Abstract
Gut microbiota and its metabolites play pivotal roles in host physiology and pathology. Short-chain fatty acids (SCFAs), as a group of metabolites, exert positive regulatory effects on energy metabolism, hormone secretion, immune inflammation, hypertension, and cancer. The functions of SCFAs are related to their activation of transmembrane G protein-coupled receptors and their inhibition of histone acetylation. Though controversial, growing evidence suggests that SCFAs, which regulate inflammation, oxidative stress, and fibrosis, have been involved in kidney disease through the activation of the gut–kidney axis; however, the molecular relationship among gut microbiota–derived metabolites, signaling pathways, and kidney disease remains to be elucidated. This review will provide an overview of the physiology and functions of SCFAs in kidney disease.
Introduction
The human intestinal tract harbors a diverse and complex microbial community, which plays a pivotal role in health. In recent years, gut microbiota–derived metabolites have been shown to influence host physiology and pathology. Changes in these metabolites exert major consequences, both harmful and beneficial, to the host’s health. On one hand, metabolites – particularly, short-chain fatty acids (SCFAs) – are generally proven to promote health.Citation1–Citation3 On the other hand, uremic toxins, including indoles, ammonia, and trimethylamine N-oxide, produced by the gut microbiota enhance the development and progression of chronic kidney disease (CKD).Citation4–Citation7 An insufficiency in gut microbiota–generated SCFAs is also associated with illnesses, including inflammatory bowel disease, obesity, type 1 and 2 diabetes mellitus, autism, major depression, colon cancer, as well as kidney diseases – the focus of the discussion.Citation8–Citation11 The functions of SCFAs are mainly related to their activation of transmembrane G protein-coupled receptors (GPCRs) and their inhibition of histone acetylation (HDAC).Citation12 Due to their positive effects, therapeutic studies of SCFAs have been carried out both in clinical and in animal studies. However, the mechanisms of SCFAs in the gut–kidney axis have yet to be fully explored. This review will provide an overview of the physiology and functions of gut microbiota–derived SCFAs in kidney disease. Before we outline the known roles of SCFAs in renal diseases, we will first review what is known regarding the basic functions and systemic roles of SCFAs.
Gut microbiota–derived SCFAs
Definition, production, and transportation of SCFAs
SCFAs are straight-chain saturated fatty acids composed of less than six carbon atoms, among which acetate (two carbons), propionate (three carbons), and butyrate (four carbons) are the most abundant in the human intestinal tract.Citation13 These SCFAs are the end products of fermentation by the microbiota from complex polysaccharides,Citation14 including non-digestible dietary fibers like inulinCitation15 and endogenous substrates like epithelial-derived mucus.Citation16 SCFAs not only exist in the gut but also could be absorbed in the bloodstream.Citation17 There are two main mechanisms of SCFA absorption from the gut to the circulatory system: anion exchange between SCFAs and HCO3− across the membrane and diffusive movement promoted by the pH gradient during the diffusion of protonated SCFAs.Citation18 When entering the circulatory system, SCFAs influence several physiology process as ligands for G-protein couple receptors (GPR41, GPR43, GPR109A, and olfactory receptor 78) or as epigenetic regulators (HDAC inhibitors).Citation2
Multiple factors affect the concentration of SCFAs in the gut, including the amount/type of fermentable carbohydrate consumption, the composition/diversity of the microbiota, and the interactions between microbes and the host. Moreover, mother-to-child transmission is involved in the gut microbiota and its metabolites; for instance, the mode of delivery (vaginal birth or caesarean section) and feeding patterns (breastfed or bottle-fed infants) play a role in the formation of intestinal microbiota and microbial products.Citation19
Receptors and epigenetic regulation related to SCFAs
The physiological roles of SCFAs are mainly to act as ligands for GPCRs or as inhibitors of HDAC.Citation2,Citation20 GPR41 and GPR43, which are the most studied, are shown to be activated by SCFAs.Citation21 GPR41 is widely distributed in adipose tissue and at low levels in the spleen, lymph nodes, bone marrow, peripheral blood mononuclear cells, and blood vessel endothelial cells,Citation21 while GPR43 is primarily expressed in immune cells, adipocytes, islets, and gastrointestinal tract. GPR43 has a potential role in inflammation and metabolic disorders.Citation22–Citation25 More importantly, GPR41 and GPR43 both are expressed in the kidney and renal arteries.Citation26 The potencies of SCFAs are different, but the rank order has remained generally consistent among different investigators ().Citation21,Citation27 Olfr78 is another key receptor for SCFAs, which is expressed on vascular smooth muscle cells, including subset of large renal vessels, renal afferent arteriole, and juxtaglomerular apparatus, where it participates in the regulation of renin secretion in response to SCFAs.Citation26,Citation28 Unlike other receptors, Olfr78 is more sensitive to acetate and propionate but not to butyrate. In addition, GPR109a is reported to express on gut epithelial cells, adipocytes, macrophages, and dendritic cells, which only respond to butyrate and not to acetate or propionate.Citation29–Citation32 Finally, when it comes to the physiological role as HDAC inhibitors, compared to propionate, butyrate is more potent in terms of pan-inhibitory activity. SCFAs affect the expression of genes with diverse functions by inhibiting the activity of HDAC to exhibit anti-tumor, anti-fibrotic, and anti-inflammatory activities.Citation20,Citation33 Additionally, SCFA-mediated inhibition of HDACs might be independent of GPCRs and GPR41 is involved in the process.Citation34
Table 1 The expression and major functions of SCFAs receptors
The functions of SCFAs
As reported, SCFAs regulate tissue-specific health, as well as systemic health, including appetite, gastrointestinal motility, colitis, metabolic syndrome, airway disease, and even carcinogenesis.Citation35–Citation37 All of these influences are derived from the complex functions of SCFAs, including influencing energy metabolism, evoking hormone release, and regulating immune inflammation and blood pressure.
