Abstract
Background
Formononetin (FMN) is an isoflavone that produces arterial vasodilation. However, the underlying molecular mechanisms are unclear.
Purpose
The purpose of this study was to explore the vasorelaxant effect and the potential mechanism of FMN in vascular endothelium in isolated rat aorta.
Methods
The thoracic aortas of Sprague Dawley rats were isolated to test the arterial reactivity in the presence of FMN with or without inhibitors. Bioinformatics analyses, including a Bioinformatics Analysis Tool for Molecular Mechanism of Traditional Chinese Medicine and molecular docking methods, were performed to predict therapeutic targets responsible for the vascular protection produced by FMN. We used rat aortic endothelial cells (RAOECs) as an in vitro model to verify the potential mechanism through molecular biological analyses. The production of nitric oxide (NO) metabolites were evaluated via an NO assay kit according to the manufacturer’s instruction. The mRNA expression of eNOS was analyzed by polymerase chain reaction, and the protein levels of PTEN, phosphorylated Akt, and eNOS were measured by Western blot.
Results
We found that FMN dilated rat aortic rings in a concentration-dependent manner, which was reduced by endothelium denudation and eNOS inhibition. The bioinformatics analyses indicated that FMN activity was associated with the PI3K/PTEN/Akt signaling pathway. Molecular biological studies demonstrated that FMN significantly elevated the levels of NO and eNOS mRNA and markedly increased the protein expression of phosphorylated Akt and eNOS in RAOECs, and decreased PTEN compared with a dimethyl sulfoxide group.
Conclusion
FMN performs vasorelaxation of the thoracic aorta through activating the PI3K/PTEN/Akt signaling pathway.
Introduction
Increased vasoconstriction largely results in hypertension.Citation1 Hypertension is present in more than 1 billion people worldwide.Citation2 It ranks as the number 1 cardiovascular risk factor for death.Citation3 In China, hypertension prevalence has doubled in the past decade and affects more than 300 million Chinese people.Citation4 Despite official guidelines and the application of different antihypertensive drug classes, merely 34% of hypertensive patients manage their blood pressure satisfactorily.Citation5,Citation6 Thereby, the development of new medications for the treatment is in high demand.Citation7 However, the underlying causal mechanisms behind the pathology of essential hypertension remains unclear.Citation8 Recent research studies concerning vessel endothelial cells, an initiating factor and carrier of the “endothelium-hypertension-cardiovascular event” chain, has attracted growing attention.Citation9–Citation11
Healthy endothelium is a major regulator of vascular tone and structure, secreting molecules that cause vessel relaxation and/or contraction.Citation12 The loss of healthy endothelial function is referred to as “endothelial dysfunction,” and this plays a crucial role in the pathogenesis of hypertension.Citation13 In endothelial dysfunction, the balance between vasodilating and vasoconstricting substances is altered, leading to an apparent reduction in endothelium-dependent relaxation.Citation14,Citation15 The best-characterized endothelium-derived relaxing factor is nitric oxide (NO). Abnormalities in vascular NO availability not only impair endothelium-dependent vasodilation but also result in a pro-inflammatory, prothrombotic, and pro-coagulant endothelial phenotype.Citation16
A recent study has reported that formononetin (FMN; ) can enhance NO production and improve endothelial function.Citation17 FMN is a methoxylated isoflavone and is represented abundantly in the Chinese herbal medicine (CHM) Astragalus membranaceus Bunge.Citation18 Herbs containing FMN have been widely used to relieve hypertension-related signs and symptoms.Citation19 Moreover, many FMN-rich complementary and alternative remedies, such as QiLiQiangXin capsules, QiShenYiQi pills, and NaoXinTong capsules, have become commercially available for treating hypertension.Citation20–Citation22 The antihypertensive effect induced by FMN includes the downregulation of α1-adrenoceptors and 5-HT2A/1B receptors.Citation23 Other studies have demonstrated that FMN evokes endothelium-dependent relaxation in rat arterial rings via the upregulation of NO in arterial endothelium through the MAPK pathway, as well as through the stimulation of potassium channels.Citation24,Citation25 However, the precise molecular mechanisms need to be further elucidated.
