Abstract
The Covid-19 pandemic driven by the SARS-CoV-2 virus continues to exert extensive humanitarian and economic stress across the world. Although antivirals active against mild disease have been identified recently, new drugs to treat moderate and severe Covid-19 patients are needed. Sphingolipids regulate key pathologic processes, including viral proliferation and pathologic host inflammation. Opaganib (aka ABC294640) is a first-in-class clinical drug targeting sphingolipid metabolism for the treatment of cancer and inflammatory diseases. Recent work demonstrates that opaganib also has antiviral activity against several viruses including SARS-CoV-2. A recently completed multinational Phase 2/3 clinical trial of opaganib in patients hospitalized with Covid-19 demonstrated that opaganib can be safely administered to these patients, and more importantly, resulted in a 62% decrease in mortality in a large subpopulation of patients with moderately severe Covid-19. Furthermore, acceleration of the clearance of the virus was observed in opaganib-treated patients. Understanding the biochemical mechanism for the anti-SARS-CoV-2 activity of opaganib is essential for optimizing Covid-19 treatment protocols. Opaganib inhibits three key enzymes in sphingolipid metabolism: sphingosine kinase-2 (SK2); dihydroceramide desaturase (DES1); and glucosylceramide synthase (GCS). Herein, we describe a tripartite model by which opaganib suppresses infection and replication of SARS-CoV-2 by inhibiting SK2, DES1 and GCS. The potential impact of modulation of sphingolipid signaling on multi-organ dysfunction in Covid-19 patients is also discussed.
Covid-19 Overview
As of June 2022, Covid-19 has caused the deaths of more than 1,000,000 people in the United States and over 6,300,000 people worldwide (https://covid19.who.int). Beyond these mortality figures, the economic and social hardships caused by the pandemic reach virtually every country and person. Although outstanding work on the development, manufacturing and distribution of vaccines targeting SARS-CoV-2 is reducing the impact of Covid-19 in many countries, the ability of the virus to mutate into potentially unresponsive variants, as well as the lack of global access to vaccines and the certainty of breakthrough infections in many vaccinated individuals make it imperative that effective and easily administered drugs are available for the treatment of Covid-19 patients. Therapeutic antibodies against SARS-CoV-2 proteins have some efficacy in the very early stages of Covid-19, but their high cost and the need for intravenous administration reduce their broad application.Citation1 Additionally, recent viral variants are less responsive to the existing monoclonal antibody drugs. Similarly, the antiviral drug remdesivir requires intravenous administration and clinical trials have reported either modestly positive (25% reduction in mortality)Citation2 or negative (WHO Solidarity trial)Citation3 results in hospitalized Covid-19 patients. More recent clinical trial data show the efficacy of two new oral antivirals (the nucleoside analog Molnupiravir and the protease inhibitor Paxlovid) in SARS-CoV-2-infected individuals if the drugs are given very shortly after infection (3–5 days). However, Molnuporavir lacks efficacy in Covid-19 patients with moderate or severe diseaseCitation4 and Paxlovid is untested in this population. Consequently, it is essential that additional drugs be identified for use in hospitalized Covid-19 patients.
Development of Opaganib
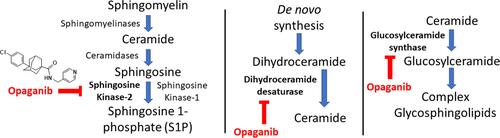
Because sphingolipids regulate key pathologic processes, including tumor cell proliferation and pathologic inflammation (recently reviewed inCitation5–Citation8), we and others sought to identify inhibitors of sphingosine kinases (SK1 and SK2) that may have efficacy as anticancer and anti-inflammatory drugs. Opaganib () is an orally active, isozyme-selective inhibitor of SK2 that is competitive with respect to sphingosine.Citation9,Citation10 Opaganib depletes sphingosine 1-phosphate (S1P) and elevates ceramides in tumor cells, thereby suppressing signaling through pERK and pAKT and promoting autophagy and/or apoptosis in tumor cells.Citation9–Citation15 Opaganib also down-regulates the expression of c-Myc in a variety of cancer cell lines.Citation14,Citation16–Citation19 Because it acts as a sphingosine mimetic, opaganib also inhibits dihydroceramide desaturase (DES1), which accounts for the substantial increases in dihydroceramides in cells treated with the drug.Citation9,Citation16 Additionally, opaganib reduces levels of hexosylceramides in cells, presumably by inhibition of glucosylceramide synthase (GCS). Therefore, opaganib has the unique ability to simultaneously target three key enzymes in the sphingolipid metabolism pathway ().
Figure 1 Multitargeting of sphingolipid metabolism by opaganib. Opaganib (aka ABC294640; Yeliva®; 3-(4-chlorophenyl)-N-(pyridin-4-ylmethyl)-1-adamantanecarboxamide, hydrochloride salt) inhibits SK2 decreasing S1P synthesis, DES1 elevating dihydroceramides, and GCS reducing hexosylceramides.
Opaganib has antitumor activity in a wide range of mouse models,Citation9,Citation13,Citation14,Citation16,Citation17,Citation20–Citation26 as well as anti-inflammatory activity in several rodent models.Citation27–Citation31 A Phase 1 clinical trial with opaganib administered to patients with advanced solid tumors was conducted to assess the drug’s safety and tolerability when given orally on a twice-daily continuous schedule to fasted patients.Citation32 This trial demonstrated that opaganib is well tolerated, with 2 patients receiving more than 40 weeks of drug treatment, including a patient with refractory cholangiocarcinoma who experienced a prolonged Partial Response. Overall, 64% of patients who completed 2 cycles of opaganib treatment had Stable Disease or better, suggesting that the drug has activity in many cancer patients. Although the Maximum Tolerated Dose was not formally defined in this study, the recommended phase 2 dose was established as 500 mg twice-daily. A subsequent food–effect study of opaganib given to healthy volunteers indicated that adverse events were milder in fed subjects than in fasted subjects. Therefore, in a following trial in patients with refractory multiple myeloma, opaganib was escalated to 750 mg twice-daily. In this trial, 58% of the evaluable patients achieved Stable Disease or better, and one developed a Very Good Partial Response that was associated with marked decreases in plasma levels of TNFα, EGF and VEGF. Opaganib is currently in Phase 2 clinical testing in patients having cholangiocarcinoma (NCT03377179) or prostate cancer (NCT04207255). To date, more than 400 people have been treated with opaganib in oncology and Covid-19 clinical trials (summarized in ), demonstrating the safety and efficacy of the drug.
Table 1 Clinical Studies of Opaganib
Anti-Viral Activity of Opaganib
Several studies have demonstrated that SKs and S1P enhance the replication of influenza, measles and hepatitis B viruses (reviewed inCitation33). We have previously shown that SK2 maintains the latency of Kaposi’s sarcoma-associated herpesvirus (KSHV)-infected endothelial cells,Citation13 and opaganib therefore suppresses KSHV-induced tumor growth in vivo.Citation13,Citation34,Citation35 Reid et al demonstrated that opaganib inhibits the replication of chikungunya virus (CHIKV), which contains a +single-stranded RNA genome as does SARS-CoV-2.Citation36 In recent studies by Xia et al, opaganib suppressed the replication of influenza A virus in A549 cells in vitro with an EC50<2 µM,Citation37 which is well below the Cmax for opaganib in cancer patients.Citation32 Furthermore, two oral doses of opaganib markedly reduced the viral load in the lungs of mice exposed to influenza A virus, and this substantially improved the survival of infected mice.Citation37 Combined activity against the influenza virus and SARS-CoV-2 could be very important for individuals with Covid-19 during the flu season. To evaluate the in vitro effects of opaganib on SARS-CoV-2 replication, opaganib was studied in a 3D tissue model of human bronchial epithelial cells (EpiAirway™) which morphologically and functionally resembles the human airway.Citation38 Opaganib treatment of cells infected with SARS-CoV-2 resulted in a dose-dependent inhibition of virus production at pharmacologically relevant concentrations (IC50~0.5 μM) 72 hours after infection without compromising cell viability. SARS-CoV-2 replication was completely blocked by <3 μM opaganib. This first study used the alpha (Washington) strain of SARS-CoV-2, and subsequent similar studies demonstrated that opaganib also inhibits the replication of the beta, gamma, delta and omicron SARS-CoV-2 variants. Because opaganib targets host proteins instead of viral proteins, mutation of the SARS-CoV-2 virus is not expected to generate opaganib-resistant variants as commonly occurs with virus-directed antivirals. Furthermore, opaganib is relatively easy to synthesize and manufacture into capsules and has exceptional chemical stability, making it a very suitable drug for global use. These preclinical studies demonstrating the anti-inflammatory and antiviral efficacies of opaganib in multiple models (summarized in ) support the potential for opaganib to help mitigate the current Covid-19 pandemic, as well as potentially successfully treat other viral diseases.
