Abstract
Non-alcoholic fatty liver disease (NAFLD), a spectrum of liver disorders from non-alcoholic fatty liver (NAFL) to the more severe non-alcoholic steatohepatitis (NASH), is the leading etiology of chronic liver disease and its global prevalence is increasing. Hepatic steatosis, a condition marked by an abnormal buildup of triglycerides in the liver, is the precursor to NAFLD. Differentiated cluster 36 (CD36), a scavenger receptor class B protein, is a membrane receptor that recognizes multiple lipid and non-lipid ligands. It is generally agreed that CD36 contributes significantly to hepatic steatosis by taking part in fatty acid uptake as well as triglyceride storage and secretion. While there has not been any conclusive research on how CD36 inhibitors prevent NAFLD from progressing and no clinically approved CD36 inhibitors are currently available for use in NAFLD, CD36 remains a target worthy of further investigation in NAFLD. In recent years, the potential role of natural products acting through CD36 in treating non-alcoholic fatty liver disease has attracted much attention. This paper offers an overview of the pathogenesis of CD36 in NAFLD and summarizes some of the natural compounds or extracts that are currently being investigated for modulating NAFLD via CD36 or the CD36 pathway, providing an alternative approach to the development of CD36-related drugs in NAFLD.
Keywords:
Introduction
NAFLD is at the forefront of liver disease with a global prevalence of 25%.Citation1 NAFLD makes a huge impact on the quality of people’s lives and has also exacted a growing economic burden.Citation2–4 There are numerous unresolved issues, and there are no recognized treatments for NAFLD although continuous progress in understanding the pathogenesis of the disorder, finding therapeutic targets, and advancing drug development.Citation5 Prevention and treatment remain increasing challenges.
NAFLD is associated with obesity, insulin resistance, type 2 diabetes, hypertension, hyperlipidemia, and metabolic syndrome.Citation6 The pathogenesis of NAFLD is a complicated process. The traditional “two-hit” mechanism sees sedentary lifestyles, high-fat diets, and insulin resistance leading to a build-up of hepatic lipid accumulation, which can make the liver sensitive and further stigmatized as a “second hit” that triggers inflammation and fibrosis.Citation7 However, the pathogenesis and progression of NAFLD are multifactorial and complex, it cannot be summarized in this simple way, and this fact has given rise to “multiple-hit”, which are thought to include a combination of insulin resistance, lipotoxicity, nutritional factors, gut microbiota, and genetics acting in parallel to trigger the NAFLD development.Citation8,Citation9
Differentiated cluster 36 (CD36), a multi-ligand receptor, is named “SR-B2”.Citation10 A variety of physiological processes are mediated by this membrane glycoprotein, which is found in hepatocytes, platelets, adipocytes, mononuclear phagocytes, myocytes, and some epithelial cells. Roles of CD36 in lipid accumulation, inflammatory signaling, energy reprogramming, and oxidative stress have been demonstrated.Citation11,Citation12 Recent research has also revealed that CD36 is a common transcription target for multiple ligand-sensing and lipogenic transcription factors, including the aryl hydrocarbon receptor,Citation13 and several nuclear hormone receptors, including progesterone X receptor (PXR), liver X receptor (LXR), and peroxisome proliferator-activated receptor γ (PPARγ).Citation14 Although CD36 expression is not high in liver cells under physiological conditions, it is highly induced when lipid overload or nuclear cell activation occurs.Citation15,Citation16 CD36 is a high-affinity receptor for long chain fatty acids (LCFAs), suggesting a potential role of CD36 in lipid metabolism. CD36 is associated with cellular uptake of free fatty acids and drives hepatic steatosis in the liver, possibly leading to the development of NASH.Citation17,Citation18 Several clinical studies have shown that Levels of CD36 are higher in patients with nonalcoholic fatty liver disease than in normal subjects.Citation19,Citation20 There are currently no FDA-approved CD36-related medications, but there have been numerous reports of natural substances treating NAFLD that work on CD36 or the CD36 pathway.Citation21,Citation22 CD36 and its transcriptional regulators are expected to be identified as new therapeutic targets for the treatment and prevention of NAFLD in the future.
The Role of CD36 in the Occurrence and Progression of NAFLD
Fatty Acid Oxidative Impairmen
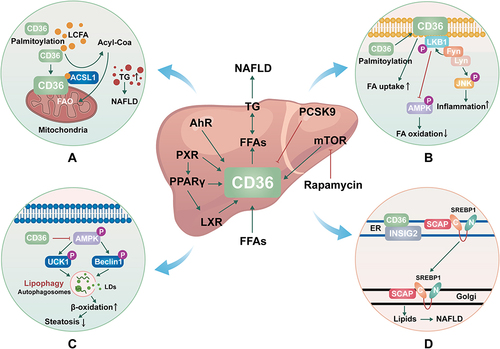
As we all know, CD36 is the main participant in metabolic tissue, primarily involved in the uptake of long-chain fatty acids (LCFAs).Citation23 CD36 is subject to various types of posttranslational modifications in a way that depends on the tissue, condition, and time-dependent manner, and one of the most common lipid modifications of CD36 is palmitoylation.Citation24 Mitochondrial fatty acid oxidative (FAO) impairment in hepatocytes leads to lipid accumulation, overproduction of mitochondrial reactive oxygen species (ROS), and oxidative damage, which further leads to NAFLD.Citation25 A recent study found that CD36 was present in hepatocyte mitochondria and that hindering CD36 palmitoylation greatly increases the distribution of CD36 to hepatocyte mitochondria. Long-chain acyl-CoA synthetase (ACSL) is a key enzyme that converts LCFAs into long-chain acyl-CoA and thus oxidizes them in mitochondria. When the localization of CD36 is increased, more ACSL1 and CD36 in the mitochondria interact to boost the transfer of additional LCFAs to ACSL1, which in turn promotes FAO and lessens NAFLD by increasing the production of long-chain acyl-CoA ().Citation26 It may be an effective method for the treatment of NAFLD to inhibit CD36 palmitoylation thereby reducing the localization of CD36 in mitochondria.