In the current study, we determined the vasoprotective role of FMN in rat thoracic aortas. On this basis, we sought to use a Bioinformatics Analysis Tool for Molecular Mechanism of Traditional Chinese Medicine (BATMAN-TCM), a well-recognized bioinformatics analysis tool for CHM, to assess the underlying molecular mechanism of FMN in affecting vascular function (BATMAN-TCM website: http://bionet.ncpsb.org/batman-tcm).Citation26,Citation27 We used a docking simulation to show an effective interaction between FMN and the drug target. Further molecular biological approaches (NO release assay, quantitative reverse transcription PCR [qRT-PCR], and Western blot) were applied to validate this prediction.
Taken together, our findings suggest that FMN increases NO production via the PI3K/PTEN/Akt signaling pathway. This work provides a potential route in the development of FMN for antihypertension treatment.
Materials and methods
Drugs and reagents
FMN was supplied by Yuanye Biotechnology Co. Ltd (Shanghai, China). Acetylcholine (ACh), and NG-nitro-l-arginine methyl ester (l-NAME) were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). Dimethyl sulfoxide (DMSO) was purchased from Dingguo Changsheng Biotechnology Co. Ltd (Beijing, China). The other reagents were of analytical grade.
Animals
Male Sprague Dawley rats weighing 240–300 g, specific pathogen free, were obtained from the Experimental Animal Center of Hunan Agricultural University, China. The rats were housed at 25°C, with a 12 hours light/dark cycle and with free access to water and food for 2 weeks prior to the experiments. All experimental steps complied with the Central South University guidelines for the care and use of animals and were approved by the Medical Ethics Committee of Central South University.
Preparation of aortic rings
The animals were euthanized through cervical dislocation, and the thoracic aorta was rapidly dissected and placed in 4°C Krebs–Henseleit (K-H) solution pre-gassed with 95% O2 and 5% CO2, which resulted in a physiological pH at 7.4. K-H solution was composed of the following: NaCl 118 mM, KCl 4.7 mM, KH2PO4 1.2 mM, MgSO4 1.2 mM, CaCl2 2.9 mM, NaHCO3 25 mM, glucose 11 mM, and EDTA-Na2 0.5 mM. The aorta was removed from connective tissues and fat, and then cut into aortic rings (2–3 mm in width). To remove the endothelium, aorta was scraped twice using sterile cotton sticks. The aortic ring was fixed by using two stainless steel triangular brackets and suspended in a 10 mL isolated organ bath filled with K-H solution with 95% O2, 5% CO2 and maintained at 37°C. The aortic ring was equilibrated for 1 hour at a resting tension of 2 g. During the equilibration period, the K-H solution was changed every 15 minutes. One bracket was attached to a tension transducer (BIOPAC TSD125C, Goleta, CA, USA) and connected with a physiological data acquisition and analysis system (BIOPAC System MP150, Goleta, CA, USA) to record data. The endothelium-intact aortic ring was balanced in 60 mM KCl, and then induced to relax by 10 μM ACh three times. Endothelial integrity was confirmed if the vasodilatation was much more than 80% compared with the vasoconstriction. For the scraped aortic ring, vasodilatation of less than 5% met the standard of endothelium denudation.
Measurement of vasorelaxation
To determine the endothelial relaxant responses of FMN, endothelium-intact and endothelium-denuded aortic rings were precontracted using 60 mM KCl. For studying the role of eNOS, tissues were pretreated with 10−4 M l-NAME for 30 minutes. After rings reached a balancing arterial tension, FMN was subsequently added into bath at an increasing concentration gradient of 10−8 to 10−3 M, and a concentration– effect curve was recorded.
Bioinformatics analysis by BATMAN-TCM
BATMAN-TCM was undertaken to identify possible mechanisms of FMN. The PubChem CID was submitted to BATMAN-TCM, and then potential KEGG pathways and diseases associated with FMN were listed on the website.