Energy metabolism
Locally, SCFAs (butyrate preferentially) are used as fuel for colonocytes and in the maintenance of the epithelium.Citation7 After absorption into the bloodstream,Citation17 circulatory SCFAs act as a primary substrate for hepatic and adipocyte lipogenesis, as well as for intestinal gluconeogenesis, and exhibit a range of metabolic effects ().Citation38,Citation39 For example, SCFAs activate AMP-activated protein kinases (AMPK) in the liver and muscle, thereby triggering the activation of peroxisome proliferator-activated receptors, and thus stimulating glucose uptake and fatty acid oxidation and improving glycemic control, at least in murine models.Citation40
Figure 1 Short-chain fatty acids to host appetite and metabolism control. Short-chain fatty acids (SCFAs) produced via microbiota fermentation of non-digestible dietary fibers or endogenous substrates can be used as fuel for colonocytes and stimulate intestinal gluconeogenesis, which improves glucose tolerance. Moreover, SCFAs can stimulate enteroendocrine L cells to release anorexigenic hormones PYY and GLP-1. These hormones promote satiety and suppress appetite which may promote weight loss. GLP-1 increases the production of insulin and decreases the production of glucagon in the pancreas, which then increases the uptake of glucose in muscle and adipose tissues. SCFAs can also decrease fatty acid synthesis and promote fatty acid oxidation in the liver. In adipose tissue, SCFAs can increase adipogenesis and inhibit lipolysis, thereby decreasing free fatty acids. Meanwhile, SCFAs can promote the secretion of leptin that suppresses appetite.
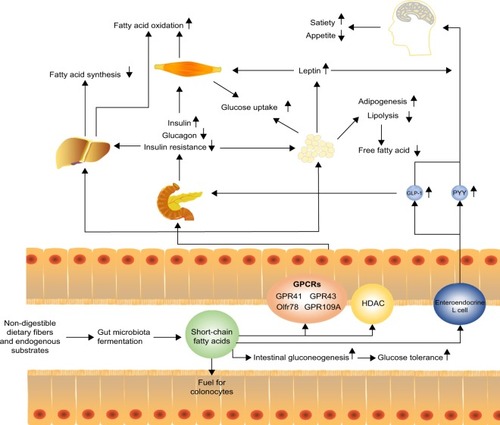
Several studies reported the benefits of regulating lipid or glucose metabolism by resistant starch, which increased SCFAs productionCitation41,Citation42 or fecal transplantation of butyrate-producing bacteria.Citation43,Citation44 Administration of acetate or propionate in adipocytes of mice reduced plasma free fatty acid levels by enhancing adipogenesis and inhibiting lipolysis via the activation of GPR43.Citation38,Citation45–Citation47 In obese hyperinsulinemia fa/fa rats, propionate lowered urinary glucose excretion and fasting blood glucose levels.Citation48 Amelioration of obesity and its comorbidities, as well as insulin resistance, was also observed in mice fed with dietary supplementation of acetate.Citation40 Not only in an experimental setup, but also in clinical studies, overweight adults supplemented with inulin-propionate ester for a longer term (which could be metabolized by the microbiota in the colon to propionate) showed a significant reduction in weight gain via appetite regulation.Citation49 Patients were advised to increase their dietary fibers, which increased concentrations of SCFAs in the gut and circulatory system; this was associated with the reduction of adverse consequences of hyperglycemia.Citation50
The benefits of SCFAs on energy metabolism could be partially explained by modulating the secretion of hormones such as peptide YY (PYY), glucagon-like peptide 1 (GLP-1), and leptin by activating GPR41 and GPR43.Citation48,Citation51,Citation52 PYY, a gut hormone derived from enteroendocrine cells, could suppress postprandial appetite, slow gastrointestinal motility, decrease insulin secretion and sensitivity, and increase glucose uptake by SCFAs-stimulated GPR41.Citation38,Citation53–Citation55 In contrast, GLP-1 influences peripheral metabolic effects by stimulating insulin secretion and increasing glucose tolerance. Further, GLP-1 exerts cardioprotective effects and induces beta-cell proliferation and plays a major role in decreasing epithelial permeability and increasing mucosal antibacterial defenses by GPR41 and GPR43 activation.Citation56–Citation60 SCFA-induced leptin is involved in regulating appetite and energy metabolism by GPCRs activation;Citation45 failure of leptin regulation is connected with obesity, hyperphagia, infertility, and immunological defects.Citation61 In general, SCFA-activated GPR41 or GPR43 promotes hormone secretion that inhibits gastric emptying and food intake and further modulates metabolic functions both locally in the gut and distally at peripheral tissues to remain systemic in metabolic health. However, a recent study in rats showed contradictory results – acetate-induced obesity and insulin resistance.Citation62 Also, SCFA concentrations were found to be higher in feces of obese humans when compared to lean controls.Citation63 This suggests that more studies are required to elucidate the true functions of SCFAs in regulating energy metabolism.
Inflammation and immune regulation
Kidney disease is often related to microinflammation and dysbiosis of immune system. Although the detailed mechanisms by which the gut microbiota regulates host health and renal health have yet to be elucidated, gut microbiota–generated SCFAs, at least partly, mediate inflammatory and immune effects ().
Figure 2 Regulation of short-chain fatty acids to host inflammation and immune. SCFAs can stimulate intestinal epithelial cells to release Muc2, which enhance the gut barrier function and heighten the response to pathogens and commensal bacteria. Moreover, SCFAs can reduce the recruitment of neutrophils under certain condition, with an increase in the levels of TGF-β, IL-10 and a decrease in the levels of IL-6, IL-1β, NO, and TNF-α to inhibit inflammation. Meanwhile, SCFAs promote T-cell production of IL-10 and Treg to prevent inflammatory responses. On the other hand, SCFAs act on DCs to limit the expression of T cell-activating molecules such as MHC II molecules and costimulatory molecules, leading to the generation of tolerogenic T cells rather than inflammatory T cells. The tolerogenic effect of SCFAs on DCs can lower inflammatory responses. However, the direct effect of SCFAs on T cells enhances the generation of Th1 and Th17 cells to boost immunity to fight pathogens, which means that activation of SCFAs for immune cells and epithelial cells may increase inflammatory responses, if not properly regulated.
