumor weights and body weights were closely monitored every 3 days. The tumor volume for each time point was calculated using the following formulation: length × width2/2.
Statistical analysis
Student’s t-test or analysis of variance was utilized to determine statistical significance among different groups. A P-value less than 0.05 was considered to be statistically significant.
Results
Synthesis and characterization of CH-Pt and HA-GEM
The formation of CH-Pt and HA-GEM was confirmed by 1H-NMR and FT-IR spectroscopy. The 1H-NMR spectra (DMSO-d6, 300 MHz) of CH-Pt showed the peaks at δ (ppm) =2.05 (–NH2 of Pt), 2.45 (–CH2–CO–N–), 3.21 (–CH–O–), 4.26–4.78 (peak of H on the sugar rings of CH), 8.03 (–CO–NH–) (). The 1H-NMR spectra of HA-GEM showed the peaks at δ (ppm) =2.01 (–OH), 3.32 (–CH2–CO–N–), 3.21 (–CH–O–C), 3.61 (–CH–OH), 3.86 (–CH–CO–N–), 4.53 (–CH–OH), 5.22 (–CH–O–C), 5.73 (–N–CH–O–), 6.67 (–N–CH=C–), 7.96 (–CO–NH–) (). The relevant peaks were marked in the spectra as well as in the structural formula. The FT-IR spectrum of CH-Pt and HA-GEM showed a peak stretching at 1,657 cm−1 and 1,644 cm−1 representing carbonyl bond of amide (–CO–NH–), respectively.
Physicochemical properties of NPs
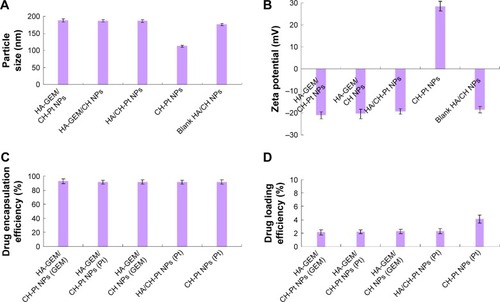
Physicochemical properties of NPs including particle morphology, particle size, zeta potential, EE and DL were characterized. TEM image of HA-GEM/CH-Pt NPs displayed a core-shell structural spherical morphology and a size of around 200 nm (). The size of HA-GEM/CH-Pt NPs, HA-GEM/CH NPs, HA/CH-Pt NPs, blank HA/CH NPs and CH-Pt NPs was 187, 186, 186, 175 and 113 nm, respectively (). The size was slightly increased when loading with drugs. However, coating of HA shell enlarged the particles to a large extent. The zeta potential of CH-Pt NPs was 28 mV, which shifted to −21 mV after coating of HA layer to form HA-GEM/CH-Pt NPs. The EE of both GEM and Pt was about 90% for all the formulations tested. The DL of GEM and Pt was between 2%–4%.
Figure 4 TEM image of HA-GEM/CH-Pt NPs.

Figure 5 The size (A), zeta potential (B), EE (C), and DL (D) of HA-GEM/CH-Pt NPs, HA-GEM/CH NPs, HA/CH-Pt NPs, blank HA/CH NPs and CH-Pt NPs.
Abbreviations: EE, drug encapsulation efficiency; DL, drug loading efficiency; HA, hyaluronic acid; GEM, gemcitabine; CH, chitosan; Pt, platinum (IV); NPs, nanoparticles.
Serum stability
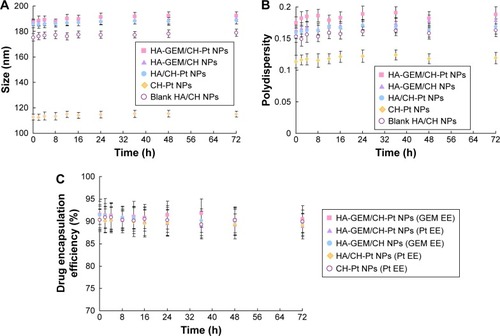
Serum stability of NPs was evaluated by measuring the size, PDI and EE of NPs in the presence of FBS. HA-GEM/CH-Pt NPs, blank HA/CH NPs and CH-Pt NPs exhibited no significant changes in size, PDI or EE (). So, the NPs prepared were considered stable during the period of 72 h.