Molecular docking
The crystal structure of PTEN (PDB ID: 1D5R) was downloaded from the RCSB Protein Data Bank. The 3D structure of FMN was obtained from PubChem Compound (PubChem CID: 5280378). The molecular modeling program Auto-dock software (version 4.2) was used to identify the main binding sites between FMN and PTEN through docking simulation. The initial protein was prepared by removing all the water molecules and adding all the hydrogen atoms. Grids centered on active sites were set at 62×64×40 with a grid spacing of 0.375 Å. The detailed parameters were set as follows: the ligand parameters including user-specified initial position and initial relative dihedral offset were set to random. The docking run options were applied by using defaults. In addition, genetic algorithm was used in the docking process.
Cell culture
Rat aortic endothelial cells (RAOECs) were purchased from Pricells (Wuhan, China). RAOECs were harvested using 0.125% trypsin-EDTA for 1 minute. The cell suspension (cell count: 1×10−5/mL) was cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10 mL of fetal bovine serum in a 37°C incubator with 5% CO2. The medium was replenished every 1–2 days. RAOECs from the third passage were used.
NO release assay
Because NO is a relatively labile-free radical with an in vivo half-life of <5 seconds, the concentration of NO is determined accurately by measuring nitrite (NO2−) and nitrate (NO3−). RAOECs were inoculated in 24-well plates at 600 μL/well and were cultured in a 37°C, 5% CO2 incubator for 12 hours, and the medium was then changed to serum-free medium for 6 hours. Then, DMSO, FMN, and l-NAME were added to the corresponding groups. The supernatant in each group was collected, and an NO assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) was used to evaluate the level of NO metabolites.
qRT-PCR
Total RNA was isolated from RAOECs with Trizol. cDNA was synthesized using 3 μg of each sample and a reverse transcription assay kit in accordance with the manufacturer’s protocol (Fermentas Co., CA, USA). PCR amplification was performed for 10 minutes at 95°C, and then for 40 cycles of 5 seconds at 95°C, 30 seconds at 56°C, and 30 seconds at 72°C. β-Actin was measured as an internal control.Citation28 The following primers (Genscript, Nanjing, China) were used (5′ to 3′): eNOS F: CGACTATCCTGTATGGCTCTGAG, eNOS R: GATCCCCATTGCCAAATGTGC, β-actin F: CATCCTGCGTCTGGACCTGG, and β-actin R: TAATGTCACGCACGATTTCC. The relative gene expression in the test group and the control group was assessed using the comparative threshold cycle (Ct) method.
Western blot assay
RAOECs were lysed in RIPA buffer on ice for 30 minutes. The lysates were then centrifuged at 12,000 rpm for 5 minutes at 4°C to collect supernatants. Protein concentrations in the supernatants were measured using a bicinchoninic acid assay. Protein samples were separated through sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride. The proteins were incubated with primary antibodies (p-eNOS, Cell Signaling Technology, Danvers, MA, USA, CST#9574; eNOS, Abcam, Cambridge, UK, ab5589; PTEN, Abcam, ab32199; p-Akt, Cell Signaling Technology, CST4060; Akt, Bioworld, Nanjing, China, AP0059; and β-actin, Proteintech, Rosemont, IL, USA, 60008-1-Ig). The membrane conjugated antibodies were detected by super chemiluminescence reagent plus (Pierce, Rockford, IL, USA) and visualized after exposure to X-ray film.
Statistical analysis
All data are expressed as mean ± standard error of the mean (SEM) and n denotes the number of rings obtained from different rats. Relaxation is expressed as a decreasing percentage of the KCl contraction level. Rmax represents the maximal relaxation induced by FMN. Significant differences were analyzed using one-way ANOVA with Tukey’s multiple comparison post hoc test or two-way ANOVA followed by a Dunnett’s test. The EC50 values refer to the concentration required to produce 50% of Rmax and were calculated by nonlinear regression analysis using GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA, USA). A value of P<0.05 was regarded as statistically significant.