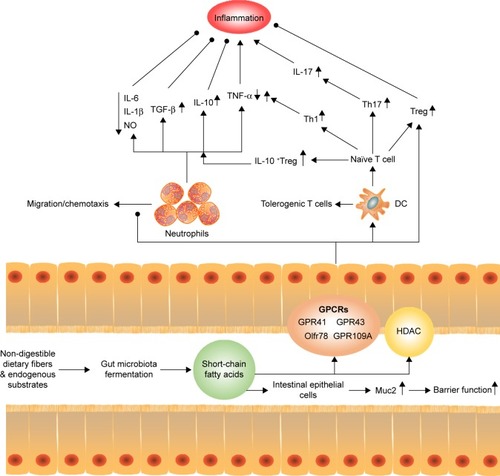
SCFAs modulate inflammation both in intestinal and in extra-intestinal environments via leukocyte recruitment and chemokines production. The anti-inflammatory effects of SCFAs have been well characterized at both the epithelial and immune cell levels. On one hand, SCFAs are involved in the expression of adhesion molecules in neutrophils and endothelial cells that reduce cell recruitment. On the other hand, SCFAs exert anti-inflammatory effects by suppressing the production of cytokines such as interleukin (IL)-6, IL-1β, tumor necrosis factor-α, and nitric oxide,Citation64–Citation69 and/or by increasing the production of anti-inflammatory cytokine IL-10Citation70 via stimulation of GPCRsCitation58,Citation71,Citation72 or inhibition of HDAC.Citation73 Moreover, SCFAs induce IL-10-expressing regulatory T cells to reduce inflammation.Citation74–Citation77 SCFAs also stimulate the migration of neutrophils by chemotaxis via activation of GPR43, which further leads to inflammatory responses.Citation72,Citation78,Citation79 In clinical investigation and animal models, SCFAs have also been demonstrated to possess protective effects on inflammatory bowel conditions, allergic airway disease, and CKD, due to their inhibitory effects on pro-inflammatory cytokines and reactive oxygen species.Citation37,Citation80,Citation81
The generation of SCFAs was also confirmed to influence innate immunity and adaptive immunity. For innate immunity, low concentrations of butyrate stimulate intestinal epithelial cells (goblet cells) to release mucin Muc2, which enhances the gut barrier function and heightens the response to pathogens and commensal bacteria, while high concentrations of butyrate diminish the intestinal barrier function.Citation82 In terms of adaptive immune system, it was illustrated that the number of colonic Tregs are influenced, or even determined, by the luminal concentration of SCFAs through GPCRs or epigenetic modification-inhibition of HDAC.Citation29,Citation59,Citation74–Citation77,Citation83–Citation85 In general, Tregs stimulated by SCFAs always decrease inflammation under certain conditions; SCFAs induce Th1 and Th17 lymphocytes productionCitation35,Citation74 by cellular bioenergetic metabolism via the conversion of SCFAs to acetyl-CoA, integration into the tricarboxylic acid, and subsequent activation of mTOR.Citation86 SCFAs also indirectly affect T-cell differentiation patterns by exerting a broadly immunosuppressive or tolerogenic effect on antigen-presenting cells. For example, SCFAs inhibit the development of myeloid dendritic cells (DCs) from their progenitors,Citation87 as well as their functional maturation,Citation88,Citation89 which then limits their ability to present antigens and cytokines to make effector T cells.Citation35 Further, SCFAs act on DCs to suppress the expression of T cell–activating molecules such as major histocompatibility complex II molecules, costimulatory molecules, and cytokines leading to generation of tolerogenic T cells rather than inflammatory T cells.Citation35,Citation37 The tolerogenic effect of SCFAs on DCs could lower inflammatory responses. Although SCFAs mostly regulate the immune system to decrease inflammation, the activity of SCFAs in immune and epithelial cells may boost inflammatory responses, if not properly regulated.Citation35 Thus, the function of SCFAs still seems to be inconsistent and complex, and future studies could provide mechanistic insights into how gut microbiota–derived metabolites contribute to immune inflammation.
In addition, SCFAs exhibit apparent impacts on cell differentiation, oxidative DNA damage, and apoptosis death through autophagy.Citation18,Citation90–Citation92 SCFAs could modulate blood pressure by regulating renin release and peripheral resistance via Olfr78 and Gpr41 expressed on the afferent arteriole (juxtaglomerular apparatus) and smooth-muscle cells of the small resistance vessels.Citation93–Citation95 So, the functions of SCFAs in most parts are contradictory; it is now becoming clear that gut microbiota–derived metabolites play a central role in host physiology.Citation96
SCFAs in kidney diseases
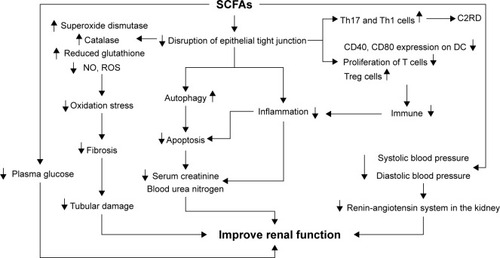
In recent years, considerable studies have explored a new and exciting area: the interaction between the gut microbiome and kidney disease, and have reported that alteration of intestinal microbiota in CKD is an important indicator of impaired renal function and progression of CKD.Citation97 Kidney disease is often related to malnutrition, hypertension or hypotension, microinflammation, dysbiosis of immune system, and multiple oxidative stress, which could be reversed by SCFAs (). Furthermore, growing evidence has highlighted that SCFAs exhibited positive effects on kidney disease in both experimental animals and patients.
Figure 3 Regulation of short-chain fatty acids to improve kidney function SCFAs can decrease the disruption of epithelial tight junction. After getting absorbed in blood, SCFAs can suppress oxidation stress by increasing superoxide dismutase, catalase, and reduced glutathione, and by decreasing nitric oxide (NO) and reactive oxygen species (ROS), which can lead to the decline of renal fibrosis and amelioration of tubular damage. In addition, SCFAs can also inhibit apoptosis by promoting autophagy and suppressing inflammation by regulating immune system, thereby decreasing serum creatine as well as blood urea nitrogen to improve renal function. Regulation of SCFAs on blood pressure and plasma glucose may help to ameliorate renal function in chronic kidney diseases. However, SCFAs can also increase Th17 and Th1 cells to promote acetate- or C2-induced renal disease (C2RD) under certain condition, as the negative outcomes of SCFAs on kidney.
