?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
The obnoxious bitter taste of orally taken antibiotics is one of the biggest problems in the treatment of children. The pediatric population cannot tolerate the bitter taste of drugs and vomit out which ultimately leads to suboptimal therapeutic value, grimace and mental stress so it is the challenging task for the formulation scientists to formulate a palatable formulation particularly to overcome address the issue.
Purpose of study
The study aimed to mask and evaluate the unpleasant bitter taste of azithro-mycin (AZ) in the dry suspension dosage form by physisorption technique.
Materials and methods
AZ was selected as an adsorbent and titanium dioxide nanoparticles as adsorbate. The AZ nanohybrids (AZN) were prepared by treating fixed amount of adsorbent with a varied amount of adsorbate, prepared separately by dispersing it in an aqueous medium. The mixture was sonicated, stirred followed by filtration and drying. The AZN produced were characterized by various techniques including scanning electron microscopy (SEM), energy dispersive X-rays (EDX), powder X-ray diffraction (PXRD), HPLC and Fourier-transformed infrared (FTIR). The optimized nanohybrid was blended with other excipients to get stable and taste masked dry suspension dosage form.
Results
The results confirmed the adsorption of titanium dioxide nanoparticles on the surface of AZ. The fabricated optimized formulation was subjected for taste masking by panel testing and accelerated stability studies. The results showed a remarkable improvement in bitter taste masking, inhibiting throat bite without affecting the dissolution rate. The product showed an excellent stability both in dry and reconstituted suspension. The optimized formulation of AZN and was found stable when subjected to physical and chemical stability studies, this is because of short and single step process which interns limits the exposure of the product to various environmental factors that could potentially affect the stability of the product. The dissolution rate of the optimized formulation of AZN was compared with its marketed counterpart, showing the same dissolution rate compared to its marketed formulation.
Conclusion
The current study concludes that, by fabricating AZ-titanium nanohybrids using physisorption can effectively mask the bitter taste of the drug. The palatability and stability of azithromycin formulation was potentially enhanced without affecting its dissolution rate.
Introduction
The oral liquid dosage form is the most convenient dosage form for the treatment of pediatric and geriatric population as it can be administered easily. Poor solubility, unpleasant taste, stability, and bioavailability are the major issues associated with oral liquid dosage form.Citation1 The obnoxious bitter taste is one of the biggest problems in completing the treatment in children and sensitive patients because most of the population cannot tolerate the bitter taste of drugs and vomit out, which ultimately leads to suboptimal therapeutic value, grimace, and mental stress.Citation2–Citation4
Taste masking is the challenging task for the formulation scientists during preformulation as well as the final formulation of liquid oral dosage form, especially suspension. Different approaches have been used to overcome these issues, which include micronization, spheronization, encapsulation, ion exchange, granulation, use of taste potentiators, taste suppressant, sweeteners, and flavors.Citation5–Citation8 Among these conventional techniques, the simplest and frequently used approach for taste masking of a pediatric formulation is the addition of flavor and sweeteners; however, it is not successful for highly bitter drugs such as quinine, azithro-mycin (AZ), clarithromycin, and vancomycin.Citation9
AZ is a macrolide antibiotic, frequently used in the pediatric population for acute bacterial sinus, otitis media, pharyngitis, tonsillitis, pneumonia, bronchitis, and skin infections.Citation10–Citation12 AZ is practically insoluble in water but soluble at saliva pH, which readily solubilizes the drug content and expose to taste buds of tongue and feel bitter.Citation13–Citation17
This study aimed to mask the bitter taste of AZ () in the form of dry suspension. In the current study, the extreme bitter taste of the drug was masked through new and novel approach of physisorption using Langmuir isotherm and ordered mixing in which very fine particles adsorb onto the surface of coarse particles due to surface energies.Citation18,Citation19 Titanium dioxide nanoparticles (TNPs) were selected as an adsorbate as it is insoluble in saliva pH, is biocompatible, have a wide range of safety margin, and act as a nanoantibiotic for oral hygiene.Citation20,Citation21 A nanoantibiotic is a nanomaterial that either show antimicrobial activity by themselves or elevate the effectiveness and safety of antibiotics administration.Citation22 AZ is treated as an adsorbent.Citation23 TNPs are adsorbed onto AZ, which acts as a shield, making a film around AZ, and as a result, drug release is retarded in the buccal cavity and the extreme bitterness is completely masked by avoiding interaction with the taste receptors of tongue; however, both the drug and the TNP adsorbed were solubilized in the intestinal pH where it releases the drug that becomes bioavailable.Citation24,Citation25
Materials and methods
Materials
AZ (Lot No HS 29415000), TNPs (Anatase; Lot No MKBC4889), and hydroxy propyl methyl cellulose (HPMC) were purchased from Sigma-Aldrich Co., St Louis, MO, USA. Xanthan gum, tribasic sodium phosphate, colloidal silicon dioxide, sodium benzoate, methyl paraben, and propyl paraben were gifted by Bryon Pharmaceutical (Pvt) Ltd, 48-Industrial Estate Peshawar, KPK, Pakistan. Analytical HPLC grade acetonitrile, methanol, tetrabu-tylammonium phosphate, phosphoric acid, and ammonium phosphate monoacid were purchased from Merck & Co., Inc., Whitehouse Station, NJ, US.