Table 2 Nonclinical Studies of Opaganib Relating to Covid-19
Safety and Efficacy of Opaganib in Covid-19 Patients
Testing for opaganib efficacy in Covid-19 patients was originally rationalized based on its ability to suppress pathologic inflammation in multiple preclinical models, its preclinical antiviral activity, and its safety in oncology clinical trials. However, the direct demonstration of the ability of opaganib to inhibit SARS-CoV-2 replication in vitroCitation38 provided compelling support for testing opaganib for the treatment and/or prevention of Covid-19. A Phase 2a proof-of-concept study of opaganib administered to hospitalized patients with severe Covid-19 pneumonia requiring supplemental oxygen was conducted in the USA in 2020. In addition to the Standard of Care treatment, patients received opaganib (n = 23) or placebo (n = 19) for up to 14 days and were followed for 28 days after their last dose. There were no material differences in adverse events between the opaganib and placebo treatment groups, indicating that opaganib can be safely administered to these patients.Citation39 Although the small study size precluded definitive demonstration of safety and/or efficacy, patients who received oral opaganib required less supplemental oxygen and achieved earlier hospital discharge than the placebo control patients.Citation39 This positive outcome supported the conduct of a Phase 2/3 multinational randomized, double-blind, parallel arm, placebo-controlled study to evaluate the ability of opaganib to improve the clinical status of hospitalized patients with severe Covid-19.Citation40 The study enrolled 475 adult patients who were randomized to either 500 mg oral opaganib every 12 hours or matching placebo, in addition to Standard of Care for 14 days. Patients were followed for 42 days from their first dose of opaganib. As with the Phase 2a study, adverse events were similar in both treatment groups, further demonstrating the safety of opaganib for Covid-19 patients. More specifically, in the setting of a 14-day course of treatment, opaganib had a favorable safety profile with mostly low-grade nausea, insomnia and anxiety being clearly treatment related adverse events, all occurring in less than 10% of patients. The pre-specified analyses for the primary clinical outcome (the proportion of patients breathing room air without oxygen support by Day 14) did not demonstrate a statistically significant treatment benefit in the entire patient population. However, the subpopulation of patients (54%) requiring at or below the median oxygen supplementation (median fraction inspired oxygen (FiO2) at baseline was 60%) demonstrated a clear clinical benefit with opaganib treatment.Citation40 Most importantly, opaganib reduced the incidences of intubation/mechanical ventilation and death by Day 42 by 62% each. Additionally, a more rapid clearance of the virus was observed in patients treated with opaganib (median = 10 days) compared to control patients receiving standard of care (median >14 days) for the entire treated population.Citation41 Overall, the data suggest therapeutic benefit from the treatment of moderately severe Covid-19 patients with opaganib. Although untested to date, it is likely that the clinical benefit of opaganib would be even greater in less-severe Covid-19 patients including non-hospitalized individuals at risk for hospitalization or long-term sequelae.
Mechanism for the Antiviral Efficacy of Opaganib
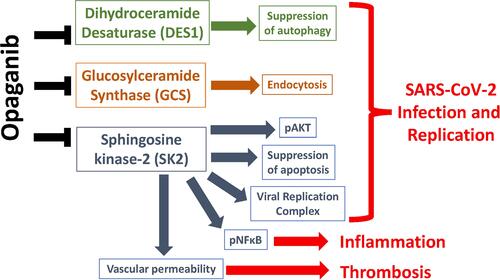
Increasing attention is being paid to the involvement of sphingolipids in viral infection and replication in general (reviewed inCitation33,Citation42,Citation43) and SARS viruses in particular.Citation44–Citation48 Entry of SARS viruses into target cells is primarily mediated by binding to angiotensin-converting enzyme 2 (ACE2), followed by proteolytic cleavage by TMPRSS2 and internalization via endocytosis mediated by lipid rafts, which are cholesterol- and sphingolipid-rich membrane domains.Citation49 McGowan et alCitation44 provided an excellent review of the roles of sphingolipid metabolism in viral replication, activation of the host immune response and maintaining vascular integrity. The authors also discussed how manipulating the SK-S1P pathway may be beneficial to Covid-19 patients and provided a brief overview of the potential for repurposing sphingolipid-directed drugs for the treatment of Covid-19, including opaganib and two other drugs in clinical trials at that time. Since its publication, clinical testing of opaganib has markedly progressed and the results from the recently completed multinational Phase 2/3 clinical trial with hospitalized severe Covid-19 patients have been reported.Citation40 Because of the positive efficacy of opaganib against Covid-19, definition of the biochemical mechanism(s) for this activity requires further investigation. We herein focus on potential antiviral mechanisms that are directly relevant to the biochemical actions of opaganib ().
Figure 2 Model for the therapeutic activity of opaganib against Covid-19. Sphingolipids regulate the ability of SARS-CoV-2 to replicate and thereby cause Covid-19. Firstly, pro-autophagic dihydroceramide levels are normally maintained at low levels by DES1. Inhibition of DES1 by opaganib elevates dihydroceramides and promotes autophagy which suppresses viral replication. Secondly, hexosylceramides are necessary for the endocytosis of virus bound to ACE2. Inhibition of GCS by opaganib reduces hexosylceramides thereby impairing the ability of the virus to enter target cells. Thirdly, SK2 regulates several signaling pathways, as well as the viral replication complex, that are required for viral replication. Inhibition of SK2 by opaganib therefore has multifaceted suppressive effects on viral infection and replication. Furthermore, opaganib suppression of inflammation and thrombosis mediated by SK2 may protect against multi-organ dysfunction in Covid-19 patients.
Inhibition of Spike Protein-ACE2 Binding
Blocking the ability of the SAR-CoV-2 spike protein to interact with ACE2 is clearly expected to inhibit infection and viral replication, and this is a likely mechanism for several antibody-based therapeutics, including convalescent plasma. Edwards et al recently demonstrated that binding of the SARS-CoV-2 spike protein to ACE2 is potently inhibited by sphingosine.Citation50 By inhibiting sphingosine phosphorylation to S1P by SK2, opaganib at least transiently elevates sphingosine levels that may be sufficient to disrupt SARS-CoV-2 binding to target cells. Alternatively, because opaganib is competitive with sphingosine for inhibition of SK2,Citation9,Citation10 ie acts as a “sphingosine mimetic”, it may directly disrupt binding of the spike protein to ACE2 thereby suppressing the internalization and replication of SARS-CoV-2.