Clinical investigations of gut microbiota–derived SFCAs
Recently, multiple studies focusing on microbiota in CKD or end-stage renal disease (ESRD) patients have reported a correlation between dysbiosis and CKD.Citation7,Citation98 Compared with control groups, patients with CKD or ESRD have altered microbiotaCitation99 with increased bacteria that possessed urease, uricase, p-cresol-, and indole-forming enzymes and reduced bacteria that possessed SCFA-forming enzymes.Citation100 Uremic toxicities produced by microbiota could not only worsen the intestinal environment and alter its composition, but also affect cardiovascular disease progression and mortality in patients with CKD and ESRD.Citation101–Citation105 For treatment of these toxicities, dietary fiber supplementation in a group of chronic hemodialysis patients was reported to reduce serum concentration of indoxyl sulfate and p-cresol sulfate,Citation106 and the use of indoxyl sulfate–binding agent, AST-120, in pre-dialysis CKD patients also improved uremic symptoms, enhanced 5-year survival rate, and potentially delayed the onset of uremia.Citation107,Citation108 Compared to gut microbiota–generated toxicities, there were few studies that focused on SCFAs in CKD/ESRD patients, and most studies were related to dietary management. For instance, in ESRD patients, reduced dietary fiber intake was associated with a reduction in the population of butyrate-forming bacteria,Citation100 and after improving dietary management, colonic-CKD pathology ameliorated.Citation109 On the whole, these observations suggest the potentially beneficial effects of fiber-rich diets on CKD progression. However, despite indirect positive effects of SCFAs on remission of CKD/ESRD, direct effects on kidney diseases are still in the experimental stage and require further study.
Experimental studies of SFCAs in animals
In recent decades, a small but growing number of animal experiments focusing on SCFAs in kidney disease have been reported using different models. However, these are mainly divided into two parts: acute kidney injury (AKI) and CKD ().
Table 2 Applications of SCFAs in animal models of kidney injury
AKI
Regarding AKI, researchers have expanded the role of acetate to explain the gut–kidney connection in several animal models, including ischemia-reperfusion injury, contrast-induced nephropathy (CIN), and gentamicin-induced nephrotoxicity. Though various mechanisms were reported, most of these studies suggest that decreasing inflammation and enhancing antioxidant activity by SCFAs may result in the improvement of renal function.
In the ischemia-reperfusion injury model, treatment with acetate or acetate-producing bacteria could reduce kidney injury.Citation2 The key mechanism of action of SCFAs against kidney injury was suggested to be the reduction of inflammatory cytokines and chemokines locally and systemically, as well as the inhibition of production of reactive oxygen species (ROS), apoptosis, and chromatin modification.Citation2 It is also interesting to note that in the ischemia-reperfusion models of other tissues, SCFAsCitation1 or SCFA-producing bacteriaCitation110 have been shown to exhibit protective effects, implying that the underlying mechanism may be common across tissues. CIN is another form of AKI with exposure contrasting that of the media. A study showed that sodium butyrate protected against kidney injury by inhibiting inflammatory and oxidative tubular damage, with nuclear factor-κB signal pathway playing key roles in the development of CIN.Citation111 However, the study did not exclude other potential mechanisms by which SCFAs may take part in inflammatory response, such as inhibition of HDAC, given that the regulatory mechanisms of SCFAs are extremely complex.
The effect of acute and chronic treatment of sodium butyrate in gentamicin-induced nephrotoxicity was also assessed. In an animal experimental model, kidney injury was attenuated by long-term oral administration of sodium butyrate via enhanced renal antioxidant enzymes activity, which promoted the expression of prohibitin proteinCitation112 and increased the levels of superoxide dismutase, catalase activity, and reduced glutathione.Citation33 Another drug-induced nephropathy, paracetamol-induced nephrotoxicity, was reported to be protected by ethyl acetate extract of Zingiber zerumbet rhizome. This process was also probably mediated by its antioxidant properties.Citation113
Taking into account all of these recent studies, they have almost included all main types of AKI experimental models and have shown the positive effects of acetate or butyrate to improve renal function. Although the results are exciting, they are still not sufficient to drop the veil of SCFAs, and the underlying mechanism still remains unclear; therefore, more studies are that focus on SCFAs are needed.
CKD
Supposing that SCFAs play a significant role in AKI, it might be of interest to define their role in the development and progression of CKD. Increasing dietary fiber in CKD rats showed similar results to clinical research, with significantly improved intestinal epithelial tight junctions, reduced oxidative stress and inflammation, and less severe renal dysfunction.Citation80 Besides increasing SCFAs in uremic rats could improve kidney function, and neutralizing bacteria-derived uremic toxin indoxyl sulfate inside the gut could also delay the progression of CKD and cardiovascular disease.Citation114 The direct effects of SCFAs on kidney disease have been studied in juvenile diabetic rats by administering butyrate post-treatment; the results showed that SCFAs not only decreased plasma glucose, creatinine, and urea but also improved renal histological alterations (including fibrosis and collagen deposition), apoptosis, and DNA damage.Citation115 Supplementation of acetate was reported to attenuate glomerular and tubulointerstitial fibrosis in the DOCA-salt mice.Citation116
However, there are still several negative outcomes of SCFAs on kidney injury. After chronically increasing oral doses of SCFAs to higher than physiological levels in mice, Th1 and Th17 cells were observed to generate in the ureteropelvic junction and proximal part of the ureter, which induced inflammation and led to kidney hydronephrosis, hereafter called acetate- or C2-induced renal disease (C2RD).Citation117 It was indicated that C2RD was not conducted by GPR41/GPR43,Citation117 but the underlying mechanism is still not clear. However, SCFAs were confirmed to play a dual role in the inflammation system depending on the stimulus concentration in kidney disease, consistent with the afore-mentioned inflammation of SCFAs. Besides C2RD, mice fed a high-fiber diet had increased gut butyrate and were more susceptible to infection with Escherichia coli,Citation118 as well as enhanced Gb3 levels in the gut and kidney, which resulted in severe kidney damage.Citation119
Despite growing interest in SCFAs, many problems involving chronic kidney injury have not yet been answered and are still disputed. Unlike the positive effects of SCFAs on AKI models that have been observed, the influence of SCFAs on CKD seems to be more controversial. This enhances the importance of SCFAs concentrations when testing the benefits of SCFAs in kidney disease and encourages further studies to identify the pharmacological concentration. Importantly, the benefits of SCFAs on diabetic nephropathy, the leading cause of ESRD worldwide, should be paid more attention to, because of the positive effects of SCFAs on regulation of energy metabolism and immune inflammation.