Methods
Preparation of AZ nanohybrids (AZN)
The AZNs were prepared by physisorption technique. In this method, 2% w/v stock suspensions of AZ and 0.5% w/v suspension of TiO2 nanosuspension were prepared separately by dispersing it in an aqueous medium. The ultrasonication of the produced suspensions was carried out for 5 minutes at ultrasonic inputs (200 W) at a pause of 3 seconds followed by stirring at 300 rpm for 30 minutes at 25°C–30°C. A number of dilutions containing fixed amount (200 mg/10 mL) of the stock suspension containing AZ were treated with varied dilutions of titanium dioxide stock suspension. Each mixture was stirred for 4 hours using magnetic stirrer at 1,500 rpm at room temperature followed by filtration (Whatman 42). The unadsorbed nanoparticles of each sample were filtered, and the residue left on the surface of filter paper was dried and kept for characterization and further studies.
Characterization
Scanning electron microscopy (SEM)
The particle size (PS) and morphology of unprocessed AZ, TNP, and AZN were analyzed by SEM (Model: Qunata 400 FEI) at 500× and 5,000× magnification levels, respectively.Citation26–Citation28 The samples of AZ and TPN were prepared for SEM studies by fixing the samples in a metal stub using an adhesive double-sided tape.
Fourier-transformed infrared (FTIR)
The molecular complex of AZ, TNP, and AZN was crushed to fine powder using an agate mortar and pestle. The fine powdered samples were mixed with potassium bromide (1% wt ratio) and pressed hydraulically under pressure of 8 tons to make a disk. Potassium bromide (KBr) disks were analyzed using FTIR (Bruker IFS-55, 140 Germany) between 4,000 and 400 cm−1 region.Citation29,Citation30
Energy-dispersive X-ray (EDX)
The optimized sample of AZN was prepared for EDX studies by fixing the samples in a metal stub using an adhesive double-sided tape. The cobalt stub was used to calibrate the machine. The parameters were included on low-vacuum beam gas with a path length of 11 m, gas type-water 4KPS as the accusation rate, and dead time selected under 50%. EDX analysis was done to determine the elemental and the phase composition of AZN.Citation28,Citation31
X-ray diffraction (XRD)
The samples of unprocessed AZ, TNP, and optimized AZNs were evaluated using X-ray powder diffraction (PANalytical, X’pert Powder). The detector was scanned over 2θ angles at a step size of 0.01° step time of 10 seconds.Citation28
Adsorption efficiency
Adsorption efficiency of drug–TiO2 was calculated by taking the various concentrations of TiO2 with a known amount of AZ to get optimized AZN. The drug adsorption efficiency of all treated nanohybrids was quantified for adsorbed nanoparti-cles using the method reported by Zubata et al.Citation32 Accurately weighed quantity of each sample of a binary system containing drug and adsorbed nanoparticles was dissolved in a solution of buffer with pH 6.8, and the final concentration was made to 5 µg/mL. The solutions were filtered and analyzed by HPLC Shimadzu Model LC-10ATVP (Shimadzu Corporation, Kyoto, Japan) with Hypersil BDS C18 (250×4.6 mm) column. The gradient mobile phase composed of acetonitrile, methanol, and buffer with pH 8 at a ratio of 20:20:60 was used. The drug adsorption efficiency was calculated.