Figure 1 The mechanisms of CD36 in NAFLD. (A). Molecular mechanisms by which CD36 palmitoylation promotes FAO and NAFLD. (B). Molecular mechanisms by which CD36 palmitoylation promotes β-oxidation and inflammation. (C). Molecular mechanisms by which CD36 inhibits autophagy. (D). Molecular mechanisms by which CD36 promotes De novo lipogenesis.
β-Oxidation and Inflammation
The function of CD36 largely depends on its position in the plasma membrane, and post-translational modification of palmitoylation can increase the modified protein’s lipophilicity, controlling the distribution and function of CD36 within the cell. The expression of CD36 enhanced as the liver progressed from normal to simple steatosis (SS) and then to NASH. When compared to SS and liver cirrhosis, the dispersion of CD36 on the cell membrane of NASH patients’ liver cells increased significantly.Citation19 This suggests that the enhanced location of CD36 on hepatocyte plasma membranes may be a crucial aspect of NASH development.Citation27 One study showed that the increase of palmitoylation of CD36 promoted the transfer of CD36 to the plasma membrane of liver cells. The formation of the CD36/Lyn/Fyn complex is aided by palmitoylated CD36, which also aids FA absorption, and initiates c-JUN N-terminal kinase (JNK) which causes inflammation in adipose tissues, promotes Fyn-mediated LKB1 phosphorylation, and inhibits LKB1-mediated AMPK activation, and impair FAβ-oxidation. Inhibiting palmitoylation of CD36 may enhance phosphorylation of AMP-activated protein kinase (AMPK) in the way of Fyn-LKB1 manner, improve FAβ-oxidation and prevent steatosis. Furthermore, JNK signaling pathway inactivation is a crucial factor in CD36-mediated inflammation. The inhibition of palmitoylation of CD36 can block JNK signaling pathway inactivation, which reduces inflammation in the liver tissue ().Citation28 These results imply that in the future, targeting the palmitoylation site of CD36 may represent a novel research avenue for the treatment and prevention of NAFLD.
Lipotoxicity
Lipotoxicity refers to the harmful effects of high concentrations of lipids and lipid derivatives on cells.Citation29 Lipotoxicity occurs when lipid metabolism is misregulated. Evidence shows that hepatic injury is one of the key events in the pathophysiology of NAFLD.Citation30 When the high levels of peripheral free fatty acids or the new fat synthesis of the liver increases, the liver’s ability to utilize, store and export free fatty acids is covered, which is the cause of lipotoxicity.Citation31 The mechanisms of lipotoxicity involve several cellular processes, such as endoplasmic reticulum stress, mitochondrial dysfunction, and lysosomal permeability, which ultimately lead to triggering apoptosis.Citation32,Citation33 The increase in intake and utilization of free fatty acids is significantly influenced by CD36. Excessive circulating free fatty acids can cause cytotoxicity and apoptosis. As mentioned earlier, palm acylation of CD36 promotes the intake of fatty acids and increases the accumulation of lipids in the liver. The relationship between palm acylation of CD36 lipid toxicity needs further study.Citation26 Gaemers IC et alCitation34 established a mouse model of NAFLD by high-fat liquid diet (HFLD) overfeeding which liver steatosis developed and NASH developed. In the mouse model, adipocytes experienced metabolic changes, which resulted in a decrease in lipid storage capacity and an increase in lipid outflow, which led to lipotoxicity in peripheral organs. And CD36 was found to be induced in the livers of mice overfed with HFLD.Citation35 In a word, it is very important to understand the molecular mechanism of CD36 for inhibiting or alleviating lipotoxicity and adverse consequences during nonalcoholic fatty liver disease.