Experimental studies of SFCAs in kidney cells
Researchers have explored not only the in vivo effects of SCFAs but also their mechanism of action on kidney cells (). When glomerular mesangial cells (GMCs) are induced by high glucose and lipopolysaccharide (LPS), the pharmacological concentrations of SCFAs accompanied with GPR43 agonist diminish renal inflammation by decreasing MCP-1 and IL-1β. Inflammation and oxidative stress are inseparably linked, as each causes and strengthens the other, which could cause glomerulosclerosis, tubular atrophy, and fibrosis.Citation120 Therefore, besides decreasing inflammation, SCFAs inhibit ROS generation induced by high glucose and LPS in GMCsCitation121 and HK-2 human kidney epithelial cells after hypoxia.Citation2 In addition to GMCs, elevated concentration of butyrate in proximal tubular epithelial cells was described to prevent TGF-β1 generation,Citation122 which is involved in renal fibrosis, and its antagonistic action has been proposed as a potential therapeutic target.Citation123–Citation125 In porcine kidney fibroblast, WT1, involved in cell proliferation and development, was markedly enhanced along with an increase in sodium butyrate levels and by prolonging the treatment.Citation126,Citation127
Table 3 Applications of SCFAs in different kidney cells
These investigations of SCFAs on kidney resident cells (glomerular cells and tubular cells), which concentrate on inflammation, ROS, and fibrosis, are in agreement with the findings from animal experiments and provide more potential pathways to understand the mechanisms. However, future studies are still required to focus more on the interaction among SCFAs-associated molecular patterns and metabolism, inflammation, and immune system in order to clarify the molecular mechanisms behind kidney injury in the pathogenesis of kidney disease.
Conclusion
In summary, the function of gut microbiota–derived SCFAs in kidney disease has become an exciting area in recent years. SCFAs play extensive roles in host physiology, such as regulation of energy metabolism, immune inflammation, and blood pressure by recognizing their receptors and inhibiting HDACs. However, the entire field of SCFAs in kidney disease is still in its infancy. It has now been reported that the main beneficial effects of SCFAs on kidney function were by decreasing inflammation and enhancing antioxidant activity. Nevertheless, there are still few related studies, and all the concerned studies are either preliminary or controversial. Thus, in coming years, more explorations will be needed to better understand these pathways and their potential implications.
Acknowledgments
The authors thank Steve Salerno, from the University of Michigan, for proofreading the manuscript and for their language support, which improved the presentation of the work. This study was supported by the National Key Research and Development Program of China (No 2016YFC1305403).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest in this work.
References
- Aguilar-NascimentoJESalomaoABNochiRJJrNascimentoMNeves JdeSIntraluminal injection of short chain fatty acids diminishes intestinal mucosa injury in experimental ischemia-reperfusionActa Cir Bras2006211 21 2516491218
- Andrade-OliveiraVAmanoMTCorrea-CostaMGut bacteria products prevent AKI induced by ischemia-reperfusionJ Am Soc Nephrol2015268 1877 188825589612
- BindelsLBPorporatoPDewulfEMGut microbiota-derived propionate reduces cancer cell proliferation in the liverBr J Cancer20121078 1337 134422976799
- VaziriNDZhaoYYPahlMVAltered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: the nature, mechanisms, consequences and potential treatmentNephrol Dial Transpl2016315 737 746
- VaziriNDYuanJNorrisKRole of urea in intestinal barrier dysfunction and disruption of epithelial tight junction in chronic kidney diseaseAm J Nephrol2013371 1 623258127
- MoraesCFouqueDAmaralACMafraDTrimethylamine N-oxide from gut microbiota in chronic kidney disease patients: focus on dietJ Ren Nutr2015256 459 46526235933
- RamezaniAMassyZAMeijersBEvenepoelPVanholderRRajDSRole of the gut microbiome in uremia: a potential therapeutic targetAm J Kidney Dis2016673 483 49826590448
- Diaz HeijtzRWangSAnuarFNormal gut microbiota modulates brain development and behaviorProc Natl Acad Sci U S A20111087 3047 305221282636
- Vijay-KumarMAitkenJDCarvalhoFAMetabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5Science20103285975 228 23120203013
- UronisJMMuhlbauerMHerfarthHHRubinasTCJonesGSJobinCModulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibilityPLoS One200946 e602619551144
- PaolaMDFilippoCDCavalieriDImpact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural AfricaProc Natl Acad Sci U S A201010733 1469120679230
- LinMYde ZoeteMRvan PuttenJPStrijbisKRedirection of epithelial immune responses by short-chain fatty acids through inhibition of histone deacetylasesFront Immunol20156 55426579129
- MaslowskiKendle MThe role of GPR43 in the immune system: a novel connection between diet, gut microbiota and immune function Available from: Unsworks.unsw.edu.auAccessed September 1, 2017
- NymanMFermentation and bulking capacity of indigestible carbohydrates: the case of inulin and oligofructoseBr J Nutr200287Suppl 2 S163 S16812088514
- KotzampassiKGiamarellosbourboulisEJStavrouGObesity as a consequence of gut bacteria and diet interactionsISRN Obes20142014196 65189524977101
- WongJMde SouzaRKendallCWEmamAJenkinsDJColonic health: fermentation and short chain fatty acidsJ Clin Gastroenterol2006403 235 24316633129
- PomareEWBranchWJCummingsJHCarbohydrate fermentation in the human colon and its relation to acetate concentrations in venous bloodJ Clin Invest1985755 1448 14543998144