Results
FMN leads to the vasodilatation in rat thoracic aortic rings
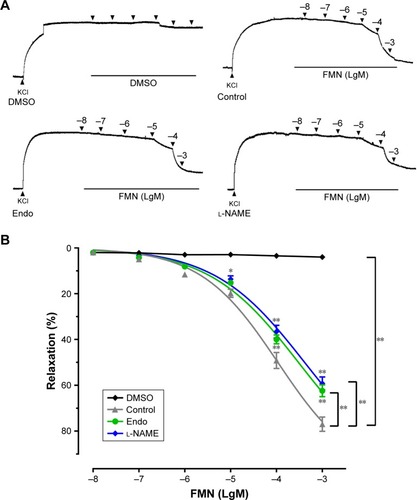
We examined the vasorelaxant effect of FMN on endothelium-intact rat aortic rings. showed that FMN induced dose-dependent vasodilation with KCl precontraction. The Rmax and EC50 values of control were 77.0%±3.1%, 107.2±11.9 μM, respectively. The DMSO group did not demonstrate an obvious relaxation effect. The removal of endothelium markedly reduced the relaxation effect of FMN (Rmax =62.4%±2.5%, EC50 =301.3±28.4 μM). This suggested that the vascular dilation produced by FMN was endothelium dependent. Next, we measured whether endothelium-derived NO was related to this vasorelaxation. l-NAME, as a nonspecific NO synthase inhibitor,Citation29 significantly suppressed FMN-evoked relaxation in endothelium-intact aortic rings (Rmax =59.2%±2.8%, EC50 =411.0±46.2 μM). eNOS played a pivotal role in the endothelium-dependent vasodilatation of FMN.
Figure 2 Effect of DMSO or cumulative doses of FMN on the relaxation of rat aortas (precontracted by 60 mM KCl), either with or without endothelium (endo) or with (l-NAME).
Abbreviations: DMSO, dimethyl sulfoxide; FMN, formononetin; l-NAME, NG-nitro-l-arginine methyl ester; SEM, standard error of the mean.
The PI3K/PTEN/Akt signaling pathway is predicted by bioinformatics analyses
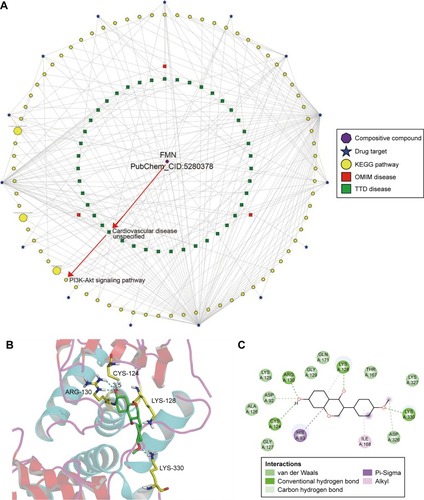
BATMAN-TCM provided valuable clues for potential pathways responsible for the antihypertensive effect of FMN. As shown in , bioinformatics analysis of the KEGG pathway predicted the PI3K-Akt signaling pathway as one of the candidate therapeutic mechanisms. To help us understand the mechanism further, molecular docking studies were carried out using Auto-Dock software. PTEN is known to be a negative regulator of the PI3K/AKT pathway in vascular smooth muscle cells and endothelium.Citation30 PTEN has lipid phosphatase activity and converts phosphatidylinositol-3,4,5-triphosphate (PIP3) to phosphatidylinositol-4,5-biphosphate (PIP2) and antagonizes the PI3K signaling pathway.Citation31 Therefore, we performed a molecular docking experiment to analyze the interaction between FMN and PTEN. The data shown in indicated that FMN could form H bonds with carboxyl oxygen residues of ARG-130, CYS-124, LYS-128, and LYS-330. The total binding free energy was −7.39 kcal/mol. The good interaction between FMN and PTEN showed that FMN might be a potential PTEN agonist. Therefore, we concluded that FMN may positively modulate PI3K/Akt signaling and lead to the vasodilation of aortic rings through inhibiting the function of PTEN.
Figure 3 The ingredient–target–pathway/disease network.
Abbreviations: CHM, Chinese herbal medicine; FMN, formononetin; KEGG, Kyoto Encyclopedia of Genes and Genomes; OMIM, Online Mendelian Inheritance in Man; TTD, Therapeutic Target Database.