Autophagy
In addition to playing a role in fatty acid uptake, CD36 has also been shown to affect lipid autophagy, which is the defense mechanism of hepatocytes against NAFLD.Citation36 This is a selective mass degradation system, which swallows solute and regulates the hepatic metabolic pathway. Lipid autophagy is a form of selective autophagy, which cuts off some lipid droplets and fuses them with lysosomes, promoting lipid decomposition, and then producing energy and ketones through β-oxidative catabolism.Citation37 It has been shown that CD36 knockdown induces autophagy in hepatocytes due to increased autophagosome formation in autophagic flow, while CD36 overexpression inhibits autophagy.Citation38 AMP-activated protein kinase (AMPK) directly phosphorylates autophagy activating kinase 1 (ULK1) and Beclin1 is critical for autophagy.Citation39,Citation40 In CD36 gene knockout hepatocytes, lipid phagocytosis increased through the AMPK-ULK1/Beclin pathway, which contributed to the increase of β-oxidation and the alleviation of steatosis. CD36 acts as a negative regulator of autophagy, which is responsible for lipid accumulation in liver cells and mice. Inhibition of CD36 can enhance fatty acid clearance by inducing autophagy, which has become a new therapeutic strategy for NAFLD and other metabolic diseases ().Citation41
De novo Lipogenesis
De novo lipogenesis (DNL) or de novo fatty acid (FA) synthesis is a metabolic pathway that syntheses fatty acids from excess carbohydrate.Citation42 Studies have shown that enhanced DNL in hepatocytes is a major cause of NAFLD.Citation43 Overexpression of CD36 is related to the exacerbation of steatosis, and its mechanism involves the increase of FFA uptake and TG storage in the hepatic. There is growing evidence that CD36 goes far beyond FFA transport.Citation41,Citation44 In a recent study, CD36 was found to have a novel role in the resynthesis of fat that goes beyond the known FFA transporter function. CD36 promotes liver lipid homeostasis by regulating Sterol regulatory element-binding protein 1 (SREBP1) processing. CD36 is coupled with insulin-induced gene-2 (INSIG2) to eliminate the interaction between INSIG2 and the SREBP cleavage-activating protein (SCAP)-SREBP complex, thereby leading to the translocation of SREBP1 from ER to Golgi for processing, leading to lipogenesis.Citation45,Citation46 Thus, CD36-mediated DNL in hepatocytes is a key driver of NAFLD development and provides an intervention strategy for the treatment of hepatic steatosis ().
Regulation of CD36 Targets in Lipid Metabolism
PCSK9
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is expressed mainly in the liver and is thought to induce the degradation of low-density lipoprotein receptor (LDLR) in the liver, thus leading to an increase in the levels of circulating LDLC.Citation47,Citation48 Recent studies have shown that PCSK9 induces CD36 degradation via intracellular or extracellular pathways, reducing CD36 levels on the cell surface, and thereby affecting the metabolism of long-chain fatty acids and triglycerides in the livers of the lysosome adipocytes and mice. PCSK9 interacts directly with CD36. Functionally, the internalization of ligands like oxidized LDL in liver cells and analogs of palmitic acid in adipocytes was considerably diminished by the lower CD36 levels. In contrast, by inhibiting PCSK9 expression with small interfering RNA, CD36 and LDLR protein levels in HepG2 hepatocytes were significantly increased. In vivo, recombinant PCSK9 was injected into wild-type C57BL/6 mice, causing the degradation of CD36 in the liver. Conversely, loss of PCSK9 resulted in a significant rise in CD36 protein expression in the liver and other tissues, which is linked to an increase in fatty acid absorption and triglyceride levels in the liver. PCSK9 induces the degradation of CD36 in the acidic compartment of the posterior endoplasmic reticulum through a proteasome sensitive mechanism. In tissues with significant lipid flow, including the visceral adipose tissue and liver, PCSK9-mediated control of CD36 levels may restrict fatty acid uptake and triglyceride accumulation and offer additional mechanistic support for the function of PCSK9 in triglyceride metabolism.Citation49 Similarly, Lebeau PF et alCitation50 found that PCSK9 prevents the uptake and accumulation of CD36-mediated FA in cultured hepatocytes. It was observed that the Increase of CD36 expression significantly led to the increase of intracellular lipid levels in liver cell lines with reduced PCSK9 expression.
Although there is a large body of data suggesting that PCSK9 affects circulating cholesterol more than it does circulate and peripheral triglyceride levels.Citation51,Citation52 However, PCSK9 can reduce CD36 levels and prevent the accumulation of long-chain fatty acids and triglycerides, thus preventing endoplasmic reticulum stress, fibrosis, and liver injury, which is still worthy of our attention and further research.
PPAR/RXR/LXR
Through engaging the lipogenic transcription factor sterol regulatory element-binding protein (SREBP-1c) and its target gene, the liver X receptor (LXR) is considered to induce adipogenesis.Citation53–55 There is increasing evidence that LXR may have additional transcription targets. It may promote steatosis by regulating CD36 directly or indirectly through peroxisome proliferator-activated receptor (PPAR)γ, this activation is liver-specific.Citation56,Citation57 Similarly, it has been demonstrated that the Pregnane X receptor (PXR) controls CD36 either directly or indirectly through PPARγ, and PXR activation of CD36 is also liver-specific.Citation58 Along with LXR and PXR, the activation of PPARγ may also encourage CD36 activation and steatosis. Recent studies have shown that LXR, PXR, and PPARγ synergistically contribute to promoting CD36 expression, which in turn promotes hepatic steatosis. In addition, Hajri T et alCitation59 showed that palmitic acid (PA) stimulation of liver cells reduced PPARγ promoter-specific DNA methylation, strongly induced CD36 and, very low-density lipoprotein (VLDL) expression, and increased liver fat storage, which further led to hepatic steatosis. To assess the efficacy of an intervention for adipogenic therapy, CD36, a common target for adipogenic nuclear receptors, maybe a trustworthy liver indicator.