- AdomDNieDRegulation of autophagy by short chain fatty acids in colon cancer cellsBaillyYAutophagy – A Double-Edged Sword – Cell Survival or DeathInTech2013
- OrrhageKNordCEFactors controlling the bacterial colonization of the intestine in breastfed infantsActa Paediatr199988430 47 57
- KohADe VadderFKovatcheva-DatcharyPBackhedFFrom dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolitesCell20161656 1332 134527259147
- UlvenTShort-chain free fatty acid receptors FFA2/GPR43 and FFA3/GPR41 as new potential therapeutic targetsFront Endocrinol20123 111
- DewulfEMGeQBindelsLBEvaluation of the relationship between GPR43 and adiposity in humanNutr Metabol2012101 11
- TangYChenYJiangHRobbinsGTNieDG-protein-coupled receptor for short-chain fatty acids suppresses colon cancerInt J Cancer20111284 847 85620979106
- KarakiSMitsuiRHayashiHShort-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestineCell Tissue Res20063243 353 36016453106
- NilssonNEKotarskyKOwmanCOldeBIdentification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acidsBiochem Biophys Res Commun20033034 1047 105212684041
- PluznickJLProtzkoRJGevorgyanHOlfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulationProc Natl Acad Sci U S A201311011 4410 441523401498
- VinoloMARodriguesHGNachbarRTCuriRRegulation of inflammation by short chain fatty acidsNutrients2011310 858 87622254083
- FlegelCManteniotisSOstholdSHattHGisselmannGExpression profile of ectopic olfactory receptors determined by deep sequencingPLoS One201382 e5536823405139
- SinghNGuravASivaprakasamSActivation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesisImmunity2014401 128 13924412617
- ThangarajuMCresciGALiuKGPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colonCancer Res2009697 2826 283219276343
- WandersDGraffECJuddRLEffects of high fat diet on GPR109A and GPR81 gene expressionBiochem Biophys Res Commun20124252 278 28322842580
- IngersollMAPotteauxSAlvarezDHutchisonSBVanRNRandolphGJNiacin inhibits skin dendritic cell mobilization in a GPR109A independent manner but has no impact on monocyte trafficking in atherosclerosisImmunobiology20122175 548 55721798616
- HuangWZhouLGuoHXuYXuYThe role of short-chain fatty acids in kidney injury induced by gut-derived inflammatory responseMetabolism201768 20 3028183450
- TanJMcKenzieCPotamitisMThorburnANMackayCRMaciaLThe role of short-chain fatty acids in health and diseaseAdv Immunol2014121 91 11924388214
- KimCHParkJKimMGut microbiota-derived short-chain fatty acids, T cells, and inflammationImmune Netw2014146 277 28825550694
- MaslowskiKMVieiraATNgARegulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43Nature20094617268 1282 128619865172
- TrompetteAGollwitzerESYadavaKGut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesisNat Med2014202 159 16624390308
- YamashitaHFujisawaKItoEImprovement of obesity and glucose tolerance by acetate in type 2 diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) ratsBiosci Biotechnol Biochem2014715 1236 1243
- BerggrenAMNymanEMLundquistIBjorckIMInfluence of orally and rectally administered propionate on cholesterol and glucose metabolism in obese ratsBr J Nutr1996762 287 2948813902
- BouterKEvan RaalteDHGroenAKNieuwdorpMRole of the gut microbiome in the pathogenesis of obesity and obesity-related metabolic dysfunctionGastroenterology20171527 1671 167828192102
- ToppingDLCliftonPMShort-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharidesPhysiol Rev2001813 1031 106411427691
- StoddartLASmithNJMilliganGInternational Union of Pharmacology. LXXI. Free fatty acid receptors FFA1, -2, and -3: pharmacology and pathophysiological functionsPharmacol Rev2008604 405 41719047536
- VriezeAVan NoodEHollemanFTransfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndromeGastroenterology20121434 913.e7 916.e722728514
- UdayappanSDHartstraAVDallinga-ThieGMNieuwdorpMIntestinal microbiota and faecal transplantation as treatment modality for insulin resistance and type 2 diabetes mellitusClin Exp Immunol20141771 2424528224
- ZaibiMSStockerCJO’DowdJRoles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acidsFEBS Lett201058411 2381 238620399779
- HongYHNishimuraYHishikawaDAcetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43Endocrinology200514612 5092 509916123168
- GeHLiXWeiszmannJActivation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acidsEndocrinology20081499 4519 452618499755
- DelzenneNMCaniPDEverardANeyrinckAMGut microorganisms as promising targets for the management of type 2 diabetesDiabetologia20155810 2206 221726224102
- ChambersESViardotAPsichasAEffects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adultsGut20156411 1744 175425500202
- ParkYSubarAFHollenbeckASchatzkinADietary fiber intake and mortality in the NIH-AARP diet and health studyArch Int Med201117112 1061 106821321288
- TolhurstGHeffronHLamYSShort-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2Diabetes2012612 364 37122190648
- RozengurtNWuSVChenMCHuangCSterniniCRozengurtEColocalization of the alpha-subunit of gustducin with PYY and GLP-1 in L cells of human colonAm J Physiol Gastrointest Liver Physiol20062915 G792 G80216728727
- FreelandKRWoleverTMAcute effects of intravenous and rectal acetate on glucagon-like peptide-1, peptide YY, ghrelin, adiponectin and tumour necrosis factor-alphaBr J Nutr20101033 460 46619818198
- PlaisanciéPDumoulinVChayvialleJACuberJCLuminal peptide YY-releasing factors in the isolated vascularly perfused rat colonJ Endocrinol19971513 421 429
- SamuelBSShaitoAMotoikeTEffects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41Proc Natl Acad Sci U S A200810543 16767 1677218931303