FMN increases NO production and eNOS expression
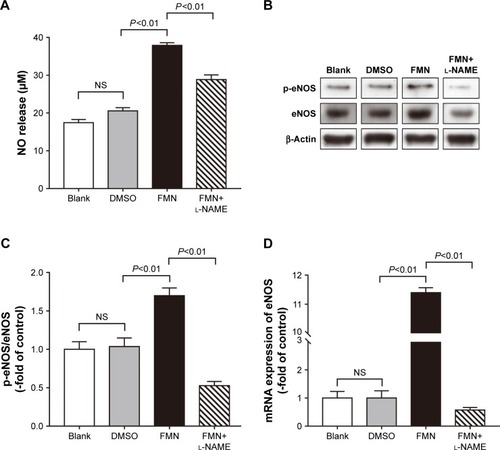
As shown in , no statistically significant difference was found between the blank group and the DMSO group. FMN increased the NO level in RAOECs significantly compared with the DMSO group, which was similar to the vasorelaxation result. In the presence of l-NAME, the NO concentration decreased markedly compared with the FMN group. These results suggested that FMN improved NO release, and this process could be abolished by l-NAME. To determine the potential roles of eNOS and its upstream regulators, we measured the mRNA and protein expression of eNOS. As shown in , FMN also affected the phosphorylation of eNOS, and l-NAME markedly inhibited p-eNOS expression. Moreover, it was observed that the eNOS mRNA level was significantly elevated by FMN treatment (). However, this effect could be reversed by l-NAME.
Figure 4 Effect of FMN on NO and eNOS production. RAOECs were incubated with medium, DMSO (5 μM) or FMN (30 μM) in the absence or presence of l-NAME (50 μM). Notes: NO release (A) is presented. Data are shown as mean ± SEM, n=6. Western blots of p-eNOS and eNOS (B), and their relative quantitation in RAOECs (C) and the eNOS mRNA levels in each group (D) are presented. Data are shown as mean ± SEM, n=5.
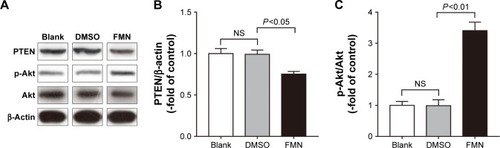
FMN inhibits PTEN expression and elevates Akt phosphorylation
To further verify whether the PI3K/PTEN/Akt pathway modulates NO activity, we examined the levels of phosphorylated pathway proteins. The results () showed that PTEN protein levels were significantly decreased in cells treated with FMN compared with DMSO-treated cells. In contrast, p-Akt protein levels were elevated in cells expressing FMN (). These results suggested that FMN promoted the activation of the PI3K/Akt pathway by downregulating PTEN.
Figure 5 The role of the PI3K/PTEN/Akt pathway in the FMN-mediated regulation of eNOS/NO.
Abbreviations: DMSO, dimethyl sulfoxide; eNOS, endothelial nitric oxide synthase; FMN, formononetin; NO, nitric oxide; NS, not significant; RAOECs, rat aortic endothelial cells; SEM, standard error of the mean.
Discussion
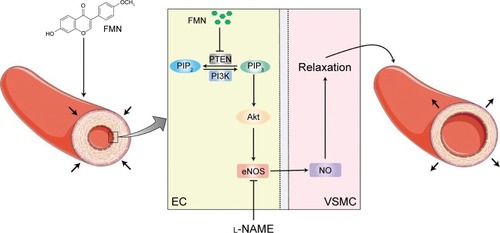
In the present study, we evaluated the potential role of FMN in ameliorating the KCl-induced vasoconstriction of rat arterial rings. Bioinformatics analyses revealed that the PI3K/PTEN/Akt pathway was involved in this process. This link was confirmed using molecular biological techniques, including NO production assays, qRT-PCR, and Western blotting. To the best of our knowledge, this is the first investigation to delineate the detailed effects of FMN in activating the PI3K/PTEN/Akt signaling pathway (). The current work reveals the underlying mechanisms of FMN in modulating vascular tone.
Figure 6 FMN-induced endothelium-dependent vasorelaxation via the PI3K/PTEN/Akt signaling pathway.