Adsorption equilibrium study
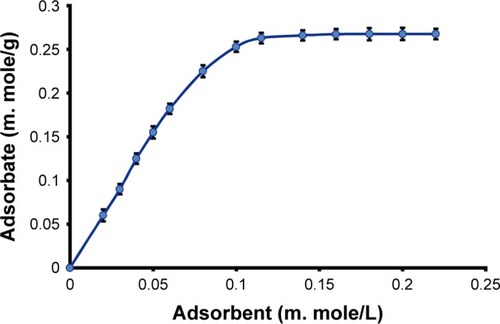
The adsorption equilibrium study was conducted using the Langmuir adsorption isotherm to calculate the adsorption potential of TNPs onto the surface of the drug macromolecule (AZ). Various concentrations of stock suspension containing AZ (0.040, 0.080, 0.120, 0.160, 0.20, 0.280, 0.360, and 0.40 mM/L) were treated with TNP as the adsorbate (0.0313, 0.0469, 0.0626, 0.0783, 0.0939, 0.1096, 0.1252, 0.1409, 0.1565, and 1.7216 mM) in separate 50-mL stoppered volumetric flasks. The flasks were then stirred at 1,500 rpm for 4 hours at 37°C±0.5°C. The samples were withdrawn at a specified time, filtered, and dried at 40°C for 2 hours. The prepared nanohybrids were then subjected to equilibrium concentration (Ceq) of the drug to get maximum adsorption of the TNP on to the surface of drug particles by constructing Langmuir adsorption isotherm model.Citation33–Citation35
Formulation of AZN
The optimized fabricated AZN-7 was selected for conversion to dry suspension. Different formulations were prepared from AZN-7 (equivalent to 200 mg of AZ) and mixed with various concentrations of excipients to get a stable, taste masked dry suspension. All the formulation components including AZN and excipients were dried at 40°C separately for 1 hour, sifted through mesh 60, and mixed thoroughly. The dry mixture was transferred to amber glass bottles for further studies.
In vitro dissolution
In vitro dissolution studies of optimized AZNs (AZN-7), its formulations (F1 to F10), and pure drug (AZ) were determined at saliva pH 6.8 and also at intestinal pH 7.4 using USP dissolution apparatus II at 50 rpm at 37°C. The samples were analyzed using the method reported by Zubata et alCitation32 as discussed above.
The reconstituted suspension containing an equivalent amount of AZ (200 mg) from each formulation, AZ, and AZN-7 were added to the dissolution medium (pH 7.4). An equivalent amount of marketed suspension was also analyzed under the same condition for comparative studies. The samples were withdrawn at the specific time of intervals (10, 20, 30, 40, 50, and 60 minutes). The sink conditions were maintained by replacing with the fresh medium of the same amount. The withdrawn samples were suitably diluted, filtered with Whatman filter paper 42, and quantitatively assayed.
Taste masking evaluation
The taste masking study was conducted using human volunteers. All the experimental work on children for taste evaluation was conducted under the approved protocols vide Ref. No LKH/Paeds/01/17 by the Ethical Committee of Agency Headquarter Hospital Landikotal, Khyber Pakhtunkhwa, Pakistan. It is also important to mention that the designed study was conducted in accordance with the Declaration of Helsinki. Prior to conduct the taste masking evaluation studies using human volunteers, the written signed consent proformas were collected from each volunteer.
In the current research, volunteers were selected by sequential method ().Citation36,Citation37 The taste identification and palatability of all batches F1 to F10 were evaluated by panel testing.Citation38
Table 1 Sequential method for the selection of trainee volunteers
The sequential test for taste evaluation was performed to analyze and interpret sensory evaluation for the selection of trainee volunteers for panel testing of the final formulation taste evaluation. In this method, stock suspension containing 3.0, 2.0, and 1.0 mL and blank suspension having no AZ (blank) were prepared by dispersing in distilled water. Each level of taste ranging from tasteless to intense bitter was given numeric values from 0 to 4.
In this evaluation method, a total of 25 volunteers divided into five groups were tested for taste threshold and correct evaluation of different tastes. In total, 5 mL of each stock suspension was given randomly to each volunteer in every set of test. The supertasters and nontasters were rejected through sequential tests. Of 25 trainees, 15 volunteers were approved.