Figure 6 Serum stability of HA-GEM/CH-Pt NPs, HA-GEM/CH NPs, HA/CH-Pt NPs, blank HA/CH NPs and CH-Pt NPs evaluated by measuring the size (A), PDI (B) and EE (C) of NPs in the presence of FBS.
Abbreviations: HA, hyaluronic acid; GEM, gemcitabine; CH, chitosan; Pt, platinum (IV); NPs, nanoparticles; PDI, polydispersity; EE, drug encapsulation efficiency; FBS, fetal bovine serum.
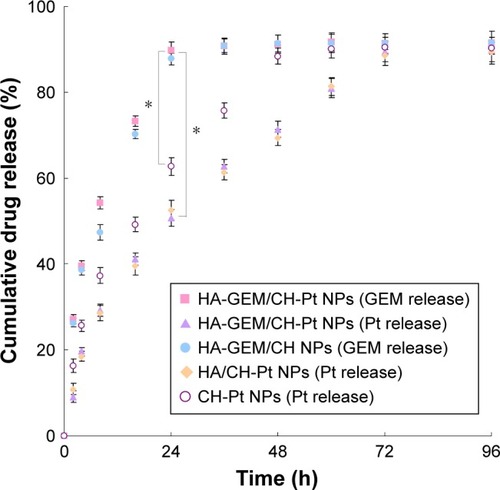
In vitro drug release
In vitro GEM and/or Pt release from HA-GEM/CH-Pt NPs and other NPs was studied and the profiles were described (). The data indicated that both drugs were released from the NPs in a sustained manner and no burst release was observed. The GEM release from HA-GEM/CH-Pt NPs and HA-GEM/CH-NPs was faster than Pt release from the NPs (P<0.05). The release of Pt from HA-GEM/CH-Pt NPs and HA/CH-Pt NPs was slower than that from CH-Pt NPs (P<0.05).
In vitro cytotoxicity and synergistic effects
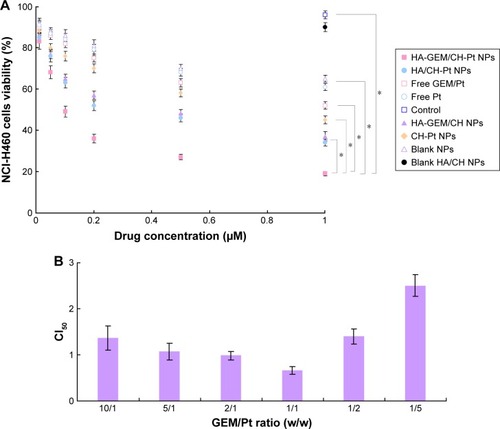
In vitro cytotoxicity of HA-GEM/CH-Pt NPs and other NPs was evaluated on NCl-H460 cells by MTT assay. At all the studied drug concentrations, the cytotoxicity of drug-loaded NPs was better than free drug solutions (P<0.05) (). To select the suitable GEM to Pt ratio to get the best synergism effect, CI50 values were calculated in HA-GEM/CH-Pt NPs. HA-GEM/CH-Pt NPs showed the best synergism when GEM to Pt ratio was 1/1 (). So, this ratio was used for the preparation and evaluation of HA-GEM/CH-Pt NPs.
Figure 8 In vitro cytotoxicity of HA-GEM/CH-Pt NPs and other NPs evaluated in NCl-H460 cells for 72 h (A). CI50 values calculated in HA-GEM/CH-Pt NPs (B).
Abbreviations: HA, hyaluronic acid; GEM, gemcitabine; CH, chitosan; Pt, platinum (IV); NPs, nanoparticles; CI, combination index.
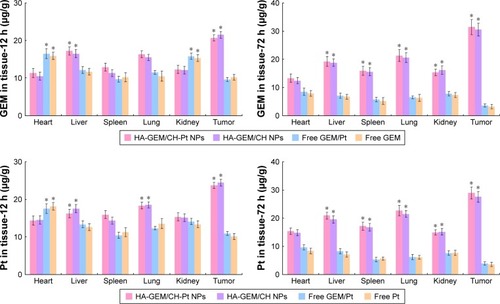
In vivo tissue distribution
In vivo GEM and Pt distribution of drug-loaded NPs were higher than drugs solutions in tumor tissues (P<0.05) (). Drugs solutions were distributed in a higher quantity in the heart and kidneys; on the contrary, the drug-loaded NPs were mainly distributed in the lungs and liver. Distribution of drug solution was relatively high when tested at 12 h. However, it decreased dramatically at 72 h post-injection.