Abbreviations: EC, endothelial cell; eNOS, endothelial nitric oxide synthase; FMN, formononetin; l-NAME, NG-nitro-l-arginine methyl ester; NO, nitric oxide; PIP2, phosphatidylinositol-4,5-biphosphate; PIP3, phosphatidylinositol-3,4,5-triphosphate; VSMC, vascular smooth muscle cell.
Vascular relaxation is particularly important in opposing vasoconstriction for homeostatic balance and reducing the impact of hypertension.Citation32 Similar to a previous study,Citation17 we found that FMN attenuated KCl-mediated pre-constriction of the aorta in a dose-dependent manner. Mechanical removal of the endothelium and addition of l-NAME suppressed this effect, which confirmed the endothelium/NO-related vasorelaxant effect of FMN. The ability of FMN to induce endothelial- and NO-dependent vasodilation has been demonstrated in several studies.Citation24,Citation25,Citation33 It has also been established that the PI3K/Akt pathway plays a critical role in eNOS expression and NO production.Citation34,Citation35 Thus, we focused on the probable vasodilative effect of FMN and its interaction with the PI3K/Akt signaling pathway in the current study.
Accordingly, we hypothesized that FMN might produce an antihypertensive effect through improving the production of NO in the endothelium via the PI3K/Akt pathway. Recently, accumulating evidence shows that the in silico prediction of drug–target interaction is a feasible and powerful way to discover novel drug candidates.Citation26,Citation36,Citation37 BATMAN-TCM, the first online bioinformatics analysis tool specifically designed for studying the molecular mechanisms of CHM, appeared to be well suited for the current study. Using this analytical method, we predicted PI3K/Akt signaling as highly likely to be functional pathway, which is consistent with our hypothesis. Molecular docking, which has an important and promising role in drug discovery,Citation38 was used to dig out the potential pharmacological targets of FMN. We performed a computational molecular docking experiment to evaluate the interaction between FMN and PTEN. The data showed that FMN demonstrated significant binding activity with PTEN. Therefore, FMN may be regarded as a PTEN suppressor, which promotes aortic relaxation via the acceleration of eNOS and NO expression through PI3K/Akt signaling.
The PI3K/Akt signaling pathway is a complex network that regulates cellular pathways involved in a myriad of physiological and pathological conditions.Citation39 Akt is primarily activated through the modulation of upstream PI3K. The degree of phosphorylated Akt indirectly reflects the levels of active PI3K.Citation40 PI3K catalyzes the production of PIP3 from PIP2, resulting in the phosphorylation of Akt and other downstream substrates.Citation41 This activity is counteracted by PTEN, the main endogenous PI3K blocker, which dephosphorylates PIP3 at the D3 position.Citation42 NO, as a key cardiovascular regulating factor, is able to expand blood vessels. On the other hand, NO inhibits the adhesion of platelets, the activation of leukocyte chemotaxis, and the proliferation of vascular smooth muscle cells.Citation43 Stimulation of Akt enhances the activity of eNOS by phosphorylating serine at 1,177 and triggers the release of NO.Citation44 To test our hypothesis, we chose a 30 μM concentration for all subsequent experiments in accordance with the dose-dependent dilation of FMN. FMN augmented cellular NO production via eNOS phosphorylation, which is evident from the suppression of this effect by l-NAME. Hence, we propose that the protective effect of FMN on endothelium, at least in part, is attributable to its activation of the eNOS/NO pathway. Moreover, FMN treatment decreased PTEN, while increasing Akt phosphorylation. The data indicate that this mechanism is PI3K/PTEN/Akt-dependent.
Conclusion
In summary, FMN exerts a vasodilatory action in the rat thoracic aorta partly through activating the PI3K/PTEN/Akt pathway. This study provides an insight into the molecular mechanisms of FMN and could help in developing antihypertensive treatment.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
This study was supported by National Natural Science Foundation of China (Nos 81673719 and 81373554).
Disclosure
The authors report no conflicts of interest in this work.