AhR
The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor belonging to the basic helix-loop-helix (bHLH)/Per-Arnt-Sim (PAS) family of proteins.Citation60 AhR and its ligands have been found to have a philanthropic effect on AhR gene expression and lipid metabolism. Lee JH et alCitation61 reported a CA-AhR transgenic mouse by eliminating the minimum ligand-binding domain of AhR (amino acid 287–422). CA-AhR transgenic mice showed spontaneously hepatic steatosis in contrast to wild mice. In addition, the steatosis effect of AhR agonists was eliminated in CD36 knockout mice, suggesting that AhR activation promotes steatosis through activation of CD36 expression. Yao L et alCitation62 found that hyperhomocysteine (HHcy) induced hepatic steatosis and increased hepatic expression of CD36, which was associated with the activation of AhR. In another study, Kawano Y et alCitation63 found that the AhR agonist 3-methylcholanthrene (3MC) enhanced CD36 expression in mouse and human hepatocellular carcinoma cells HepG2 and induced hepatic steatosis. In addition, the study by Yuan P and others found that compared with low-dose TCDF (a kind of have to human body health of toxic organic pollutants 2,3,7,8-tetrachlorodibenzofuran) treatment in mice, high dose treatment showed the ratio of liver to body weight of mice increased significantly, as well as a significant rise in the liver triglyceride levels, TCDF exposure activates AhR and its target genes Cyp1a1 and CD36.Citation64 Jin J et alCitation65 found a qualitative increase in liver steatosis in Ahre/e mice (a type of systemic AhR excised mouse). CD36 was increased by PCB126 (activators of AhR) exposure and Ahre/e genotype. Liver steatosis and injury were increased in Ahre/e mice. This may be partly due to increased lipid uptake of liver CD36 receptors. These studies may be helpful to establish AhR and its target CD36 as new targets for the therapy and averting the development of NAFLD.
mTOR
The mammalian target of rapamycin (mTOR) has been proved to play a role in the activation of inflammatory stress and metabolic syndrome.Citation66 Rapamycin, a specific inhibitor of mTOR in mammalian cells, has been used as an immunosuppressant to prevent transplant rejection. Research has indicated that the lipid metabolism enzymes SREBP1c, SREBP2, FASn, acetyl-coa carboxylase (ACC), stearoyl-coa desaturase-1 (SCD1), and LDLR can be inhibited by rapamycin, which also reduces hepatic steatosis.Citation67–70 Rapamycin has been shown in studies to have a pleiotropic anti-lipid deposition impact in hepatic steatosis.Citation71 Rapamycin significantly reduced lipid accumulation in the liver of HepG2 hepatocytes treated with palmitic acid or C57BL/6J mice fed with HFD, which indicated that rapamycin had a protective effect on reducing hepatic steatosis.Citation72 Although CD36 mRNA expression was not affected by rapamycin, CD36 protein expression was. Rapamycin altered the expression of the CD36 protein in the liver at the post-transcriptional level as opposed to the transcriptional level because CD36 is essential for the rapamycin-mediated remission of induced hepatic steatosis. Rapamycin inhibits the mTOR signaling pathway’s partial inflammatory stress-induced increased phosphorylation and reduced CD36 translational efficiency, which lowers the quantity of CD36 protein. mTOR signaling pathway mediates inflammatory stress and enhance the expression of CD36 protein. In addition, rapamycin can significantly reduce the intake of FFA in vitro and in vivo, reduce the lipid accumulation in the liver, overcomes the influence of inflammatory stress, and provide a protective effect in reducing liver steatosis.Citation73 Inflammation significantly increases FFA uptake, while rapamycin reduced FFA uptake, which is one of the mechanisms of fat accumulation in cells in addition to the fat formation, expressed by CD36. Rapamycin reduces CD36 translation efficiency and mTOR signaling, which may represent a novel molecular mechanism of hepatic steatosis and adds to the data supporting the use of mTOR inhibitors to treat NAFLD in patients with metabolic syndrome.