Inhibition of Akt Signaling
A strong body of evidence demonstrates that viruses commonly commandeer host machinery required for viral replication, including activation of the PI3K/AKT/mTOR signaling pathway (reviewed inCitation51). For example, Mizutani et al demonstrated that infection of Vero E6 cells by coronavirus activates the AKT signaling pathway,Citation52 and that pAKT is essential for establishing persistent viral infection of these cells.Citation53 Recent work confirms Akt activation by SARS-CoV-2,Citation54 and further shows that inhibition of AKT by GSK690693 blocks viral-induction of cytokine and chemokine expression by lung epithelial cells.Citation55 Additionally, the pan-AKT inhibitor capivasertib was shown to inhibit the entry of SAR-CoV-2 into Vero cells.Citation56 Similarly, the AKT inhibitor MK-2206 suppressed viral replication, most likely by inducing autophagy.Citation54,Citation57 Together, these results led to the proposal that inhibitors of the PI3K/AKT/mTOR signaling pathway may be effective for Covid-19 therapy;Citation51,Citation58 however, none of the clinical trials assessing such drugs have reported positive data to date.Citation51 Beyond direct inhibition of viral replication, AKT inhibitors may attenuate excessive inflammation, cytokine storm, fibrosis, and thrombosis in Covid-19 patients.Citation58 We and others have demonstrated that opaganib efficiently inhibits AKT phosphorylation in multiple cell types.Citation9,Citation10,Citation12,Citation14,Citation16,Citation17,Citation20,Citation22–Citation25,Citation59–Citation63 The biochemical mechanism for reduction of pAKT by opaganib has not been fully defined, but likely involves the stimulation of protein phosphatase 2A (PP2A)-mediated pAKT dephosphorylation due to reduction of S1P levels (which suppress PP2A activityCitation64) and/or inactivation of Inhibitor 2 of PP2A (SET) due to elevation of ceramide levels.Citation65,Citation66 It is also possible that opaganib directly binds to and inhibits SET as has been suggested for fingolimod (FTY720).Citation67 Finally, AKT contains an N-terminal pleckstrin homology (PH) domain which enables its membrane translocation and subsequent activation by upstream kinases. Because SK2 also contains a PH domain that directs its localization to membranes,Citation68 opaganib may alter potential SK2-AKT co-localization necessary to allow viral replication. Through one or more of these mechanisms, inhibition of AKT phosphorylation may underlie the ability of opaganib to suppress infection by SARS-CoV-2.
Induction of Autophagy
The primary roles of autophagy in normal cells are to recycle intracellular materials and to eliminate intracellular pathogens. Viruses, including coronaviruses, suppress autophagy to promote their own replication, and consequently activation of autophagy can effectively limit viral replication.Citation69–Citation72 SARS-CoV-2 blocks autophagy, leading to the suggestion that autophagy-inducing agents may overcome infection by reactivating intracellular viral destruction.Citation73–Citation79 Supporting this concept, SARS-CoV-2 was shown to promote Beclin-1 degradation,Citation80 and several autophagy-modifying compounds suppress SAR-CoV-2 replication in vitro (reviewed inCitation75). It is established that: dihydroceramides induce autophagy;Citation81–Citation87 that opaganib elevates dihydroceramides by inhibition of DES1;Citation16,Citation88,Citation89 and that opaganib promotes autophagy.Citation11,Citation15,Citation34 Therefore, inhibition of DES1 may be involved in the ability of opaganib to suppress infection by SARS-CoV-2 through the induction of protective autophagy in the host cells.
Induction of Apoptosis
One mechanism that limits viral spread is the induction of apoptosis of infected host cells, and consequently viruses frequently suppress apoptosis to facilitate their replication. Coronavirus infection modulates both the extrinsic and intrinsic apoptosis pathways via the Death Domain (DD) superfamily of proteins and the Bcl-2 family of proteins, respectively.Citation90 For example, Zhong et al demonstrated that the avian coronavirus infectious bronchitis virus (IBV) induces the expression of Mcl-1 (which inhibits apoptosis) and Bak (which promotes apoptosis), and that genetic ablation of Mcl-1 accelerates IBV-induced apoptosis.Citation91 Furthermore, viruses, including SARS-CoV-2, activate signaling through NFκB which suppresses apoptosis via both pathways. Sphingosine kinase-2 plays important roles in regulating apoptosis and NFκB pathways, and the effects of opaganib on these processes have been examined. For example, SK2 contains a BH3 domain, and therefore overexpression of SK2 induces apoptosis.Citation92 Opaganib promotes apoptosis alone and in combination with the DD ligand TRAIL in cancer cellsCitation7,Citation93 and suppresses NFκB activation both in vitro and in vivo.Citation12,Citation94–Citation96 Mechanistically, opaganib promotes apoptosis by suppressing the expression of Mcl-1Citation17,Citation97,Citation98 and survivin.Citation99 Furthermore, we have previously shown that in conjunction with suppressing its kinase activity, opaganib increases SK2 expression, and this overexpression of the BH3 domain could provide a magnified pro-death stimulus.Citation10 Therefore, similar to studies with KSHV-infected cells,Citation13,Citation35 opaganib may suppress Covid-19 through the induction of protective apoptosis in SARS-CoV-2-infected host cells.
Inhibition of Endocytosis
Entry of SARS-CoV into cells occurs via ACE2-mediated, pH-dependent endocytosis that does not involve clathrin or caveolae, but does require sphingolipid-containing membrane lipid rafts.Citation49 More specifically, glycosphingolipids are abundant components of the extracellular surface of the plasma membrane that are essential for endocytosis,Citation100–Citation103 and so play major roles in the penetration of target cells by viruses. For example, exposure of cells to influenza virus elevates levels of sphingomyelin and glucosylceramide,Citation104,Citation105 and inhibition of GCS suppresses influenza virus infectionCitation106 and maturation.Citation107 More specifically, GCS activity is required for the entry of influenza virusCitation108 and thrombocytopenia syndrome virusCitation109 via endocytosis. This concept was extended by Vitner et al who demonstrated that SARS-CoV-2 infection of Vero E6 cells significantly elevated glycosphingolipid and sphinganine levels, and that similar increases occurred in mice infected with the virus.Citation110 Importantly, two GCS inhibitors strongly suppressed an early step in the replication of influenza virus and SARS-CoV-2 in Vero E6 cells,Citation111 demonstrating that GCS is a potential new target for anti-Covid-19 drugs. Therefore, the ability of opaganib to inhibit GCS may be involved in the ability of opaganib to suppress replication of SARS-CoV-2.
Association with the Viral Replication-Transcription Complex (RTC)
SARS-CoV-2 RNA synthesis is conducted using RTC, which is anchored by the transmembrane viral proteins Nsp3, Nsp4 and Nsp6. The importance of sphingolipids in determining the structure and function of membrane domains such as lipid rafts is well established, and consequently, alteration of sphingolipids by opaganib may disrupt the ability of SARS-CoV-2 to efficiently establish functional RTCs. Furthermore, Reid et al demonstrated that SK2 is a host factor that co-localizes with the RTC of CHIKV by interaction with Nsp3.Citation36 This appears to be essential for optimal function of the RTC because inhibiting SK2 by opaganib or genetic knockout inhibited CHIKV transcription.Citation36 Conversely, overexpression of SK2 in response to opaganib may further suppress viral replication by competing with G3BP binding to the RTC.Citation112 Nsp3 plays a parallel membrane anchoring role for SARS-CoV-2 replication,Citation113 and therefore, disruption of the viral RTC may be involved in the ability of opaganib to suppress replication by SARS-CoV-2.