The selected 15 volunteers were again divided into five groups and each group consisted of three volunteers. The panel testing method was chosen to evaluate the bitterness of all formulations (F1–F10). In this method, the same procedure as that of sequential method was followed and ranking made on the scale of perception ranging from 0 to 3, with 0 being tasteless and 3 marked as bitter.
Stability studies
Chemical and accelerated stability studies were performed on the optimized formulation of F6 for both dry and reconstituted suspension as per ICH guidelines.Citation39 The required quantity of optimized formulation (F6) batch was kept for physical and chemical stability in a tightly closed amber glass bottle at 40°C±2°C and 75%±5% RH in the stability chamber for 90 days. The reconstituted samples were kept at refrigeration for 14 days, and the physical and chemical stabilities were analyzed quantitatively for active contents as per schedule.
Results and discussion
Optimized AZNs
The AZNs were prepared by physisorption technique. A known quantity of AZ was treated with the varied dilution of TNP. The theoretical drug loading, experimental drug loading, and percent drug entrapment efficiency were calculated for 10 samples as shown in . The results in exhibited that the AZN-7 was found effective having maximum drug entrapment capacity when the concentration of TiO2 was 93.6%±1.3%. This showed that TiO2 nanoparticles were sufficiently adsorbed onto the surface of AZ particles as reported by Khan et al,Citation40 which in case of nanoparticles adsorption onto the carrier particles, at a particular concentration of the adsorbate, the surface of adsorbent becomes saturated where there is no chance for the small particles to be further attached onto the surface of adsorbent. The optimized nanohybrid (AZN-7) was selected for further studies including its formulation on the basis of maximum adsorption of nanoparticles on the surface of the drug with the help of entrapment efficiency. The effectiveness of the adsorption using entrapment efficacy was also previously studied by Aboutaleb et al.Citation41
Table 2 Preparation of AZNs
SEM
The SEM images of all samples of AZNs and TNPs were taken. The studies showed that the PS of TiO2 NPs was found in the range of 70–200 nm. It appears in agglomerates as shown in . The cubic-shaped crystals with sharp and elongated edges were observed for AZ having average dimensions between 10 and 150 µm as shown in . The SEM studies showed that the TNPs were successfully adsorbed on the surface of AZ (). Furthermore, the SEM image of the recovered AZN () also showed that the surface has been covered by a whitish sheet of TNPs. The adsorption isotherm also confirmed the adsorption.Citation33,Citation34 The equilibrium time adsorption of TNPs on to the surface of macromolecule (AZ) was achieved within 2 hours without loss of active drug content and adhesion to the wall of glasswares like flasks and other accessories. The maximum adsorption of the TNPs was recorded as 2.6 mM/g, whereas the Ceq of the drug was established at 0.115 mM/L of the solution as shown in . The Ceqs of the drug and adsorbate (TNP) were 0.280 and 0.1252 mM/g, respectively, which corresponds to 10 mg of adsorbed TNP onto the surface of 200 mg of AZ. Furthermore, after this concentration (10 mg), no free sites were available on the surface of AZ to adsorb any more TNP.
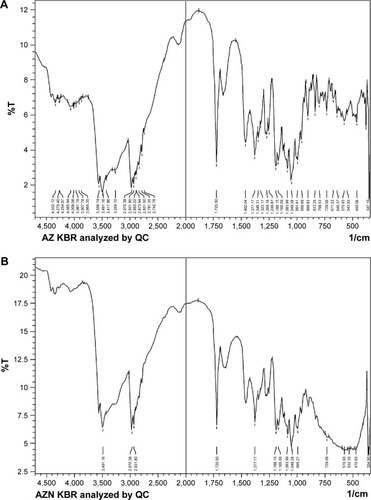
Evaluation of AZ–TNP complex by FTIR
The results of AZNs () showed distinct peaks at 3,491.16, 2,970.38, 2,931.80, 1,720.50, 1,377.17, 1,188.15, 1,165.0, 1,083.99, 1,049.28, and 995.27 cm−1. Similarly, the FTIR spectrum of unprocessed AZ () showed distinct peaks at the same position as that observed for AZN, ie, 3,556.74, 3,491.16, 2,970.38, 2,931.80, 1,720.50, 1,462.04, 1,377.17, 1,188.15, 1,083.99, 1,049.28, and 991.41 cm−1, respectively. The results clearly showed and confirmed that no interaction occurred due to adsorption of TNPs () on AZ. The same distinct peaks for AZN and TiO2 have also been previously reported by other researchers.Citation30,Citation42
EDX
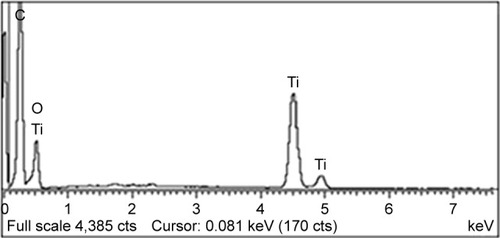
EDX confirmed the presence of both AZ and TiO2 compounds. The peaks of titanium were observed at 0.5, 4.51, and 4.9 keV as this energy is found in the electron transition of TiO2.Citation43 Peaks of carbon (C) and oxygen (O) were seen as these two elements were found in AZ adsorbed by titanium dioxide, as shown in .Citation44 In addition to microscopic and HPLC studies, the EDX study was also found very useful to confirm the presence of both adsorbate and adsorbent in the recovered samples.