In addition, hepatic hypoxia-inducible factor 2α (HIF-2α) was identified as a major regulator of hepatic lipid metabolism through the upregulation of CD36.Citation74 Likewise, krueppel-like factor 9 (KLF9) can regulate hepatic lipid metabolism and development of NAFLD by promoting the expression of CD36,Citation75 sex determining region Y-box 2 (SOX2) was proved to play a role in FFA-induced lipid accumulation in hepatocytes by up-regulating CD36 expression.Citation76 Other studies have found that some microRNAs (miRNAs) potentially target CD36 and influence liver lipid accumulation by modulating the receptor. Among them, miR-29a was found to improve hepatocellular steatosis and liver fibrosis by targeting its 3’-untranslated region (UTR) to inhibit CD36 expression.Citation77 Similarly, MiR-20a-5p inhibits CD36 expression by binding to CD36’s 3 ‘UTR.Citation78 Another study found that a protective role of miR-100 in HFD induced metabolic syndrome and liver steatosis, partially mediated by the direct repression of CD36 and attenuation of hepatic lipid storage.Citation79
Natural Products That Affect the Occurrence and Development of NAFLD by Acting on CD36
Berberine (BBR) is an isoquinoline alkaloid derived from Rhizoma Coptidis. Data from extensive research have conclusively shown that BBR can enhance insulin sensitivity and improve glucose metabolism.Citation80 Yu M et alCitation81 found that BBR significantly reversed the HFD-fed mice liver CD36 protein level elevation. Besides, the mice intestinal protein levels of CD36 elevation caused by HFD-fed were also reversed by the treatment of BBR. BBR relieves hepatic steatosis by inhibiting the protein levels of hepatic and intestinal CD36 fatty acid uptake. It is worth noting, Choi YJ et alCitation82 found that BBR induces phosphorylation of AMPK in HepG2 cells and mouse primary hepatocytes, leading to the activation of extracellular signal-regulated kinases 1/2 (ERK1/2) and CCAAT/enhancer-binding protein β (C/EBPβ), resulting in increased CD36 expression. Similarly, prolonged activation of AMPK by BBR in HepG2 cells and mice increased CD36 expression and fatty acid uptake, which may lead to hepatocellular lipid accumulation and fatty liver. This difference may be ascribed to the use of different dosages of berberine in the two studies, which may be one of the keys to determining the toxicity of berberine. Further research is needed to clarify the distinction between the beneficial and harmful effects of berberine ().
Table 1 Natural products which treat NAFLD through CD36
Curcumin, a polyphenol derived from turmeric roots, has been shown in several studies to reduce inflammation, atherosclerosis, and obesity.Citation83–85 In recent years, curcumin has been shown to affect lipid metabolism in mouse models of NAFLD. Liu Y et alCitation86 found that curcumin significantly reversed the liver relative mRNA level of CD36 in mice fed with a high-fat and high-fructose diet (HFHFr)-diet, and reduced fatty acid uptake.
Oxidative stress is considered a key component in NASH pathogenesis.Citation87,Citation88 An increase in oxygen consumption in the liver has been linked to oxidative stress and activation of hypoxia-inducible factor (HIF) leading to CD36 overexpression.Citation89 β-patchoulene (β-PAE) is one of the active natural tricyclic sesquiterpene isolated from the essential oil of patchouli. It has exhibited unexpected anti-inflammatory and antioxidative effects.Citation90 Wu J et alCitation91 found that β-PAE decreased body weight gain, liver index, and epididymal adipocyte weight in NASH rats, and decreased serum CD36 content. In the liver of NASH rats, β-PAE treatment resulted in reduced expression of the CD36 proteins. In addition, when CD36 was highly expressed, AMPK activation in NASH rats is significantly inhibited, and β-PAE therapy reverses this trend. It was suggested that β-PAE might reduce hypoxia in liver tissue and improve hepatic lipid metabolism balance through CD36/AMPK signaling pathway.
Naringin, a naturally occurring flavonoid compound, extracted from traditional Chinese medicine (TCM), is believed to promote anti-inflammatory, anti-oxidative, and prevention of fibrosis in the liver.Citation92,Citation93 The latest research suggests that naringin can adjust NAFLD caused by a high fat diet in mice of the intestinal flora and metabolic function to give play the role of lipid-lowering. Zhang X et alCitation94 studies show that naringin reduced the accumulation of lipid droplets within the liver cells, decreased intracellular triglyceride levels and inhibited alanine transaminase (ALT) elevation. CD36 mRNA and protein levels in tissue-engineered fatty (TEF) livers were downregulated by Naringin treatment. Additionally, by molecular docking, naringin was proved to bind directly to the CD36 domain and inhibits FFA uptake.
Syzygium simile is a kind of the Myrtaceae family, distributed in Taiwan and the Philippines and other regions. Yen CH et alCitation95 by utilizing an image-based high-through put screening found that the extract of Syzygium simile leaves (SSLE) can reduce, including liver, bowel and macrophages of several cell types in the accumulation of lipid droplets. Further research proves that SSLE suppresses the mRNA and protein expression in vitro in a dose-dependent manner, blocking fatty acid uptake and subsequently preventing fatty acid uptake by liver cells. In addition, the lipid accumulation and CD36 expression in macrophages and intestinal epithelial cell lines were also inhibited after SSLE treatment.
Siphonaxanthin (SPX) is a marine carotenoid abundant in green algae, which has been reported to have antiangio-genesis, anti-degranulation, and anti-obesity effects. Zheng J et alCitation96 showed that SPX significantly inhibited the accumulation of TAG in HepG2 hepatocytes in a concentration-dependent manner. CD36 mRNA levels are significantly downregulated by SPX.
Hung WL et alCitation97 Dolichos lablab water extract (DLL-Ex) can protect liver in an in vitro model of NAFLD. THE study found that DLL-Ex inhibited lipid accumulation induced by free fatty acids in HepG2 hepatocytes and reduced FFA uptake in HepG2 hepatocytes. The expression levels of CD36 were significantly and dose-dependently decreased when HepG2 cells are treated with the DLL-Ex (100 and 250 lg/mL).