Potential for Opaganib to Attenuate Multi-Organ Dysfunction in Covid-19 Patients
Sphingolipid metabolism is critically involved in the pathogenesis of lung damage, including pulmonary failure in Covid-19 patients (reviewed inCitation114). Therefore, beyond the focused antiviral effects discussed above, the ability of opaganib to suppress pathologic inflammation is expected to benefit Covid-19 patients by limiting multi-organ damage due to excessive cytokine production and activity. For example, several studies have examined the role of SK2 in a murine Pseudomonas aeruginosa (PA)-induced pneumonia lung inflammation model. Genetic deletion of SK2, but not SK1, suppressed NADPH oxidase 4 induction,Citation115 and decreased levels of inflammatory cytokines, proteins and cell counts in bronchoalveolar lavage, as well as neutrophil infiltration into the alveolar space, in mice exposed to intratracheal PA.Citation116 This was associated with reduced expression of NFκB-regulated inflammation-associated genes in the lung tissue.Citation117 Most importantly, the administration of opaganib to mice nearly completely ameliorated PA-induced lung injury,Citation118 specifically by decreasing inflammatory cell infiltration on histologic examination, markedly reducing infection-induced increases in TNFα, IL-6, and H2O2 in bronchoalveolar lavage fluids, and improving survival of infected mice. Additionally, opaganib inhibits IL-6 secretion from human bronchial epithelial cells in vitro (RedHill Biopharma unpublished data). Consequently, opaganib may suppress Acute Respiratory Distress Syndrome and subsequent pulmonary fibrosis in Covid-19 patients.
Sphingolipids also have critical roles in acute kidney injury (AKI) and renal fibrosis (reviewed inCitation119,Citation120). For example, Park et al demonstrated that genetic knockout of SK2 decreased AKI following ischemia-reperfusion, whereas knockout of SK1 increased injury.Citation121 Additionally, Bajwa et al showed that the SK2 inhibitor SLP120701 reduced folic acid-induced renal fibrosis in mice,Citation122 and Zhu et al demonstrated that opaganib inhibits extracellular matrix deposition in human kidney fibroblasts.Citation62 Furthermore, opaganib reduced fibrosis and decreased inflammatory cell infiltration in the kidneys of mice subjected to unilateral ureteral obstruction (RedHill Biopharma unpublished data). Therefore, opaganib may suppress AKI and subsequent renal failure in patients with severe Covid-19.
Finally, the roles of sphingolipids in thrombosis have been studied since 1995, when Yatomi et al demonstrated that S1P promotes platelet aggregation.Citation123 Interestingly, genetic knockout of SK2, but not SK1, markedly reduced S1P levels in platelets, and strongly attenuated platelet aggregation in vitro and in vivo thrombus formation in ferric chloride-treated mice.Citation124 This is consistent with preliminary studies that demonstrate that opaganib administration reduces in vivo thrombus formation in the ferric chloride model (RedHill Biopharma unpublished data). Additionally, tissue factor-mediated coagulation following SARS-CoV-2 infection has been linked to stimulated sphingolipid metabolism.Citation125 Consequently, opaganib treatment may provide an anticoagulant benefit to Covid-19 patients resulting in a lower risk of thrombosis.
Conclusion
Clinical experience with opaganib demonstrates that it can be safely administered to severely compromised patients with cancer or Covid-19. Data from the completed Phase 2/3 clinical trial indicate efficacy of opaganib in a subset of severe Covid-19 patients. Antiviral and anticancer therapy usually involve multiple drugs targeting different key proteins to achieve clinical benefit. Opaganib appears to be uniquely situated to simultaneously inhibit three sphingolipid-metabolizing enzymes in human cells, ie SK2, DES1 and GCS (). While additional studies are needed to elucidate which of these enzymes mediate its antiviral activity, opaganib has the potential to suppress a range of viruses, including SARS-CoV-2. Because of this tripartite targeting, it is unlikely that viral resistance to opaganib will be encountered either through adaptive mutation during therapy or by random mutation to generate additional viral variants.
Currently, the primary treatments available for patients with a score of 5 on the WHO Ordinal Scale for Clinical Recovery are dexamethasone and remdesivir. Dexamethasone is an anti-inflammatory medication that was shown in the RECOVERY study to be most effective for patients who are on mechanical ventilation, with a smaller effect for hospitalized patients on oxygen but not mechanically ventilated.Citation126 Remdesivir is an anti-viral nucleoside analogue that was initially shown to be most effective in time to recovery in patients with the equivalent of WHO 4 status with little to no effect in patients with the equivalent of WHO 5.Citation2 In the larger WHO SOLIDARITY trial, there was a small effect on mortality in the combined WHO 4 and 5 groups receiving remdesivir.Citation3 It is also important to note that remdesivir is only given by intravenous infusion. Opaganib would present an oral alternative with both anti-viral and anti-inflammatory properties for patients in the WHO 5 category. In addition, in a prespecified substrata analysis, when given on top of dexamethasone and remdesivir standard of care, opaganib was nominally superior to placebo. Overall, opaganib may provide an important oral drug for the treatment of patients with severe Covid-19.
Acknowledgments
Apogee Biotechnology Corporation holds patents on opaganib and related compounds, which have been licensed to RedHill Biopharm LTD. Both companies have provided funding for laboratory and clinical research discussed in this manuscript.
Disclosure
Charles D. Smith, Lynn W. Maines and Staci N. Keller are current employees and own stock in Apogee Biotechnology Corporation. In addition, Dr Charles D Smith has patents (7,338,961; 8,063,248; 8,557,800) licensed to RedHill Biopharma LTD. Dr Lynn W Maines reports patents (8324237; 8685936) issued to RedHill Biopharma. Vered Katz Ben-Yair, Reza Fathi, Terry F. Plasse and Mark L. Levitt are currently paid consultants to RedHill Biopharma LTD. The authors report no other conflicts of interest in this work.
References
- Li D, Sempowski GD, Saunders KO, Acharya P, Haynes BF. SARS-CoV-2 Neutralizing Antibodies for COVID-19 Prevention and Treatment. Annu Rev Med. 2021;73:1–16. doi:10.1146/annurev-med-042420-113838
- Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020;383(19):1813–1826. doi:10.1056/NEJMoa2007764
- Pan H, Peto R, Henao-Restrepo AM, et al.; Consortium WHOST. Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N Engl J Med. 2021;384(6):497–511.
- Singh AK, Singh A, Singh R, Misra A. Molnupiravir in COVID-19: A systematic review of literature. Diabetes Metab Syndr. 2021;15(6):102329. doi:10.1016/j.dsx.2021.102329
- Ogretmen B. Sphingolipid metabolism in cancer signalling and therapy. Nat Rev Cancer. 2018;18(1):33–50. doi:10.1038/nrc.2017.96
- Companioni O, Mir C, Garcia-Mayea Y, Me LL. Targeting Sphingolipids for Cancer Therapy. Front Oncol. 2021;11:745092. doi:10.3389/fonc.2021.745092
- Lewis CS, Voelkel-Johnson C, Smith CD. Targeting Sphingosine Kinases for the Treatment of Cancer. Adv Cancer Res. 2018;140:295–325.
- Stepanovska B, Huwiler A. Targeting the S1P receptor signaling pathways as a promising approach for treatment of autoimmune and inflammatory diseases. Pharmacol Res. 2020;154:104170. doi:10.1016/j.phrs.2019.02.009
- French KJ, Zhuang Y, Maines LW, et al. Pharmacology and antitumor activity of ABC294640, a selective inhibitor of sphingosine kinase-2. J Pharmacol Exp Ther. 2010;333(1):129–139. doi:10.1124/jpet.109.163444
- Gao P, Peterson YK, Smith RA, Smith CD. Characterization of isoenzyme-selective inhibitors of human sphingosine kinases. PLoS One. 2012;7(9):e44543. doi:10.1371/journal.pone.0044543
- Beljanski V, Knaak C, Smith CD. A novel sphingosine kinase inhibitor induces autophagy in tumor cells. J Pharmacol Exp Ther. 2010;333(2):454–464. doi:10.1124/jpet.109.163337
- Antoon JW, White MD, Slaughter EM, et al. Targeting NFkB mediated breast cancer chemoresistance through selective inhibition of sphingosine kinase-2. Cancer Biol Ther. 2011;11(7):678–689. doi:10.4161/cbt.11.7.14903
- Qin Z, Dai L, Trillo-Tinoco J, et al. Targeting sphingosine kinase induces apoptosis and tumor regression for KSHV-associated primary effusion lymphoma. Mol Cancer Ther. 2014;13(1):154–164. doi:10.1158/1535-7163.MCT-13-0466
- Schrecengost RS, Keller SN, Schiewer MJ, Knudsen KE, Smith CD. Downregulation of Critical Oncogenes by the Selective SK2 Inhibitor ABC294640 Hinders Prostate Cancer Progression. Mol Cancer Res. 2015;13(12):1591–1601. doi:10.1158/1541-7786.MCR-14-0626
- Ding X, Chaiteerakij R, Moser CD, et al. Antitumor effect of the novel sphingosine kinase 2 inhibitor ABC294640 is enhanced by inhibition of autophagy and by sorafenib in human cholangiocarcinoma cells. Oncotarget. 2016;7(15):20080.