References
- DoroszkoAJanusASzahidewicz-KrupskaEMazurGDerkaczAResistant HypertensionAdv Clin Exp Med201625117318326935512
- Rodriguez-IturbeBPonsHJohnsonRJRole of the Immune System in HypertensionPhysiol Rev20179731127116428566539
- YangLBaiYLiNVascular VPO1 expression is related to the endothelial dysfunction in spontaneously hypertensive ratsBiochem Biophys Res Commun2013439451151624021280
- HuangKSongYTHeYHFengXLHealth system strengthening and hypertension management in ChinaGlob Health Res Policy201611329202062
- Al DisiSSAnwarMAEidAHAnti-hypertensive Herbs and their Mechanisms of Action: Part IFront Pharmacol2016632326834637
- MarantaFSpoladoreRFragassoGPathophysiological mechanisms and correlates of therapeutic pharmacological interventions in essential arterial hypertensionAdv Exp Med Biol2017956375927864806
- YuanTYChenYCZhangHFDL0805-2, a novel indazole derivative, relaxes angiotensin II-induced contractions of rat aortic rings by inhibiting Rho kinase and calcium fluxesActa Pharmacol Sin201637560461627041459
- CarthyERAutonomic dysfunction in essential hypertension: A systematic reviewAnn Med Surg (Lond)2014312725568776
- XiongXYangXLiuYZhangYWangPWangJChinese herbal formulas for treating hypertension in traditional Chinese medicine: perspective of modern scienceHypertens Res201336757057923552514
- VanhouttePMShimokawaHFeletouMTangEHEndothelial dysfunction and vascular disease – a 30th anniversary updateActa Physiol (Oxf)20172191229626706498
- Khaddaj MallatRMathew JohnCKendrickDJBraunAPThe vascular endothelium: A regulator of arterial tone and interface for the immune systemCrit Rev Clin Lab Sci2017547–845847029084470
- KonukogluDUzunHEndothelial dysfunction and hypertensionAdv Exp Med Biol201795651154028035582
- VersariDDaghiniEVirdisAGhiadoniLTaddeiSEndothelium-dependent contractions and endothelial dysfunction in human hypertensionBr J Pharmacol2009157452753619630832
- NagyJCsikyBKovácsTWittmannIEndothelial dysfunctionOrv Hetil20011423116671672 Hungarian11556260
- MiaoQWangQDongLWangYTanYZhangXThe expression of p66shc in peripheral blood monocytes is increased in patients with coronary heart disease and correlated with endothelium-dependent vasodilatationHeart Vessels201530445145724676406
- LandmesserUDrexlerHEndothelial function and hypertensionCurr Opin Cardiol200722431632017556884
- ZhaoYChenBNWangSBWangSHDuGHVasorelaxant effect of formononetin in the rat thoracic aorta and its mechanismsJ Asian Nat Prod Res2012141465422263593
- LuoLYFanMXZhaoHYLiMXWuXGaoWYPharmacokinetics and Bioavailability of the Isoflavones Formononetin and Ononin and Their in Vitro Absorption in Ussing Chamber and Caco-2 Cell ModelsJ Agric Food Chem201866112917292429504397
- HaoPJiangFChengJMaLZhangYZhaoYTraditional Chinese medicine for cardiovascular disease: evidence and potential mechanismsJ Am Coll Cardiol201769242952296628619197
- ZhangYShiPYaoHShaoQFanXMetabolite profiling and pharmacokinetics of herbal compounds following oral administration of a cardiovascular multi-herb medicine (Qishen yiqi pills) in ratsCurr Drug Metab201213551052322292791
- HeYduBFanHBeneficial Effects of Qili Qiangxin Capsule on Lung Structural Remodeling in Ischemic Heart Failure via TGF-β1/Smad3 PathwayEvid Based Complement Alternat Med2015201529863126604970
- HanJYLiQMaZZFanJYEffects and mechanisms of compound Chinese medicine and major ingredients on microcirculatory dysfunction and organ injury induced by ischemia/reperfusionPharmacol Ther201717714617328322971
- SunTWangJHuangLHCaoYXAntihypertensive effect of for-mononetin through regulating the expressions of eNOS, 5-HT2A/1B receptors and α1-adrenoceptors in spontaneously rat arteriesEur J Pharmacol20136991–324124923123056