Cinnamic acid (CA), a kind of natural polyphenols, contains nine carbon atoms, was reported to have antibacterial, anti-inflammatory, antioxidant, and the effect of anti-diabetes.Citation98–100 Research has shown that cinnamon acid on a high-fat diet induced animal models of obesity and lipid levels, Adisakwattana et alCitation101 found that cinnamic acid decreased liver cancer cell accumulation of lipid droplets, after cinnamic acid treatment, the mRNA expression of CD36 in obese rats were significantly down-regulated, and fatty acid uptake in hepatocytes was inhibited.
Alisol B is a natural compound derived from the plant Alisma orientalis (Sam). Zhao Z et alCitation102 discovered that Alisol B reduced hepatic steatosis, inflammation, and fibrosis in NASH mice induced by a high-fat diet plus carbon tetrachloride (DIO+CCl4) and choline-deficient and amino acid-defined (CDA) diet. Alisol B dramatically de-creased CD36 expression and controlled retinol metabolism in DIO+CCl4 and CDA-diet-induced NASH mice. Similarly, Alisol B inhibited the mRNA and protein levels of CD36 in a dose-dependent manner in mouse primary hepatocytes. Alisol B Inhibited Oxidative Stress and Inflammation in Primary Hepatocytes in a CD36-Dependent Manner. Further research has found that Alisol B has previously unknown therapeutic benefits against NASH via a unique mechanism by modulating the RAR-PPAR-CD36 cascade, indicating Alisol B as a prospective lead chemical for the treatment of NASH.
Quercetin, the special subclass of flavonoid, has anti-inflammatory and anti-oxidant actions. Liu L et alCitation103 found that quercetin inhibited the mRNA and protein expression of CD36 in HFD-fed mice, thereby decreasing excessive deposition of hepatic oxidized low-density lipoprotein (ox-LDL), and alleviating long-term HFD-induced liver damage.
Gypenosides (GP) extracted from Gynostemma pentaphyllum Makino have a significant role in reducing serum lipid levels and treating fatty liver diseases. Huang XGP et alCitation104 showed that administration of GP alleviates steatohepatitis and reversed the upregulated CD36 levels in mice fed an HFHC (high-fat and high-cholesterol) diet.
Raw Bowl Tea polyphenols (RBTP) contain numerous polyphenolic compounds, including Gallic acid, (-)-epigallocatechin, catechin, L-epicatechin, (-)-epigallocatechin gallate, (-)-gallocatechin gallate, and (-)-epicatechin gallate (ECG), and high levels of caffeine, (-)-epigallocatechin (EGC), and ECG. Liu B showed et alCitation105 showed that RBTP could effectively down-regulated the expression of CD36 located in the brush border membrane of small intestine villus cells of NAFLD mice, and prevented NAFLD by regulating intestinal function.
Lee W et alCitation106 showed that the aqueous extract of the aerial part of Angelica tenuissima Nakai (ATX) inhibited oleic acid–induced neutral lipid accumulation in HepG2 cells, and decreased the mRNA and protein levels of CD36.
Piceatannol, a natural stilbene, has an effect on cancer prevention, neuro-protection, and anti-diabetic. Yang J et alCitation107 found that piceatannol significantly reduced fat accumulation and inhibited fatty acid uptake by reducing CD36 mRNA expression in fat-induced HepG2 cells.
Magnolol (MG) is a bioactive polyphenolic compound isolated from the Magnolia officinalis that exert the accumulation of oxidative stress and plays a significant role via a variety of mechanisms. Kuo NC et alCitation108 found that MG reversed the elevated mRNA levels of CD36 in NAFLD Rats induced by Tyloxapol (a nonionic surfactant that blocks plasma lipolytic functions) treatment. In addition, MG inhibited fatty acid uptake by reducing CD36 mRNA expression in PA-induced steatotic HepG2 cells.
Pfohl M et alCitation109 showed that pomegranate fruit extract (PE) alleviated diet-induced fatty liver and suppressed the mRNA levels of CD36 in HFD-fed mice.
Gong X et alCitation110 showed that the Cordycepin (CRD), a nucleotide analogue derived from traditional Chinese medicine cordyceps, suppresses the mRNA and protein expression of CD36 on HFD-induced obesity with NAFLD mice and reduces hepatic lipid accumulation.
Liriope platyphylla is traditional medicine, which suggested a protective role of LPE in anti-obesity and anti-inflammatory effects.Citation111 Le TNH et alCitation112 showed that the treatment with Liriope platyphylla root ethanolic extract (LPE) reduced the protein expression of CD36 both in vivo and in vitro, leading to reduced fatty acid uptake.
Swertiamarin is a kind of secoiridoid glycoside, which is the active ingredient of Swertia chinensis. Yang et alCitation113 found that the mRNA level of CD36 is markedly decreased in mice with sweroside treatment, which may ameliorate obesity with fatty liver via the regulation of lipid metabolism and inflammatory responses.
Gao Y et alCitation114 found that Ginsenoside Rg1 (G-Rg1) inhibits FFA-induced lipid uptake to reduce hepatic steatosis of HepG2 cells by downregulating the mRNA and protein expression of CD36.