- Venant H, Rahmaniyan M, Jones EE, et al. The Sphingosine Kinase 2 Inhibitor ABC294640 Reduces the Growth of Prostate Cancer Cells and Results in Accumulation of Dihydroceramides In Vitro and In Vivo. Mol Cancer Ther. 2015;14(12):2744–2752. doi:10.1158/1535-7163.MCT-15-0279
- Venkata JK, An N, Stuart R, et al. Inhibition of sphingosine kinase 2 downregulates the expression of c-Myc and Mcl-1 and induces apoptosis in multiple myeloma. Blood. 2014;124(12):1915–1925. doi:10.1182/blood-2014-03-559385
- Wallington-Beddoe CT, Powell JA, Tong D, Pitson SM, Bradstock KF, Bendall LJ. Sphingosine kinase 2 promotes acute lymphoblastic leukemia by enhancing MYC expression. Cancer Res. 2014;74(10):2803–2815. doi:10.1158/0008-5472.CAN-13-2732
- Lewis CS, Voelkel-Johnson C, Smith CD. Suppression of c-Myc and RRM2 expression in pancreatic cancer cells by the sphingosine kinase-2 inhibitor ABC294640. Oncotarget. 2016;7(37):60181–60192. doi:10.18632/oncotarget.11112
- Antoon JW, White MD, Driver JL, Burow ME, Beckman BS. Sphingosine kinase isoforms as a therapeutic target in endocrine therapy resistant luminal and basal-A breast cancer. Exp Biol Med. 2012;237(7):832–844. doi:10.1258/ebm.2012.012028
- Antoon JW, White MD, Meacham WD, et al. Antiestrogenic effects of the novel sphingosine kinase-2 inhibitor ABC294640. Endocrinology. 2010;151(11):5124–5135. doi:10.1210/en.2010-0420
- Beljanski V, Knaak C, Zhuang Y, Smith CD. Combined anticancer effects of sphingosine kinase inhibitors and sorafenib. Invest New Drugs. 2011;29(6):1132–1142. doi:10.1007/s10637-010-9452-0
- Beljanski V, Lewis CS, Smith CD. Antitumor activity of sphingosine kinase 2 inhibitor ABC294640 and sorafenib in hepatocellular carcinoma xenografts. Cancer Biol Ther. 2011;11(5):524–534. doi:10.4161/cbt.11.5.14677
- Chumanevich AA, Poudyal D, Cui X, et al. Suppression of colitis-driven colon cancer in mice by a novel small molecule inhibitor of sphingosine kinase. Carcinogenesis. 2010;31(10):1787–1793. doi:10.1093/carcin/bgq158
- Xun C, Chen MB, Qi L, et al. Targeting sphingosine kinase 2 (SphK2) by ABC294640 inhibits colorectal cancer cell growth in vitro and in vivo. J Exp Clin Cancer Res. 2015;34(1):94. doi:10.1186/s13046-015-0205-y
- Panneer Selvam S, De Palma RM, Oaks JJ, et al. Binding of the sphingolipid S1P to hTERT stabilizes telomerase at the nuclear periphery by allosterically mimicking protein phosphorylation. Sci Signal. 2015;8(381):ra58. doi:10.1126/scisignal.aaa4998
- Fitzpatrick LR, Green CL, Maines LW, Smith CD. Experimental osteoarthritis in rats is attenuated by ABC294640, a selective inhibitor of sphingosine kinase-2. Pharmacology. 2011;87(3–4):135–143. doi:10.1159/000323911
- Fitzpatrick LR, Green CL, Frauenhoffer EE, et al. Attenuation of arthritis in rodents by a novel orally-available inhibitor of sphingosine kinase. Inflammopharmacology. 2010;19(2):75–87. doi:10.1007/s10787-010-0060-6
- Maines LW, Fitzpatrick LR, French KJ, et al. Suppression of ulcerative colitis in mice by orally available inhibitors of sphingosine kinase. Dig Dis Sci. 2008;53(4):997–1012. doi:10.1007/s10620-007-0133-6
- Maines LW, Fitzpatrick LR, Green CL, Zhuang Y, Smith CD. Efficacy of a novel sphingosine kinase inhibitor in experimental Crohn’s disease. Inflammopharmacology. 2010;18(2):73–85. doi:10.1007/s10787-010-0032-x
- Maines LW, French KJ, Wolpert EB, Antonetti DA, Smith CD. Pharmacologic manipulation of sphingosine kinase in retinal endothelial cells: implications for angiogenic ocular diseases. Invest Ophthalmol Vis Sci. 2006;47(11):5022–5031. doi:10.1167/iovs.05-1236
- Britten CD, Garrett-Mayer E, Chin SH, et al. A Phase I Study of ABC294640, a First-in-Class Sphingosine Kinase-2 Inhibitor, in Patients with Advanced Solid Tumors. Clin Cancer Res. 2017;23(16):4642–4650. doi:10.1158/1078-0432.CCR-16-2363
- Bezgovsek J, Gulbins E, Friedrich SK, Lang KS, Duhan V. Sphingolipids in early viral replication and innate immune activation. Biol Chem. 2018;399(10):1115–1123. doi:10.1515/hsz-2018-0181
- Dai L, Bai A, Smith CD, Rodriguez PC, Yu F, Qin Z. ABC294640, A Novel Sphingosine Kinase 2 Inhibitor, Induces Oncogenic Virus-Infected Cell Autophagic Death and Represses Tumor Growth. Mol Cancer Ther. 2017;16(12):2724–2734. doi:10.1158/1535-7163.MCT-17-0485
- Dai L, Trillo-Tinoco J, Bai A, et al. Ceramides promote apoptosis for virus-infected lymphoma cells through induction of ceramide synthases and viral lytic gene expression. Oncotarget. 2015;6(27):24246–24260. doi:10.18632/oncotarget.4759
- Reid SP, Tritsch SR, Kota K, et al. Sphingosine kinase 2 is a chikungunya virus host factor co-localized with the viral replication complex. Emerg. Microbes Infect. 2015;4(10):e61. doi:10.1038/emi.2015.61
- Xia C, Seo YJ, Studstill CJ, Vijayan M, Wolf JJ, Hahm B. Transient inhibition of sphingosine kinases confers protection to influenza A virus infected mice. Antiviral Res. 2018;158:171–177. doi:10.1016/j.antiviral.2018.08.010
- RedHill Biopharma. RedHill Biopharma’s Opaganib Demonstrates Complete Inhibition of SARS-CoV-2; 2020. Available from: https://www.redhillbio.com/news/news-details/2020/RedHill-Biopharmas-Opaganib-Demonstrates-Complete-Inhibition-of-SARS-CoV-2/default.aspx. Accessed July 5, 2022.
- Winthrop KL, Skolnick AW, Rafiq AM, et al. Opaganib in COVID-19 pneumonia: Results of a randomized, placebo-controlled Phase 2a trial. Open Forum Infect Dis. 2022:ofac232. doi:10.1093/ofid/ofac232
- RedHill Biopharma. RedHill Biopharma Reports Further Analysis of Phase 2/3 Data Including a 62% Reduction in Mortality with Oral Opaganib in Moderately Severe COVID-19 Patients; 2021. Available from: https://www.redhillbio.com/news/news-details/2021/RedHill-Biopharma-Reports-Further-Analysis-of-Phase-23-Data-Including-a-62-Reduction-in-Mortality-with-Oral-Opaganib-in-Moderately-Severe-COVID-19-Patients/default.aspx. Accessed December 22, 2021.