- SunTCaoLPingNNWuYLiuDZCaoYXFormononetin upregulates nitric oxide synthase in arterial endothelium through estrogen receptors and MAPK pathwaysJ Pharm Pharmacol201668334235126786718
- TsengHHVongCTLeungGPCalycosin and Formononetin Induce Endothelium-Dependent Vasodilation by the Activation of Large-Conductance Ca2+-Activated K+ Channels (BKCa)Evid Based Complement Alternat Med20162016527253127994632
- LiuZGuoFWangYBATMAN-TCM: a Bioinformatics Analysis Tool for Molecular mechANism of Traditional Chinese MedicineSci Rep201662114626879404
- ZhouJLiuTCuiHXuefu zhuyu decoction improves cognitive impairment in experimental traumatic brain injury via synaptic regulationOncotarget2017842720697208129069769
- WuJKeXMaNFormononetin, an active compound of Astragalus membranaceus (Fisch) Bunge, inhibits hypoxia-induced retinal neovascularization via the HIF-1α/VEGF signaling pathwayDrug Des Devel Ther20161030713081
- LiSXuJYaoWSevoflurane pretreatment attenuates TNF-α-induced human endothelial cell dysfunction through activating eNOS/NO pathwayBiochem Biophys Res Commun2015460387988625838201
- FuJChenYLiFAttenuation of MicroRNA-495 Derepressed PTEN to Effectively Protect Rat Cardiomyocytes from HypertrophyCardiology2018139424525429566365
- NakakidoMDengZSuzukiTDohmaeNNakamuraYHamamotoRDysregulation of AKT Pathway by SMYD2-Mediated Lysine Methylation on PTENNeoplasia201517436737325925379
- SiangjongLGoldmanDHKriskaTVascular hepoxilin and trioxilins mediate vasorelaxation through TP receptor inhibition in mouse arteriesActa Physiol (Oxf)2017219118820126666460
- WuJHLiQWuMYFormononetin, an isoflavone, relaxes rat isolated aorta through endothelium-dependent and endothelium-independent pathwaysJ Nutr Biochem201021761362019570671
- TrottDWLuttrellMJSeawrightJWWoodmanCRAging impairs PI3K/Akt signaling and NO-mediated dilation in soleus muscle feed arteriesEur J Appl Physiol201311382039204623563601
- LauYSLingWCMuruganDKwanCYMustafaMREndothelium-Dependent Relaxation Effect of Apocynum venetum Leaf Extract via Src/PI3K/Akt Signalling PathwayNutrients2015775239525326133970
- van LaarhovenTNabuursSBMarchioriEGaussian interaction profile kernels for predicting drug-target interactionBioinformatics201127213036304321893517
- ChengTHaoMTakedaTBryantSHWangYLarge-Scale Prediction of Drug-Target Interaction: a Data-Centric ReviewAAPS J20171951264127528577120
- RizviSMShakilSZeeshanMAn enzoinformatics study targeting polo-like kinases-1 enzyme: Comparative assessment of anticancer potential of compounds isolated from leaves of Ageratum houstonianumPharmacogn Mag201410Suppl 1S14S2124914294
- HuangJJShiYQLiRLAngiogenesis effect of therapeutic ultrasound on HUVECs through activation of the PI3K-Akt-eNOS signal pathwayAm J Transl Res2015761106111526279754
- XingZXiaZPengWXuefu Zhuyu decoction, a traditional Chinese medicine, provides neuroprotection in a rat model of traumatic brain injury via an anti-inflammatory pathwaySci Rep201662004026818584
- YuneTYByunJYExpression of PTEN and phosphorylated Akt in human cholesteatoma epitheliumActa Otolaryngol2009129550150618615333
- GhigoALiMPhosphoinositide 3-kinase: friend and foe in cardiovascular diseaseFront Pharmacol2015616926321955
- ZhangYWangSJHanZHPI3K/AKT signaling pathway plays a role in enhancement of eNOS activity by recombinant human angiotensin converting enzyme 2 in human umbilical vein endothelial cellsInt J Clin Exp Pathol20147118112811725550859
- AnwarMASamahaAABallanSSalehAIIratniREidAHSalvia fruticosa Induces Vasorelaxation In Rat Isolated Thoracic Aorta: Role of the PI3K/Akt/eNOS/NO/cGMP Signaling PathwaySci Rep20177168628386068