In vivo antitumor effect and systemic toxicity
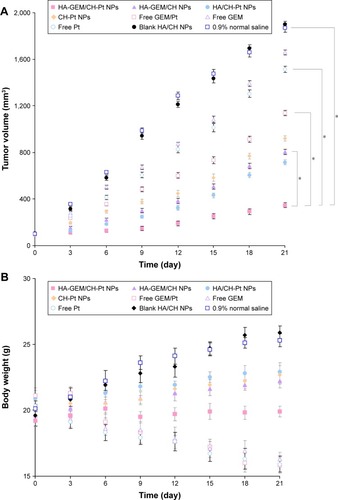
In vivo antitumor effect and systemic toxicity were conducted on NCl-H460 cells of mouse models (). The tumors treated with HA-GEM/CH-Pt NPs were significantly smaller than those treated with single-drug-loaded NPs and drug solutions (P<0.05). By contrast, the tumors treated with blank NPs were similar to those treated with saline (). Body weight changes were observed to determine the systemic toxicity of the systems (). Obviously, increases in mean body weights were found in 0.9% saline and blank NPs groups, and weight decrease was found in drug solution groups. No obvious changes of body weights were found in drug-loaded NPs groups.
Figure 10 In vivo antitumor effect and systemic toxicity conducted in NCl-H460 cells in mouse models. (A) Tumor growth inhibition curves; (B) body weight changes.
Abbreviations: HA, hyaluronic acid; GEM, gemcitabine; CH, chitosan; Pt, platinum (IV); NPs, nanoparticles.
Discussion
In this study, CH-Pt and HA-GEM prodrugs were synthesized. LbL NPs were prepared by using CH-Pt as positive charged core and HA-GEM as negatively charged shell. The physicochemical properties of the NPs and biological efficiency on cancer cells and cancer-bearing animal models were studied.
CH-Pt was synthesized by linking the carboxyl groups of Pt (IV) complex with the amino groups of CH. Pt (IV) prodrugs are typically designed based on cytotoxic Pt (II) constructs and commonly include CIS, carboplatin, oxaliplatin and [Pt(dach)Cl2] structures.Citation24 Pt (II) could be oxidized by treatment with hydrogen peroxide or ozone. The trans-dihydroxo Pt (IV) derivative can be synthesized by treating the trans-dihydroxo complex with acid anhydrides or acid chlorides (). HA-GEM was synthesized by conjugating HA with GEM using amido linkage (). HA-GEM exhibited strongly negative charges due to the hydroxyl and carboxyl groups in HA.Citation17
Enhanced permeability and retention (EPR) effect in tumor tissues could be utilized for nanosized drug delivery.Citation36 Drug could accumulate at the tumor site and enhance tumor targeting based on the EPR effect, thereby reducing the drug dose needed to obtain a greater antitumor effect and minimizing toxicity. Particle sizes smaller than 200 nm are conducive to drug accumulation at the tumor site.Citation37 The sizes of the HA-GEM/CH-Pt NPs, blank HA/CH NPs and CH-Pt NPs were less than 200 nm in this study. Zeta potential is an important parameter among the physicochemical characteristics of NPs. It affects the cellular and tissue uptake efficiency and toxic effect on cells. For positively charged NPs, cytotoxicity remains a problem especially in vivo.Citation38 For instance, cationic NPs may cause cell shrinking, reduced number of cell mitoses and vacuolization of the cytoplasm. To overcome these limitations, anionic NPs were developed to reduce the cytotoxicity.Citation39 The zeta potential of CH-Pt NPs was 28 mV, which shifted to −21 mV after coating of HA layer to form HA-GEM/CH-Pt NPs. EE was over 90% for the two drugs loaded in different NPs. High and comparable EE of each component is a prerequisite for controlled loading of several modalities in the same NP. Serum stability of NPs in 10% FBS was tested to simulate the in vivo hemocompatibility of systems.Citation40 The size, PDI and EE of all kinds of particles in serum showed no obvious change during 72 h of incubation. This could be the evidence that the NPs are stable in the presence of serum, and the particles may not aggregate following in vivo administration.