- RedHill Biopharma. RedHill Biopharma's Oral Opaganib Significantly Improves Viral Clearance in Phase 2/3 Study in Severely Ill Hospitalized COVID-19 Patients; 2022. Available from: https://www.redhillbio.com/news/news-details/2022/RedHill-Biopharmas-Oral-Opaganib-Significantly-Improves-Viral-Clearance-in-Phase-23-Study-in-Severely-Ill-Hospitalized-COVID-19-Patients/default.aspx. Accessed July 5, 2022.
- Schneider-Schaulies J, Schneider-Schaulies S. Sphingolipids in viral infection. Biol Chem. 2015;396(6–7):585–595. doi:10.1515/hsz-2014-0273
- Beckmann N, Becker KA. Ceramide and Related Molecules in Viral Infections. Int J Mol Sci. 2021;22(11):5676. doi:10.3390/ijms22115676
- McGowan EM, Haddadi N, Nassif NT, Lin Y. Targeting the SphK-S1P-SIPR Pathway as a Potential Therapeutic Approach for COVID-19. Int J Mol Sci. 2020;21(19):7189. doi:10.3390/ijms21197189
- Abu-Farha M, Thanaraj TA, Qaddoumi MG, Hashem A, Abubaker J, Al-Mulla F. The Role of Lipid Metabolism in COVID-19 Virus Infection and as a Drug Target. Int J Mol Sci. 2020;21(10):10. doi:10.3390/ijms21103544
- Meacci E, Garcia-Gil M, Pierucci F. SARS-CoV-2 Infection: A Role for S1P/S1P Receptor Signaling in the Nervous System? Int J Mol Sci. 2020;21(18):18. doi:10.3390/ijms21186773
- Pan Y, Gao F, Zhao S, Han J, Chen F. Role of the SphK-S1P-S1PRs pathway in invasion of the nervous system by SARS-CoV-2 infection. Clin Exp Pharmacol Physiol. 2021;48(5):637–650. doi:10.1111/1440-1681.13483
- Tornquist K, Asghar MY, Srinivasan V, Korhonen L, Lindholm D. Sphingolipids as Modulators of SARS-CoV-2 Infection. Front Cell Dev Biol. 2021;9:689854. doi:10.3389/fcell.2021.689854
- Wang H, Yang P, Liu K, et al. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008;18(2):290–301. doi:10.1038/cr.2008.15
- Edwards MJ, Becker KA, Gripp B, et al. Sphingosine prevents binding of SARS-CoV-2 spike to its cellular receptor ACE2. J Biol Chem. 2020;295(45):15174–15182. doi:10.1074/jbc.RA120.015249
- Basile MS, Cavalli E, McCubrey J, et al. The PI3K/Akt/mTOR pathway: A potential pharmacological target in COVID-19. Drug Discov Today. 2021;27(3):848–856. doi:10.1016/j.drudis.2021.11.002
- Mizutani T, Fukushi S, Saijo M, Kurane I, Morikawa S. Importance of Akt signaling pathway for apoptosis in SARS-CoV-infected Vero E6 cells. Virology. 2004;327(2):169–174. doi:10.1016/j.virol.2004.07.005
- Mizutani T, Fukushi S, Saijo M, Kurane I, Morikawa S. JNK and PI3k/Akt signaling pathways are required for establishing persistent SARS-CoV infection in Vero E6 cells. Biochim Biophys Acta. 2005;1741(1–2):4–10. doi:10.1016/j.bbadis.2005.04.004
- Appelberg S, Gupta S, Svensson Akusjarvi S, et al. Dysregulation in Akt/mTOR/HIF-1 signaling identified by proteo-transcriptomics of SARS-CoV-2 infected cells. Emerg. Microbes Infect. 2020;9(1):1748–1760. doi:10.1080/22221751.2020.1799723
- Callahan V, Hawks S, Crawford MA, et al. The Pro-Inflammatory Chemokines CXCL9, CXCL10 and CXCL11 Are Upregulated Following SARS-CoV-2 Infection in an AKT-Dependent Manner. Viruses. 2021;13(6):1062. doi:10.3390/v13061062
- Sun F, Mu C, Kwok HF, et al. Capivasertib restricts SARS-CoV-2 cellular entry: a potential clinical application for COVID-19. Int J Biol Sci. 2021;17(9):2348–2355. doi:10.7150/ijbs.57810
- Gassen NC, Papies J, Bajaj T, et al. SARS-CoV-2-mediated dysregulation of metabolism and autophagy uncovers host-targeting antivirals. Nat Commun. 2021;12(1):3818. doi:10.1038/s41467-021-24007-w
- Somanath PR. Is targeting Akt a viable option to treat advanced-stage COVID-19 patients? Am J Physiol Lung Cell Mol Physiol. 2020;319(1):L45–L47. doi:10.1152/ajplung.00124.2020
- Bhat VK, Bernhart E, Plastira I, et al. Pharmacological Inhibition of Serine Palmitoyl Transferase and Sphingosine Kinase-1/-2 Inhibits Merkel Cell Carcinoma Cell Proliferation. J Invest Dermatol. 2019;139(4):807–817. doi:10.1016/j.jid.2018.10.024
- Grbcic P, Eichmann TO, Kraljevic Pavelic S, Sedic M. The Sphingosine Kinase 2 Inhibitor ABC294640 Restores the Sensitivity of BRAFV600E Mutant Colon Cancer Cells to Vemurafenib by Reducing AKT-Mediated Expression of Nucleophosmin and Translationally-Controlled Tumour Protein. Int J Mol Sci. 2021;22(19):19. doi:10.3390/ijms221910767
- Zhou J, Chen J, Yu H. Targeting sphingosine kinase 2 by ABC294640 inhibits human skin squamous cell carcinoma cell growth. Biochem Biophys Res Commun. 2018;497(2):535–542. doi:10.1016/j.bbrc.2018.02.075
- Zhu X, Shi D, Cao K, et al. Sphingosine kinase 2 cooperating with Fyn promotes kidney fibroblast activation and fibrosis via STAT3 and AKT. Biochim Biophys Acta Mol Basis Dis. 2018;1864(11):3824–3836. doi:10.1016/j.bbadis.2018.09.007
- White MD, Chan L, Antoon JW, Beckman BS. Targeting ovarian cancer and chemoresistance through selective inhibition of sphingosine kinase-2 with ABC294640. Anticancer Res. 2013;33(9):3573–3579.
- Oaks JJ, Santhanam R, Walker CJ, et al. Antagonistic activities of the immunomodulator and PP2A-activating drug FTY720 (Fingolimod, Gilenya) in Jak2-driven hematologic malignancies. Blood. 2013;122(11):1923–1934. doi:10.1182/blood-2013-03-492181
- Mukhopadhyay A, Saddoughi SA, Song P, et al. Direct interaction between the inhibitor 2 and ceramide via sphingolipid-protein binding is involved in the regulation of protein phosphatase 2A activity and signaling. FASEB J. 2009;23(3):751–763. doi:10.1096/fj.08-120550
- Oaks J, Ogretmen B. Regulation of PP2A by Sphingolipid Metabolism and Signaling. Front Oncol. 2014;4:388. doi:10.3389/fonc.2014.00388
- Saddoughi SA, Gencer S, Peterson YK, et al. Sphingosine analogue drug FTY720 targets I2PP2A/SET and mediates lung tumour suppression via activation of PP2A-RIPK1-dependent necroptosis. EMBO Mol Med. 2013;5(1):105–121. doi:10.1002/emmm.201201283
- Don AS, Rosen H. A lipid binding domain in sphingosine kinase 2. Biochem Biophys Res Commun. 2009;380(1):87–92. doi:10.1016/j.bbrc.2009.01.075
- Dinesh Kumar N, Smit JM, Reggiori F. Strategies employed by viruses to manipulate autophagy. Prog Mol Biol Transl Sci. 2020;172:203–237.