- HolstJJThe physiology of glucagon-like peptide 1Physiol Rev2007874 1409 143917928588
- RossSAEkoeJMIncretin agents in type 2 diabetesCan Fam Physician2010567 639 64820631270
- KimMHKangSGParkJHYanagisawaMKimCHShort-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in miceGastroenterology20131452 396 406 e1 e1023665276
- MorrisGBerkMCarvalhoAThe role of the microbial metabolites including tryptophan catabolites and short chain fatty acids in the pathophysiology of immune-inflammatory and neuroimmune diseaseMol Neurobiol2017546 4432 445127349436
- EverardACaniPDGut microbiota and GLP-1Rev Endocri Metab Disord2014153 189 196
- FujikawaTCoppariRLiving without insulin: the role of leptin signaling in the hypothalamusFront Neurosci20159 10825870537
- PerryRJPengLBarryNAAcetate mediates a microbiome-brain-beta-cell axis to promote metabolic syndromeNature20165347606 213 21727279214
- SchwiertzATarasDSchaferKMicrobiota and SCFA in lean and overweight healthy subjectsObesity2010181 190 19519498350
- TedelindSWestbergFKjerrulfMVidalAAnti-inflammatory properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel diseaseWorld J Gastroenterol20071320 2826 283217569118
- ParkJSLeeEJLeeJCKimWKKimHSAnti-inflammatory effects of short chain fatty acids in IFN-gamma-stimulated RAW 264.7 murine macrophage cells: involvement of NF-kappaB and ERK signaling pathwaysInt Immunopharmacol200771 70 7717161819
- VinoloMARodriguesHGHatanakaESatoFTSampaioSCCuriRSuppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophilsJ Nutr Biochem2011229 849 85521167700
- LuhrsHGerkeTMullerJGButyrate inhibits NF-kappaB activation in lamina propria macrophages of patients with ulcerative colitisScand J Gastroenterol2002374 458 46611989838
- MillardALMertesPMItteletDVillardFJeannessonPBernardJButyrate affects differentiation, maturation and function of human monocyte-derived dendritic cells and macrophagesClin Exp Immunol20021302 245 25512390312
- KaczmarczykMMMillerMJFreundGGThe health benefits of dietary fiber: beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancerMetabolism2012618 1058 106622401879
- LiuTLiJLiuYXiaoNSuoHXieKShort-chain fatty acids suppress lipopolysaccharide-induced production of nitric oxide and proinflammatory cytokines through inhibition of NF-κB pathway in RAW264.7 cellsInflammation2012355 1676 168422669487
- VoltoliniCBattersbySEtheringtonSLPetragliaFNormanJEJabbourHNA novel antiinflammatory role for the short-chain fatty acids in human laborEndocrinology20121531 395 40322186417
- VinoloMARFergusonGJKulkarniSSCFAs induce mouse neutrophil chemotaxis through the GPR43 receptorPLoS One201166 2235 2239
- TazoeHOtomoYKarakiSExpression of short-chain fatty acid receptor GPR41 in the human colonBiomed Res2009303 149 15619574715
- ParkJKimMKangSGShort-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR S6K pathwayMucosal Immunol201581 80 9324917457
- ArpaiaNCampbellCFanXMetabolites produced by commensal bacteria promote peripheral regulatory T-cell generationNature20135047480 451 45524226773
- SmithPMHowittMRPanikovNThe microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasisScience20133416145 569 57323828891
- FukudaSCommensal microbe-derived butyrate induces the differentiation of colonic regulatory T cellsNature20135047480 446 45024226770
- Le PoulELoisonCStruyfSFunctional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activationJ Biol Chem200327828 25481 2548912711604
- VinoloMARodriguesHGHatanakaEHebedaCBFarskySHCuriRShort-chain fatty acids stimulate the migration of neutrophils to inflammatory sitesClin Sci20091179 331 33819335337
- VaziriNDLiuSMLauWLHigh amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney diseasePLoS One2014912 e11488125490712
- GalvezJRodriguez-CabezasMEZarzueloAEffects of dietary fiber on inflammatory bowel diseaseMol Nutr Food Res2005496 601 60815841496
- Burger-VanPNVincentAPuimanPJThe regulation of intestinal mucin MUC2 expression by short-chain fatty acids: implications for epithelial protectionBiochem J20094202 211 21919228118
- HinnebuschBFMengSWuJTArcherSYHodinRAThe effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylationJ Nutr20021325 1012 101711983830
- HaberlandMMontgomeryRLOlsonENThe many roles of histone deacetylases in development and physiology: implications for disease and therapyNat Rev Genet2009101 32 4219065135
- ZengHChiHMetabolic control of regulatory T cell development and functionTrends Immunol2015361 3 1225248463
- DelgoffeGMKoleTPZhengYThe mTOR kinase differentially regulates effector and regulatory T cell lineage commitmentImmunity2009306 832 84419538929
- SinghNThangarajuMPrasadPDBlockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter (Slc5a8)-dependent inhibition of histone deacetylasesJ Biol Chem2010285285 27601 2760820601425
- BradfordBEZhangMOwyangSYButyrate increases IL-23 production by stimulated dendritic cellsAm J Physiol201230312 G1384
- FrikecheJSimonTBrissotEGregoireMGauglerBMohtyMImpact of valproic acid on dendritic cells functionImmunobiology20122177 704 71022209114
- HovhannisyanGAroutiounianRGleiMButyrate reduces the frequency of micronuclei in human colon carcinoma cells in vitroToxicol In Vitro2009236 1028 103319539745
- VecchiaMGCarnelos FilhoMFellipeCRCuriRNewsholmeEAAcetate and propionate potentiate the antiproliferative effect of butyrate on RBL-2H3 growthGen Pharmacol1997295 725 7289347317
- SakataTvon EngelhardtWStimulatory effect of short chain fatty acids on the epithelial cell proliferation in rat large intestineComp Biochem Physiol A Comp Physiol1983742 459 4626131789