Powder XRD studies
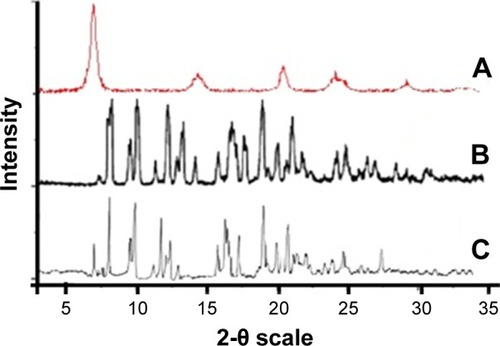
The XRD results showed that the unprocessed AZ was crystalline in nature (). However, the peak intensities of AZNs were relatively low compared to its unprocessed API (). This is due to the adsorption of TNP that has a smaller PS, which causes the reduction in peak intensity of AZN as shown in . The results clearly showed that AZ maintained its crystallinity after physisorption with TNP.Citation40,Citation45 Furthermore, in nanocomposite-type structures, where the small particles become the components of the microparticles, the peak intensities can potentially be reduced.
Formulation of optimized AZN
The optimized AZN was formulated in the form of dry suspension by using different excipients in various concentrations. The results in show that F6 formulation exhibited excellent results when subjected to different studies including physicochemical, content uniformity, and dissolution studies. As shown in , formulation F6 showed excellent results of the active content assay, in vitro dissolution (), and taste acceptability ( and ).
Figure 7 (A) Dissolution rate of formulations (F1–F5) and AZN at pH 7.4. (B) Dissolution rate of formulations (F6–F10) and AZN. (C) Comparative dissolution rate of optimized formulation (F6), marketed drug, AZN, and unprocessed AZ at pH 7.4. (D) Comparative dissolution rate of unprocessed AZ, AZN, standard (marketed) AZN, and optimized formulation (F6) at saliva pH.