Sustained release of drugs from both HA-GEM/CH-Pt NPs and CH-Pt NPs was observed in the in vitro drug release study. The core-shell structured NPs affected the release behavior of the systems. The GEM release from HA-GEM/CH-Pt NPs was the fastest, since GEM was released from the outer shell of the NPs. The release of Pt from HA-GEM/CH-Pt NPs was slower than that from CH-Pt NPs, which indicate that the HA-GEM layer at the interface of the CH-Pt core acts as a molecular fence that helps to retain the drugs inside the NPs.Citation31 The sustained release manner of HA-GEM/CH-Pt NPs could protect the drugs for a relatively longer time from being degraded in the circulation system, prevent premature drug release prior to reaching the tumor sites and thus may perform persistent therapeutic effect.Citation41
The in vitro cytotoxicity of both drug-loaded NPs and free drugs reduced cell viability in a concentration-dependent manner. Higher cytotoxicity of drug-loaded NPs was better than free drug, indicating that NPs delivery systems can enhance the cytotoxicity in vitro. The single-drug-loaded NPs show similar effect to free dual drugs GEM/Pt in vitro; this may be due to the synergistic effects of the two drugs used together. However, the double/single-drug-loaded NPs show significant effect to the free double/single-drug groups (P<0.05). In addition, blank NPs showed negligible toxicity (data not shown). To validate the synergistic effect of drugs co-loaded with NPs, the CI was further determined using the isobologram equation of Lv et al.Citation42 HA-GEM/CH-Pt NPs displayed an overall CI value <1 when GEM to Pt ratio was 1/1; in this ratio, the combined antitumor effect of the dual-drug-loaded NPs was better than the single-drug-loaded ones and could develop the ability of the drugs to a large extent.
Solid tumors have leakage microvasculatures. Nanosized particles may be delivered to the tumor tissue owing to the EPR effects.Citation29 EPR effects also made the entry of the NPs into the tumor more easily, thus prevented the entry of the system in the normal tissue. In vivo tissue distribution results showed that the administration of drug-loaded NPs led to a dramatic increase of drug accumulation in the tumor tissue than the free drug solutions. NPs of this size are typically found in the liver and spleen, and have low signal in other organs. The high signal from kidney is in the 72 h of the drugs loaded NPs may prove the long circulation time of the NPs and the disintegrate happened at the end of the 72 h after injection. This behavior may help with the performance of systems for the in vivo antitumor therapy.
In vivo tumor growth was more prominently inhibited by HA-GEM/CH-Pt NPs than the single-drug-loaded NPs and drug solutions. This could be the evidence that dual-drug-loaded NPs could be synergetic in the treatment of lung cancer in vivo, which is in accordance with the results of the in vitro cytotoxicity and synergistic effects studies. To be noticed, improved anticancer activity of HA-GEM/CH-Pt NPs was obtained along with a lower toxicity than the drugs injected alone or mixed together, which could be observed by the loss of body weight of the animals.Citation36 According to the tissue distribution results, drug solutions were distributed in a higher quantity in the heart and kidneys; on the contrary, the drug-loaded NPs were mainly distributed in the tumor, lungs and liver. Drug distribution in the heart and kidney may cause systemic toxicity; distribution in a lower quantity in the heart and kidneys could decrease the side effects and lead to better antitumor therapeutic efficiency. These studies demonstrated that dual drugs co-loaded lay-by-layer with HA-GEM/CH-Pt NPs, with the most significant synergistic therapeutic efficacy, exhibited no significant toxicity to major organs and tissues. Therefore, the combination therapy system is a promising strategy in enhancing the lung cancer chemotherapy.
Conclusion
In the present study, LbL NP co-loading GEM and Pt (IV) prodrugs were designed for synergistic combination therapy of lung cancer. The LbL NPs consisted of the CH-Pt core and the HA-GEM layer. LbL HA-GEM/CH-Pt NPs exhibited the most significant synergistic therapeutic efficacy but showed no significant toxicity to major organs and tissues. Therefore, the combination therapy system is a promising strategy in enhancing the lung cancer chemotherapy.