- PluznickJA novel SCFA receptor, the microbiota, and blood pressure regulationGut Microbes201452 202 20724429443
- PluznickJLGut microbiota in renal physiology: focus on short-chain fatty acids and their receptorsKidney Int2016906 1191 119827575555
- PluznickJLMicrobial short-chain fatty acids and blood pressure regulationCurr Hypertens Rep2017194 2528315048
- NehraVAllenJMMailingLJKashyapPCWoodsJAGut microbiota: modulation of host physiology in obesityPhysiology2016315 327 33527511459
- FelizardoRJCastoldiAAndrade-OliveiraVCamaraNOThe microbiota and chronic kidney diseases: a double-edged swordClin Transl Immunology201656 e8627757226
- RamezaniARajDSThe gut microbiome, kidney disease, and targeted interventionsJ Am Soc Nephrol2014254 657 67024231662
- VaziriNDWongJPahlMChronic kidney disease alters intestinal microbial floraKidney Int2013832 308 31522992469
- WongJPicenoYMDesantisTZPahlMAndersenGLVaziriNDExpansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRDAm J Nephrol2014393 230 23724643131
- AndersHJAndersenKStecherBThe intestinal microbiota, a leaky gut, and abnormal immunity in kidney diseaseKidney Int2013836 1010 101623325079
- PoesenRClaesKEvenepoelPMicrobiota-derived phenylacetylglutamine associates with overall mortality and cardiovascu-lar disease in patients with CKDJ Am SocNephrol20162711 3479 3487
- PoesenRWindeyKNevenEThe influence of CKD on colonic microbial metabolismJ Am Soc Nephrol2016275 1389 139926400570
- BammensBEvenepoelPKeuleersHVerbekeKVanrenterghemYFree serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patientsKidney Int2006696 1081 108716421516
- MeijersBKBammensBDe MoorBVerbekeKVanrenterghemYEvenepoelPFree p-cresol is associated with cardiovascular disease in hemodialysis patientsKidney Int20087310 1174 118018305466
- SirichTLPlummerNSGardnerCDHostetterTHMeyerTWEffect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patientsClin J Am Soc Nephrol201499 1603 161025147155
- UedaHShibaharaNTakagiSInoueTKatsuokaYAST-120 treatment in pre-dialysis period affects the prognosis in patients on hemodialysisRen Fail2008309 856 86018925523
- SchulmanGAgarwalRAcharyaMBerlTBlumenthalSKopytNA multicenter, randomized, double-blind, placebo-controlled, dose-ranging study of AST-120 (Kremezin) in patients with moderate to severe CKDAm J Kidney Dis2006474 565 57716564934
- PahlMVVaziriNDThe chronic kidney sisease – colonic axisSemin Dial2015285 459 46325855516
- WangHZhangWZuoLBifidobacteria may be beneficial to intestinal microbiota and reduction of bacterial translocation in mice following ischaemia and reperfusion injuryBr J Nutr201310911 199023122253
- MachadoRAConstantino LdeSTomasiCDSodium butyrate decreases the activation of NF-kappaB reducing inflammation and oxidative damage in the kidney of rats subjected to contrast-induced nephropathyNephrol Dial Transplant2012278 3136 314022273669
- SunXZhangBHongXZhangXKongXHistone deacetylase inhibitor, sodium butyrate, attenuates gentamicin-induced nephrotoxicity by increasing prohibitin protein expression in ratsEur J Pharmacol20137071–3 147 15423528351
- Abdul HamidZBudinSBWen JieNHamidAHusainKMohamedJNephroprotective effects of Zingiber zerumbet Smith ethyl acetate extract against paracetamol-induced nephrotoxicity and oxidative stress in ratsJ Zhejiang Univ Sci B2012133 176 18522374609
- NiwaTRole of indoxyl sulfate in the progression of chronic kidney disease and cardiovascular disease: experimental and clinical effects of oral sorbent AST-120Ther Apher Dial2011152 120 12421426500
- KhanSJenaGSodium butyrate, a HDAC inhibitor ameliorates eNOS, iNOS and TGF-beta1-induced fibrogenesis, apoptosis and DNA damage in the kidney of juvenile diabetic ratsFood Chem Toxicol201473 127 13925158305
- MarquesFZNelsonEMChuPYHigh fibre diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in DOCA-salt hypertensive miceCirculation201713510 964 97727927713
- ParkJGoergenCJHogenEschHKimCHChronically elevated levels of short-chain fatty acids induce T cell-mediated ureteritis and hydronephrosisJ Immunol20161965 2388 240026819206
- ZumbrunSDMelton-CelsaARSmithMAGilbreathJJMerrellDSO’BrienADDietary choice affects Shiga toxin-producing Escherichia coli (STEC) O157:H7 colonization and diseaseProc Natl Acad Sci U S A201311023 E2126 E213323690602
- ZumbrunSDMelton-CelsaARO’BrienADWhen a healthy diet turns deadlyGut Microbes201451 40 4323989728
- ShakeelMRecent advances in understanding the role of oxidative stress in diabetic neuropathyDiabetes Metab Syndr201594 373 37825470637
- HuangWGuoHLDengXShort-chain fatty acids inhibit oxidative stress and inflammation in mesangial cells induced by high glucose and lipopolysaccharideExp Clin Endocrinol Diabetes20171252 98 10528049222
- MatsumotoNRileySFraserDButyrate modulates TGF-beta1 generation and function: potential renal benefit for Acacia(sen) SUPERGUM (gum arabic)?Kidney Int2006692 257 26516408114
- IslamMBurkeJFMcgowanTAEffect of anti-transforming growth factor-βbgr antibodies in cyclosporine-induced renal dysfunctionKidney Int2001592 49811168932
- IsakaYTsujieMAndoYTransforming growth factor-β1 antisense oligodeoxynucleotides block interstitial fibrosis in unilateral ureteral obstructionKidney Int2000585 1885 189211044208
- PetersHBorderWANobleNATargeting TGF-βbgr overexpression in renal disease: maximizing the antifibrotic action of angiotensin II blockadeKidney Int1998545 1570 15809844133
- RiveraMNHaberDAWilms’ tumour: connecting tumorigenesis and organ development in the kidneyNat Rev Cancer200559 699 71216110318
- TanWChenYAnPSodium butyrate-induced histone hyperacetylation up-regulating WT1 expression in porcine kidney fibroblastsBiotechnol Lett2015376 1195 120225700826