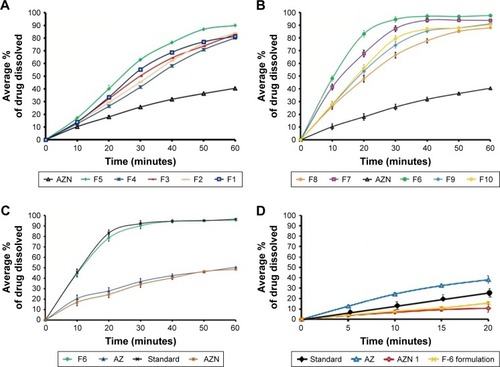
Table 3 Formulation of AZN
Table 4 Physicochemical stability of azithromycin as dry suspension (F6)
Table 5 Panel testing for taste evaluation of various formulations
In vitro dissolution
The drug release pattern of AZ, optimized nanohybrid (AZN-7), and its various formulations (F1–F10) were ana-lyzed using phosphate buffer pH 7.4. The formulation (F6) showed an excellent dissolution rate among all formulation and declared as optimized formulation, as mentioned in , whereas formulations F1 to F5 showed lesser dissolution rate, as shown in , due to the lesser ratio of xanthan gum and HPMC used. The dissolution rate of optimized formulation was compared with its marketed formulation at intestinal pH (7.4), as shown in . The dissolution rate of optimized formulation was initially slightly delayed in the first 30 minutes, while at the end of dissolution process (60 minutes), both the AZN optimized formulation and its marketed drug exhibited the same dissolution rate. This delay in dissolution rate is due to adsorption of TNP on the surface of AZ. However, this compensation of the dissolution rate was due to the faster rate of dissolution of the TNPs at pH 7.4, which resulted in maximum availability of the drug (AZ) in the medium. At the same time, the sample containing equivalent amount of AZN and AZ raw material was also run, which showed a retarded and delayed dissolution rate when compared with that of optimized formulation and marketed drug taken as standard for comparison. The excipients have shown a positive effect on dissolution rate in all developed dry suspension dosage forms. In addition, the designed dissolution studies to evaluate the impact of adsorbed nanoparticles on masking the bitter taste of AZ resulted in retarded rate at saliva pH both for the AZN and the respective developed dry suspension (F6). The AZN and for the optimized formulation F6 showed a delayed and retarded release when analyzed at saliva pH 6.8 (). This retarded dissolution rate is due to insolubility of the adsorbed TNP onto the surface of the AZ at saliva pH 6.8.Citation46–Citation48 Owing to complete coverage of the surface of AZ by TNPs, the interaction of AZ and receptors in taste buds can potentially be reduced and consequently the bitter taste is masked. In addition, there was also observed retarded dissolution rate for the marketed formulation of AZ compared with the bare AZ. However, the retarded dissolution rate of marketed formulation of AZ was less compared with F6 formulation and AZN. This shows that in our formulations, the surface of AZ was strongly protected by adsorption of TNPs from the outer medium compared with the marketed formulation.
Stability studies
The results showed that both the dry suspension and reconstituted optimized formulation (F6) met the specification and were found stable when subjected to physical and chemical stability studies ( and ). The stability of the AZN formulation is because of short and single-step process, which in turn limits the exposure of the product to various environmental factors that could potentially affect the stability of the product. In addition to the above factors, TNP that physically covers the surface of AZ also added to its stability. To get maximum stability, the dry suspension is recommended to be stored below 30°C away from the light and moisture, whereas the reconstituted suspension is advised to keep at 2°C–8°C.Citation28,Citation49,Citation50
Table 6 Stability study of optimized formulation (F6) after reconstitution kept at 8°C–15°C
Taste masking evaluation
The selected volunteers were provided the prepared formulations for panel testing to get the optimized product. As shown in and , the optimized formulation (F6) exhibited an excellent result and showed excellent palatability compared to its counterparts. This study substantiated the results of dissolution studies, where AZN and F6 showed retarded dissolution rates at saliva pH compared with the bare AZ and marketed drug. It was also confirmed from the SEM images () that TNP was completely adsorbed on the surface of AZ, which effectively forms a layer, thereby masking the intense bitter taste of AZ.
Conclusion
The novel approach of physisorption was successfully used to fabricate AZN in the form of dry suspension using bio-compatible TNPs as adsorbate; the palatability of AZ was potentially enhanced when evaluated on numbers of volunteers after passing the sequential test. The AZ optimized formulation (F6) showed a similar dissolution rate compared to its marketed product at intestinal pH, whereas the release rate of the optimized formulation was retarded at saliva pH. This retarded dissolution rate was due to TiO2, which acts as a protective layer on AZ, and due to short residual time in the oral cavity that is 20–40 seconds for pediatric oral dosage form. The adsorbed nanoparticle completely acts as a barrier between drug and taste buds of the tongue, thus inhibiting the bitter taste. It is also concluded that due to the adsorption of TNP, the product showed long-term physico-chemical stability when stored for 90 days. The reconstituted suspension meets the specification when kept at refrigerated conditions for 15 days; this may be due to the protection of drug from the light due to the adsorption of nanoparticles. It has been concluded from the current study that controlling the processes and condition for optimization is the key for fabrication of the AZN to achieve maximum palatability.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group no. RGP-262. The authors gratefully acknowledge Department of Pharmacy, University of Malakand, Chakdara, Dir (L), Khyber Pakhtunkhwa, Pakistan and Department of Pharmacy, Faculty of Life Sciences, Sarhad University, Peshawar, Khyber Pakhtunkhwa, Pakistan.