Quan T et alCitation115 found that Ficus hirta Vahl. (FV) suppressed the expression and activity of CD36 in HepG2 cell lines induced by palmitate (PA) and mouse model fed with a high-fat diet (HFD), and FV treatment can reverse the exacerbated effects of CD36 on lipid metabolism and inflammation.
Betaine is widely present in plants and microorganisms exerting beneficial effects on the organism. Li Y et alCitation116 found that betaine reduced both mRNA and protein levels of CD36. Betaine attenuates hepatic steatosis of HFD-fed mice via targeting the PPARγ/CD36 pathway in the liver.
Abelmoschus esculentus (AE) fruit is a traditional functional food that has a significant effect on possessing hypoglycemic and anti-oxidative qualities.Citation117 A study by Peng CH et alCitation118 found that subfractions F2 (having large amounts of carbohydrates and polysaccharides) isolated from AE extract demonstrate a superior effect to down-regulate the lipid uptake. F2 downregulates OAPA (oleic acid and palmitic acid with the ratio of 2:1)-induced elevation of the CD36 mRNA and protein expression in HepG2 cells. The study also found that F2 has better effects at 5 μg/mL doses.
Dillenia indica L. is a medicinal plant from the Dilleniaceae family, traditionally used to treat jaundice, dysentery, and other diseases.Citation119 The latest research by Poornima MS et alCitation120 found that the hydroethanolic extract of Dillenia indica leaf (DI-HET) can significantly lower intracellular lipid accumulation in OA-treated HepG2 cells. After DI-HET treatment, the level of CD36 protein decreased and its activity decreased.
Salvianolic acid B (SalB) is a polyphenolic compound isolated from the root of Salvia miltiorrhiza, which has many pharmacological effects, such as protecting vascular endothelium, anti-fibrosis, anti-liver injury, etc.Citation121 Meng LC et alCitation122 found that SalB might prevent NAFLD by inhibiting the accumulation of lipids. SalB decreased the mRNA levels of CD36 and reduced lipid accumulation in the liver of ob/ob mice. SalB decreased the mRNA levels of CD36 and reduce lipid accumulation in the liver of ob/ob mice.
Puerarin, one of the main isoflavonoid components of the root of Pueraria lobata, has anti-inflammation, antioxidant, and insulin resistance-reducing effects, and is commonly used for the treatment of liver damage, allergic diseases, and neuronal protective.Citation123 Zhou J et alCitation124 found that puerarin ameliorated the levels of lipids in the serum and liver. Puerarin reversed the high-fat and high-fructose diet (HFFD) resulting in increased expressions of CD36 in rats and reduced the rate of fatty acid uptake by the liver.
Gallic acid (GA), a natural polyphenol, has a wide range of pharmacological effects on anti-obesity, anti-inflammation, and anti-cancer activities.Citation125 A recent study by Tanaka M al.Citation126 found that GA inhibits lipid accumulation, capable of preventing NASH progression. GA effectively suppressed the mRNA and protein expression of CD36 in HepG2 cells and prevent hepatic steatosis.
Theobromine is a methylxanthine that occurs in a variety of plants such as Theobroma cacao, Cola aluminate, Paullinia cupana, and Ilex aquifolium.Citation127 Theobromine has a variety of pharmacological activities and therapeutic effects, including anti-inflammatory, antioxidant stress, and antimicrobial activity.Citation128 A recent study showed that theobromine regulated lipid metabolism in hepatocytes in vivo and in vitro. Theobromine reversed HFD-diet-induced elevation of CD36 mRNA and protein expression in mice. Similar results were obtained in experiments in vitro. Theobromine also reduced the mRNA and protein expression CD36 in AML-12 cells.Citation129
Conclusions and Prospects
Nonalcoholic fatty liver disease (NAFLD) is now a leading cause of liver disease worldwide and a worldwide health problem.Citation1–3 Although the pathogenesis and therapeutic targets of NAFLD have been further studied and some progress has been made in drug development, there are still some problems to be solved, for example, no approved medical treatment exists so far.Citation130 CD36 is a membrane glycoprotein widely found in platelets, monocytes, adipocytes and skeletal muscle, etc.Citation131 It plays an important role in lipid accumulation, inflammatory signaling, energy reprogramming, and oxidative stress.Citation11,Citation12 The mechanism of CD36 action is partially elucidated. However, the exact roles of CD36 in NAFLD remain to be determined. Natural compounds derived from plants are an important resource for drug development, and targeting CD36 may be a potential therapy for non-alcoholic fatty liver disease. Some natural compounds have been found to alleviate NAFLD by targeting CD36 or through the CD36 pathway, presenting a promising therapeutic prospect, Cinnamic acid,Citation101 Alisol B,Citation102 Magnolol,Citation108 etc., are potential CD36 inhibitors. Compared with the standard drug, natural compounds do not need synthetic, readily available.Citation132 Although the role of these natural compounds in vivo or in vitro was widely studied, clinical application research is still less, and future research can be aimed at improving pharmacokinetic and pharmacodynamic properties of these drugs, make it a useful drug for the treatment and reverse NAFLD.