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statistics, 2016CA Cancer J Clin2016661 7 3026742998
- ShiCYuHSunDCisplatin-loaded polymeric nanoparticles: characterization and potential exploitation for the treatment of non-small cell lung carcinomaActa Biomater201518 68 7625707922
- ReckMHeigenerDFMokTSoriaJCRabeKFManagement of non-small-cell lung cancer: recent developmentsLancet20133829893 709 71923972814
- HegerZPolanskaHKrizkovaSCo-delivery of VP-16 and Bcl-2-targeted antisense on PEG-grafted oMWCNTs for synergistic in vitro anti-cancer effects in non-small and small cell lung cancerColloids Surf B Biointerfaces2017150 131 14027907860
- GrecoFVicentMJCombination therapy: opportunities and challenges for polymer-drug conjugates as anticancer nanomedicinesAdv Drug Deliv Rev20096113 1203 121319699247
- MörthCValachisASingle-agent versus combination chemotherapy as first-line treatment for patients with advanced non-small cell lung cancer and performance status 2: a literature-based meta-analysis of randomized studiesLung Cancer2014843 209 21424702946
- MastersGATeminSAzzoliCGAmerican Society of Clinical Oncology Clinical PracticeSystemic therapy for stage IV non-small-cell lung cancer: American society of clinical oncology clinical practice guideline updateJ Clin Oncol20153330 3488 351526324367
- ScagliottiGVParikhPvon PawelJPhase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancerJ Clin Oncol20082621 3543 355118506025
- CarteiGBinatoSSaccoCSimplified gemcitabine and platin regimen in patients with advanced or metastatic non-small cell lung cancer (NSCLC) to be proposed as neoadjuvant therapyAnn Oncol200617Suppl 5 v47 v5116807462
- HaxtonKJBurtHMPolymeric drug delivery of platinum-based anticancer agentsJ Pharm Sci2009987 2299 231619009590
- NajjarARajabiNKaramanRRecent approaches to platinum(IV) prodrugs: a variety of strategies for enhanced delivery and efficacyCurr Pharm Des20172316 2366 237628155621
- JohnstoneTCSuntharalingamKLippardSJThe next generation of platinum drugs: targeted Pt(II) agents, nanoparticle delivery, and Pt(IV) prodrugsChem Rev20161165 3436 348626865551
- MarcatoPDFávaroWJDuránNCisplatin properties in a nanobiotechnological approach to cancer: a mini-reviewCurr Cancer Drug Targets2014145 458 47624806969
- DubeyRDSanejaAGuptaPKGuptaPNRecent advances in drug delivery strategies for improved therapeutic efficacy of gemcitabineEur J Pharm Sci201693 147 16227531553
- LuoCSunJSunBHeZProdrug-based nanoparticulate drug delivery strategies for cancer therapyTrends Pharmacol Sci20143511 556 56625441774
- FangJYAl-SuwayehSANanoparticles as delivery carriers for anticancer prodrugsExpert Opin Drug Deliv201296 657 66922507134
- NohIKimHOChoiJCo-delivery of paclitaxel and gemcitabine via CD44-targeting nanocarriers as a prodrug with synergistic antitumor activity against human biliary cancerBiomaterials201553 763 77425890771
- MortonSWPoonZHammondPTThe architecture and biological performance of drug-loaded LbL nanoparticlesBiomaterials20133421 5328 533523618629
- RamasamyTHaidarZSTranTHLayer-by-layer assembly of liposomal nanoparticles with PEGylated polyelectrolytes enhances systemic delivery of multiple anticancer drugsActa Biomater20141012 5116 512725169256
- SuhMSShenJKuhnLTBurgessDJLayer-by-layer nanoparticle platform for cancer active targetingInt J Pharm20175171–2 58 6627923697
- ZhouDXiaoHMengFLayer-by-layer assembled polypeptide capsules for platinum-based pro-drug deliveryBioconjug Chem20122312 2335 234323176570