Disclosure
The authors report no conflicts of interest in this work.
References
- NunnTWilliamsJFormulation of medicines for childrenBr J Clin Pharmacol200559667467615948931
- SteinerJEHuman facial expressions in response to taste and smell stimulationAdv Child Dev Behav197913257295484324
- SuzukiHOnishiHTakahashiYIwataMMachidaYDevelopment of oral acetaminophen chewable tablets with inhibited bitter tasteInt J Pharm20032511–212313212527182
- KendrickJGMaKDezorziPHamiltonDVomiting of oral medications by pediatric patients: survey of medication redosing practicesCan J Hosp Pharm201265319620122783030
- SharmaSLewisSTaste masking technologies: a reviewInt J Pharm Pharm Sci201022613
- BarnardASZapolPCurtissLAModeling the morphology and phase stability of TiO2 nanocrystals in waterJ Chem Theory Comput20051110711626641122
- AbrahamJMathewFTaste masking of paediatric formulation: a review on technologies, recent trends and regulatory aspectsInt J Pharm Pharm Sci2014611219
- GaneshGManjushaPGowthamarajanKDesign and development of buccal drug delivery system for labetalol using natural polymerInt J Pharmaceut Res Dev2011333749
- PanovskáZŠediváAJedelskáMPokornýJEffect of ethanol on interactions of bitter and sweet tastes in aqueous solutionsCzech J Food Sci2008262139145
- WallachJVStructure Activity Relationship (SAR) Studies of Arylcycloalkylamines as N-Methyl-D-Aspartate Receptor AntagonistsPh.D. thesisPhiladelphia, PAUniversity of the Sciences2014
- MaskellJPSeftonAMWilliamsJDComparative in-vitro activity of azithromycin and erythromycin against Gram-positive cocci, Hae-mophilus influenzae and anaerobesJ Antimicrob Chemother199025Suppl_A19242154433
- FieseEFSteffenSHComparison of the acid stability of azithromycin and erythromycin AJ Antimicrob Chemother199025Suppl_A3947
- NgTBPAuditors’ decisions on audit differences that affect significant earnings thresholdsAudit J Pract Theor20072617189
- WoldumHSLarsenKLMadsenFCyclodextrin controlled release of poorly water-soluble drugs from hydrogelsDrug Deliv2008151698018197526
- AroraSCSharmaPKIrchhaiyaRKhatkarASinghNGagoriaJDevelopment, characterization and solubility study of solid dispersions of cefuroxime axetil by the solvent evaporation methodJ Adv Pharm Technol Res20101332622247865
- ApsJKMartensLCReview: the physiology of saliva and transfer of drugs into salivaForensic Sci Int20051502–311913115944052
- AframianDJDavidowitzTBenolielRThe distribution of oral mucosal pH values in healthy saliva secretorsOral Dis200612442042316792729
- IsmailMWengCNRahmanHAZakariaNAFreundlich isotherm equilibrium equastions in determining effectiveness a low cost absorbent to heavy metal removal in wastewater (leachate) at Teluk Kitang Landfill, Pengkalan Chepa, Kelantan, MalaysiaJ Geogr Earth Sci20131118
- SaharanVAKukkarVKatariaMKharbVChoudhuryPOrdered mixing: mechanism, process and applications in pharmaceutical formulationsAsian J Pharm Sci200836240259
- KhanSTAl-KhedhairyAAMusarratJZnO and TiO2 nanoparticles as novel antimicrobial agents for oral hygiene: a reviewJ Nanopart Res2015176276
- KalaiarasiSJoseMStreptomycin loaded TiO2 nanoparticles: preparation, characterization and antibacterial applicationsJ Nanostructure Chem2017714753
- HuhAJKwonYJ“Nanoantibiotics”: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant eraJ Control Release2011156212814521763369
- WeirATitanium Dioxide Nanomaterials: Human Exposure and Environmental Releasemaster’s thesisTempe, AZArizona State University2011
- RocheEJJohnson and Johnson Consumer Inc, assigneeTaste masking and sustained release coatings for pharmaceuticalsUnited States patents US507511419911224
- BalarakDMostafapourFAzarpiraHJoghataeiALangmuir, Freundlich, Temkin and Dubinin–radushkevich isotherms studies of equilibrium sorption of ampicilin unto montmorillonite nanoparticlesJ Pharm Res Int201720219