- Mao J, Lin E, He L, Yu J, Tan P, Zhou Y. Autophagy and Viral Infection. Adv Exp Med Biol. 2019;1209:55–78.
- Suares A, Medina MV, Coso O. Autophagy in Viral Development and Progression of Cancer. Front Oncol. 2021;11:603224. doi:10.3389/fonc.2021.603224
- Zhao Z, Lu K, Mao B, et al. The interplay between emerging human coronavirus infections and autophagy. Emerg. Microbes Infect. 2021;10(1):196–205. doi:10.1080/22221751.2021.1872353
- Bello-Perez M, Sola I, Novoa B, Klionsky DJ, Falco A. Canonical and Noncanonical Autophagy as Potential Targets for COVID-19. Cells. 2020;9(7):1619. doi:10.3390/cells9071619
- Fecchi K, Anticoli S, Peruzzu D, et al. Coronavirus Interplay With Lipid Rafts and Autophagy Unveils Promising Therapeutic Targets. Front Microbiol. 2020;11:1821. doi:10.3389/fmicb.2020.01821
- Pereira G, Leao A, Erustes AG, et al. Pharmacological Modulators of Autophagy as a Potential Strategy for the Treatment of COVID-19. Int J Mol Sci. 2021;22(8):4067.
- Prestes EB, Bruno JCP, Travassos LH, Carneiro LAM. The Unfolded Protein Response and Autophagy on the Crossroads of Coronaviruses Infections. Front Cell Infect Microbiol. 2021;11:668034. doi:10.3389/fcimb.2021.668034
- Sargazi S, Sheervalilou R, Rokni M, Shirvaliloo M, Shahraki O, Rezaei N. The role of autophagy in controlling SARS-CoV-2 infection: An overview on virophagy-mediated molecular drug targets. Cell Biol Int. 2021;45(8):1599–1612. doi:10.1002/cbin.11609
- Vidoni C, Fuzimoto A, Ferraresi A, Isidoro C. Targeting autophagy with natural products to prevent SARS-CoV-2 infection. J Tradit Complement Med. 2022;12(1):55–68. doi:10.1016/j.jtcme.2021.10.003
- Yang N, Shen HM. Targeting the Endocytic Pathway and Autophagy Process as a Novel Therapeutic Strategy in COVID-19. Int J Biol Sci. 2020;16(10):1724–1731. doi:10.7150/ijbs.45498
- Gassen NC, Papies J, Bajaj T, et al. Analysis of SARS-CoV-2-controlled autophagy reveals spermidine, MK-2206, and niclosamide as putative antiviral therapeutics. bioRxiv. 2020. doi:10.1101/2020.04.15.997254
- Casasampere M, Ordonez YF, Casas J, Fabrias G. Dihydroceramide desaturase inhibitors induce autophagy via dihydroceramide-dependent and independent mechanisms. Biochim Biophys Acta Gen Subj. 2017;1861(2):264–275. doi:10.1016/j.bbagen.2016.11.033
- Gagliostro V, Casas J, Caretti A, et al. Dihydroceramide delays cell cycle G1/S transition via activation of ER stress and induction of autophagy. Int J Biochem Cell Biol. 2012;44(12):2135–2143. doi:10.1016/j.biocel.2012.08.025
- Hernandez-Tiedra S, Fabrias G, Davila D, et al. Dihydroceramide accumulation mediates cytotoxic autophagy of cancer cells via autolysosome destabilization. Autophagy. 2016;12(11):2213–2229. doi:10.1080/15548627.2016.1213927
- Lee AY, Lee JW, Kim JE, et al. Dihydroceramide is a key metabolite that regulates autophagy and promotes fibrosis in hepatic steatosis model. Biochem Biophys Res Commun. 2017;494(3–4):460–469. doi:10.1016/j.bbrc.2017.10.110
- Munoz-Guardiola P, Casas J, Megias-Roda E, et al. The anti-cancer drug ABTL0812 induces ER stress-mediated cytotoxic autophagy by increasing dihydroceramide levels in cancer cells. Autophagy. 2021;17(6):1349–1366. doi:10.1080/15548627.2020.1761651
- Signorelli P, Munoz-Olaya JM, Gagliostro V, Casas J, Ghidoni R, Fabrias G. Dihydroceramide intracellular increase in response to resveratrol treatment mediates autophagy in gastric cancer cells. Cancer Lett. 2009;282(2):238–243. doi:10.1016/j.canlet.2009.03.020
- Wu CY, Jhang JG, Lin WS, et al. Dihydroceramide desaturase promotes the formation of intraluminal vesicles and inhibits autophagy to increase exosome production. iScience. 2021;24(12):103437. doi:10.1016/j.isci.2021.103437
- McNaughton M, Pitman M, Pitson SM, Pyne NJ, Pyne S. Proteasomal degradation of sphingosine kinase 1 and inhibition of dihydroceramide desaturase by the sphingosine kinase inhibitors, SKi or ABC294640, induces growth arrest in androgen-independent LNCaP-AI prostate cancer cells. Oncotarget. 2016;7(13):16663–16675. doi:10.18632/oncotarget.7693
- Shin SH, Kim HY, Yoon HS, et al. A Novel Selective Sphingosine Kinase 2 Inhibitor, HWG-35D, Ameliorates the Severity of Imiquimod-Induced Psoriasis Model by Blocking Th17 Differentiation of Naive CD4 T Lymphocytes. Int J Mol Sci. 2020;21(21):21. doi:10.3390/ijms21218371
- Ivanisenko NV, Seyrek K, Kolchanov NA, Ivanisenko VA, Lavrik IN. The role of death domain proteins in host response upon SARS-CoV-2 infection: modulation of programmed cell death and translational applications. Cell Death Discov. 2020;6(1):101. doi:10.1038/s41420-020-00331-w
- Zhong Y, Liao Y, Fang S, Tam JP, Liu DX. Up-regulation of Mcl-1 and Bak by coronavirus infection of human, avian and animal cells modulates apoptosis and viral replication. PLoS One. 2012;7(1):e30191. doi:10.1371/journal.pone.0030191
- Maceyka M, Sankala H, Hait NC, et al. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J Biol Chem. 2005;280(44):37118–37129. doi:10.1074/jbc.M502207200
- Yang J, Yang C, Zhang S, et al. ABC294640, a sphingosine kinase 2 inhibitor, enhances the antitumor effects of TRAIL in non-small cell lung cancer. Cancer Biol Ther. 2015;16(8):1194–1204. doi:10.1080/15384047.2015.1056944
- Liu Q, Rehman H, Shi Y, et al. Inhibition of sphingosine kinase-2 suppresses inflammation and attenuates graft injury after liver transplantation in rats. PLoS One. 2012;7(7):e41834. doi:10.1371/journal.pone.0041834
- Shi W, Zhang S, Ma D, et al. Targeting SphK2 Reverses Acquired Resistance of Regorafenib in Hepatocellular Carcinoma. Front Oncol. 2020;10:694. doi:10.3389/fonc.2020.00694
- Shi Y, Rehman H, Ramshesh VK, et al. Sphingosine kinase-2 inhibition improves mitochondrial function and survival after hepatic ischemia-reperfusion. J Hepatol. 2012;56(1):137–145. doi:10.1016/j.jhep.2011.05.025
- LeBlanc FR, Pearson JM, Tan SF, et al. Sphingosine kinase-2 is overexpressed in large granular lymphocyte leukaemia and promotes survival through Mcl-1. Br J Haematol. 2020;190(3):405–417. doi:10.1111/bjh.16530
- Sundaramoorthy P, Gasparetto C, Kang Y. The combination of a sphingosine kinase 2 inhibitor (ABC294640) and a Bcl-2 inhibitor (ABT-199) displays synergistic anti-myeloma effects in myeloma cells without a t(11;14) translocation. Cancer Med. 2018;7(7):3257–3268. doi:10.1002/cam4.1543
- Hasanifard L, Samadi N, Rashtchizadeh N, Dastmalchi S, Karimi P. Sphingosine kinase-2 Inhibitor ABC294640 Enhances Doxorubicin-Induced Apoptosis of NSCLC Cells via Altering Survivin Expression. Drug Res. 2018;68(1):45–53. doi:10.1055/s-0043-117181
- Johannes L, Billet A. Glycosylation and raft endocytosis in cancer. Cancer Metastasis Rev. 2020;39(2):375–396. doi:10.1007/s10555-020-09880-z
- Johannes L, Wunder C, Shafaq-Zadah M. Glycolipids and Lectins in Endocytic Uptake Processes. J Mol Biol. 2016. doi:10.1016/j.jmb.2016.10.027
- Pontier SM, Schweisguth F. Glycosphingolipids in signaling and development: from liposomes to model organisms. Dev Dyn. 2012;241(1):92–106. doi:10.1002/dvdy.22766
- Schneider-Schaulies J, Schneider-Schaulies S. Viral infections and sphingolipids. Handb Exp Pharmacol. 2013;216:321–340.