- FicaiDSonmezMAlbuMGMihaiescuDEFicaiABleotuCAntitumoral materials with regenerative function obtained using a layer-by-layer techniqueDrug Des Devel Ther20159 1269 1279
- MortonSWShahNJQuadirMADengZJPoonZHammondPTOsteotropic therapy via targeted layer-by-layer nanoparticlesAdv Healthc Mater201436 867 87524124132
- ChinCFWongDYJothibasuRAngWHAnticancer platinum (IV) prodrugs with novel modes of activityCurr Top Med Chem20111121 2602 261222039869
- LuZSuJLiZZhanYYeDHyaluronic acid-coated, prodrug-based nanostructured lipid carriers for enhanced pancreatic cancer therapyDrug Dev Ind Pharm2017431 160 17027553814
- FabianoABizzarriRZambitoYThermosensitive hydrogel based on chitosan and its derivatives containing medicated nanoparticles for transcorneal administration of 5-fluorouracilInt J Nanomedicine201712 633 64328144144
- BabuAWangQMuralidharanRShankerMMunshiARameshRChitosan coated polylactic acid nanoparticle-mediated combinatorial delivery of cisplatin and siRNA/Plasmid DNA chemosensitizes cisplatin-resistant human ovarian cancer cellsMol Pharm2014118 2720 273324922589
- NiSQiuLZhangGZhouHHanYLymph cancer chemotherapy: delivery of doxorubicin-gemcitabine prodrug and vincristine by nanostructured lipid carriersInt J Nanomedicine201712 1565 157628280326
- LiCGeXWangLConstruction and comparison of different nanocarriers for co-delivery of cisplatin and curcumin: a synergistic combination nanotherapy for cervical cancerBiomed Pharmacother201786 628 63628027539
- ChenWGuoMWangSAnti prostate cancer using PEGylated bombesin containing, cabazitaxel loading nanosized drug delivery systemDrug Dev Ind Pharm20164212 1968 197627143168
- MiaoLGuoSZhangJKimWYHuangLNanoparticles with precise ratiometric co-loading and co-delivery of gemcitabine monophosphate and cisplatin for treatment of bladder cancerAdv Funct Mater20142442 6601 661125395922
- ZhangJMiaoLGuoSSynergistic anti-tumor effects of combined gemcitabine and cisplatin nanoparticles in a stroma-rich bladder carcinoma modelJ Control Release2014182 90 9624637468
- ChouTCTalalayPQuantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitorsAdv Enzyme Regul198422 27 556382953
- XiongYZhaoYMiaoLLinCMHuangLCo-delivery of polymeric metformin and cisplatin by self-assembled core-membrane nanoparticles to treat non-small cell lung cancerJ Control Release2016244Pt A 63 7327840166
- TaiWMoRLuYJiangTGuZFolding graft copolymer with pendant drug segments for co-delivery of anticancer drugsBiomaterials20143525 7194 720324875756
- ZhuBYuLYueQCo-delivery of vincristine and quercetin by nanocarriers for lymphoma combination chemotherapyBiomed Pharmacother201791 287 29428463792
- PradosJMelguizoCOrtizRDoxorubicin-loaded nanoparticles: new advances in breast cancer therapyAnticancer Agents Med Chem2012129 1058 107022339066
- YeJWangALiuCChenZZhangNAnionic solid lipid nanoparticles supported on protamine/DNA complexesNanotechnology20081928 28570821828742
- ToriiYSugimuraNMitomoHNiikuraKIjiroKpH-responsive coassembly of oligo(ethylene glycol)-coated gold nanoparticles with external anionic polymers via hydrogen bondingLangmuir20173322 5537 554428505438
- MaPLiTXingHLocal anesthetic effects of bupivacaine loaded lipid-polymer hybrid nanoparticles: in vitro and in vivo evaluationBiomed Pharmacother201789 689 69528267672
- PoonCHeCLiuDLuKLinWSelf-assembled nanoscale coordination polymers carrying oxaliplatin and gemcitabine for synergistic combination therapy of pancreatic cancerJ Control Release2015201 90 9925620067
- LvSTangZLiMCo-delivery of doxorubicin and paclitaxel by PEG-polypeptide nanovehicle for the treatment of non-small cell lung cancerBiomaterials20143523 6118 612924794923