- WenTGaoJShenJZhouZPreparation and characterization of TiO2 thin films by the sol-gel processJ Mater Sci2001362459235926
- FagundesJGJrda Silva ManeraRTokimatsuRCVentrellaVAGallegoJApplication of titanium machining chips in welding consumables for wear-resistant hardfacingWeld Int2016307520526
- RahimHSadiqAKhanSAceclofenac nanocrystals with enhanced in vitro, in vivo performance: formulation optimization, char-acterization, analgesic and acute toxicity studiesDrug Des Devel Ther20171124432452
- ShetNVaidyaIFormulation and evaluation of taste masked suspension of azithromycin dihydrateCurr Pharma Res2013411072
- KaussTGaubertABoyerCPharmaceutical development and optimization of azithromycin suppository for paediatric useInt J Pharm20134411–221822623220079
- BoehmeMFuGIonescuEEnsingerWFabrication of anatase titanium dioxide nanotubes by electroless deposition using polycarbonate for separate casting methodNanomicro Lett2010212630
- ZubataPCeresoleRRosascoMAPizzornoMTA new HPLC method for azithromycin quantitationJ Pharm Biomed Anal200227583383611814725
- NgCLossoJNMarshallWERaoRMFreundlich adsorption isotherms of agricultural by-product-based powdered activated carbons in a geosmin-water systemBioresour Technol200285213113512227536
- SteveKErikaTReynoldTPaulMActivated carbon: a unit operations and processes of activated carbonEnviron Eng199825350749
- GiammarDEMausCJXieLEffects of particle size and crystalline phase on lead adsorption to titanium dioxide nanoparticlesEnviron Eng Sci20072418595
- VahčićNHruškarMSekvencijalni testovi u odabiru članova panel grupeMljekarstvo: časopis za unaprjeđenje proizvodnje i prerade mlijeka199848297104
- MeilgaardMCCarrBTCivilleGVSensory Evaluation TechniquesBoca Raton, FLCRC Press1999
- ShishuKamalpreetKapoorVRDevelopment of taste masked oral formulation of ornidazoleIndian J Pharm Sci201072221120838525
- GrimmWExtension of the International Conference on Harmonization Tripartite Guideline for Stability Testing of New Drug Substances and Products to countries of climatic zones III and IVDrug Dev Ind Pharm19982443133259876591
- KhanSde MatasMPlakkotSAnwarJNanocrystal recovery by use of carrier particlesCryst Growth Des201414310031009
- AboutalebAAbdel-RahmanSAhmedMYounisMEnhancement of domperidone dissolution rate via formulation of adsorbates and co-adsorbatesInt J Pharm Sci Res201673951
- KotechaRKBhadraDSRajeshKSFormulation & process development of azithromycin ophthalmic nanosuspensionInt J Pharm Pharma Sci201354490497
- SheikhFAMacossayJKanjwalMAbdal-HayATantryMAKimHTitanium dioxide nanofibers and microparticles containing nickel nanoparticlesISRN Nanomater2012201218
- BöhmeMFuGIonescuEEnsingerWFabrication of anatase titanium dioxide nanotubes by electroless deposition using polycarbonate for separate casting methodNano-Micro Lett201322630
- ShahSMUllahFKhanSSmart nanocrystals of artemether: fabrication, characterization, and comparative in vitro and in vivo antimalarial evaluationDrug Des Devel Ther20161038373850
- AucampMOdendaalRLiebenbergWHammanJAmorphous azithromycin with improved aqueous solubility and intestinal membrane permeabilityDrug Dev Ind Pharm20154171100110824980913
- SchmidtJVogelsbergerWDissolution kinetics of titanium dioxide nanoparticles: the observation of an unusual kinetic size effectJ Phys Chem B200611093955396316509682
- KramerSMGorichevIGLainerYAArtamonovaIVTerekhovaMVCalculation of the solubility of TiO2 and titanates in sulfuric acid solutionsRuss Metall201420149704707
- FreitasCMüllerRHEffect of light and temperature on zeta potential and physical stability in solid lipid nanoparticle (SLN™) dispersionsInt J Pharm19981682221229
- MatthewsBRRegulatory aspects of stability testing in EuropeDrug Dev Ind Pharm199925783185610459489