- Tanner LB, Chng C, Guan XL, Lei Z, Rozen SG, Wenk MR. Lipidomics identifies a requirement for peroxisomal function during influenza virus replication. J Lipid Res. 2014;55(7):1357–1365. doi:10.1194/jlr.M049148
- Achdout H, Manaster I, Mandelboim O. Influenza virus infection augments NK cell inhibition through reorganization of major histocompatibility complex class I proteins. J Virol. 2008;82(16):8030–8037. doi:10.1128/JVI.00870-08
- Drews K, Calgi MP, Harrison WC, et al. Glucosylceramidase Maintains Influenza Virus Infection by Regulating Endocytosis. J Virol. 2019;93(12):12. doi:10.1128/JVI.00017-19
- Hidari KI, Suzuki Y, Suzuki T. Suppression of the biosynthesis of cellular sphingolipids results in the inhibition of the maturation of influenza virus particles in MDCK cells. Biol Pharm Bull. 2006;29(8):1575–1579. doi:10.1248/bpb.29.1575
- Drews K, Calgi MP, Harrison WC, et al. Glucosylceramide synthase maintains influenza virus entry and infection. PLoS One. 2020;15(2):e0228735. doi:10.1371/journal.pone.0228735
- Drake MJ, Brennan B, Briley K Jr., et al. A role for glycolipid biosynthesis in severe fever with thrombocytopenia syndrome virus entry. PLoS Pathog. 2017;13(4):e1006316. doi:10.1371/journal.ppat.1006316
- Vitner EB, Avraham R, Politi B, Melamed S, Israely T. Elevation in sphingolipid upon SARS-CoV-2 infection: possible implications for COVID-19 pathology. Life Sci Alliance. 2022;5(1):e202101168. doi:10.26508/lsa.202101168
- Vitner EB, Achdout H, Avraham R, et al. Glucosylceramide synthase inhibitors prevent replication of SARS-CoV-2 and influenza virus. J Biol Chem. 2021;296:100470. doi:10.1016/j.jbc.2021.100470
- Oyewole OO, Reid SP. Sphingosine kinase 2 associates with the nsP3 of chikungunya virus and is required for replication. bioRxiv. 2020;2020. doi:10.1101/2020.09.10.291682
- Mariano G, Farthing RJ, Lale-Farjat SLM, Bergeron JRC. Structural Characterization of SARS-CoV-2: Where We Are, and Where We Need to Be. Front Mol Biosci. 2020;7:605236. doi:10.3389/fmolb.2020.605236
- Khan SA, Goliwas KF, Deshane JS. Sphingolipids in Lung Pathology in the Coronavirus Disease Era: A Review of Sphingolipid Involvement in the Pathogenesis of Lung Damage. Front Physiol. 2021;12:760638. doi:10.3389/fphys.2021.760638
- Fu P, Ramchandran R, Sudhadevi T, et al. NOX4 Mediates Pseudomonas aeruginosa-Induced Nuclear Reactive Oxygen Species Generation and Chromatin Remodeling in Lung Epithelium. Antioxidants. 2021;10(3):477. doi:10.3390/antiox10030477
- Ebenezer DL, Fu P, Suryadevara V, Zhao Y, Natarajan V. Epigenetic regulation of pro-inflammatory cytokine secretion by sphingosine 1-phosphate (S1P) in acute lung injury: Role of S1P lyase. Adv Biol Regul. 2017;63:156–166. doi:10.1016/j.jbior.2016.09.007
- Ebenezer DL, Fu P, Krishnan Y, et al. Genetic deletion of Sphk2 confers protection against Pseudomonas aeruginosa mediated differential expression of genes related to virulent infection and inflammation in mouse lung. BMC Genom. 2019;20(1):984. doi:10.1186/s12864-019-6367-9
- Ebenezer DL, Berdyshev EV, Bronova IA, et al. Pseudomonas aeruginosa stimulates nuclear sphingosine-1-phosphate generation and epigenetic regulation of lung inflammatory injury. Thorax. 2019;74(6):579–591. doi:10.1136/thoraxjnl-2018-212378
- Dupre TV, Siskind LJ. The role of sphingolipids in acute kidney injury. Adv Biol Regul. 2018;70:31–39. doi:10.1016/j.jbior.2018.11.003
- Zhang X, Ritter JK, Li N. Sphingosine-1-phosphate pathway in renal fibrosis. Am J Physiol Renal Physiol. 2018;315(4):F752–F756. doi:10.1152/ajprenal.00596.2017
- Park SW, Kim M, Kim M, D’Agati VD, Lee HT. Sphingosine kinase 1 protects against renal ischemia-reperfusion injury in mice by sphingosine-1-phosphate1 receptor activation. Kidney Int. 2011;80(12):1315–1327. doi:10.1038/ki.2011.281
- Bajwa A, Huang L, Kurmaeva E, et al. Sphingosine Kinase 2 Deficiency Attenuates Kidney Fibrosis via IFN-gamma. J Am Soc Nephrol. 2017;28(4):1145–1161. doi:10.1681/ASN.2016030306
- Yatomi Y, Ruan F, Hakomori S, Igarashi Y. Sphingosine-1-phosphate: a platelet-activating sphingolipid released from agonist-stimulated human platelets. Blood. 1995;86(1):193–202. doi:10.1182/blood.V86.1.193.bloodjournal861193
- Urtz N, Gaertner F, von Bruehl ML, et al. Sphingosine 1-Phosphate Produced by Sphingosine Kinase 2 Intrinsically Controls Platelet Aggregation In Vitro and In Vivo. Circ Res. 2015;117(4):376–387. doi:10.1161/CIRCRESAHA.115.306901
- Wang J, Pendurthi UR, Yi G, Rao LVM. SARS-CoV-2 infection induces the activation of tissue factor-mediated coagulation via activation of acid sphingomyelinase. Blood. 2021;138(4):344–349. doi:10.1182/blood.2021010685
- Horby P, Lim WS; Group RC. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384(8):693–704.
- Snider AJ, Ruiz P, Obeid LM, Oates JC. Inhibition of sphingosine kinase-2 in a murine model of lupus nephritis. PLoS One. 2013;8(1):e53521. doi:10.1371/journal.pone.0053521
- Shin SH, Cho KA, Hahn S, et al. Inhibiting Sphingosine Kinase 2 Derived-sphingosine-1-phosphate Ameliorates Psoriasis-like Skin Disease via Blocking Th17 Differentiation of Naive CD4 T Lymphocytes in Mice. Acta Derm Venereol. 2019;99(6):594–601. doi:10.2340/00015555-3160