By summarizing current experimental evidence, we believe that natural products that inhibit CD36 have potential clinical effects in preventing NAFLD. It cannot be ignored that some but not all studies are limited to in vitro studies, such as Siphonaxanthin,Citation96 Piceatannol,Citation107 Ginsenoside Rg1,Citation114 etc. The effectiveness of natural inhibitors of CD36 in treating NAFLD should also be addressed by using a more reliable and appropriate in vivo model of NAFLD. Besides, considering separating the single compound from extracts is difficult, our study includes not only single compounds but extracts, such as Dolichos lablab water extract,Citation97 the aqueous extract of the aerial part of Angelica tenuissima Nakai,Citation106 pomegranate fruit extract,Citation109 etc. The drug activity of these extracts is usually attributed to the synergistic and simultaneous action of multiple compounds.Citation133,Citation134 It will be interesting in future research to focus on this synergy.
Nevertheless, the safety of natural inhibitors must also be carefully evaluated to ensure their safety. For example, berberine can increase CD36 expression and fatty acid uptake when it exceeds its optimal dosage, which means that the possible safety, dose-limiting toxicity, and maximum tolerated dose determination of these compounds also need further evaluation.Citation82 In addition, it will also be very interesting to research the synergistic effects of synthetic compounds with natural products for NAFLD treatment. Natural inhibitors of CD36 mentioned in this review are not drugs in themselves, but they provided new ideas for developing new NAFLD drugs.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
We wish to express our sincere thanks to Zipeng Wang for his guidance in making the mechanism diagram. This paper was supported by the National Natural Science Foundation of China (No. 81573945), the Science and Technology Development Project of Shandong Province (No. 2013GSF11902), the National Prestigious Chinese Medicine Doctor Studio of Xinlu Wang Project ([2016]47), and the Science and Technology Development Project of Traditional Chinese Medicine in Shandong Province (Nos. 2013-081 and 2019-0093).
References
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, And outcomes. Hepatology. 2016;64(1):73–84. doi:10.1002/hep.28431
- Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in The United States and Europe. Hepatology. 2016;64(5):1577–1586. doi:10.1002/hep.28785
- Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, Predictions, Risk Factors and Prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. doi:10.1038/nrgastro.2017.109
- Paik JM, Golabi P, Younossi Y, Mishra A, Younossi ZM. Changes in the Global Burden of Chronic Liver Diseases From 2012 to 2017: growing Impact of NAFLD. Hepatology. 2020;72(5):1605–1616. doi:10.1002/hep.31173
- Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24(7):908–922. doi:10.1038/s41591-018-0104-9
- Loomba R, Sanyal AJ. The Global NAFLD Epidemic. Nat Rev Gastroenterol Hepatol. 2013;10(11):686–690. doi:10.1038/nrgastro.2013.171
- Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114(4):842–845. doi:10.1016/s0016-5085(98)70599-2
- Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of Non-alcoholic fatty liver disease (NAFLD). Metabolism: Clinical and Experimental. 2016;65(8):1038–1048. doi:10.1016/j.metabol.2015.12.012
- Gentilini A, Caligiuri A, Marra F. Molecular Pathogenesis of NASH. Int J Mol Sci. 2016;17(9):1575. doi:10.3390/IJMS17091575
- Prabhudas M, Bowdish D, Drickamer K, et al. Standardization of Scavenger receptor nomenclature. J Immunol. 2014;192(5):1997–2006. doi:10.4049/jimmunol.1490003
- Pepino MY, Kuda O, Samovski D. Structure- Function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annu Rev Nutr. 2014;34:1–303. doi:10.1146/annurev-nutr-071812-161220
- Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis. Sci Signal. 2009;2(72):re3. doi:10.1126/scisignal.272re3
- He J, Lee JH, Febbraio M, Xie W. The emerging roles of fatty acid translocase/CD36 and the aryl hydrocarbon receptor in fatty liver disease. Biol Med. 2011;236(10):1116–1121. doi:10.1258/ebm.2011.011128
- Zhou J, Febbraio M, Wada T, et al. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR. Gastroenterology. 2008;134(2):556–567. doi:10.1053/j.gastro.2007.11.037
- Garcia-monzon C, Lo Iacono O, Crespo J. Increased soluble CD36 is linked to advanced steatosis in nonalcoholic fatty liver disease. Eur J Clin Invest. 2014;44(1):65–73. doi:10.1111/eci.12192
- Greco D, Kotronen A, Westerbacka J, et al. Gene expression in human NAFLD. Am J Physiol Gastrointest Liver Physiol. 2008;2(5):294 G1281–G1287. doi:10.1152/ajpgi.00074.2008
- Febbraio M, Abumrad NA, Hajjar DP. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem. 1999;274(27):19055–19062. doi:10.1074/JBC274.27.19055
- Karunakaran U, Elumalai S, Moon JS, Won KC. CD36 Signal Transduction in Metabolic Diseases: novel Insights and Therapeutic Targeting. Cells. 2021;10(7):1833. doi:10.3390/cells10071833
- Miquilena-Colina ME, Lima-Cabello E, Sanchez-Campos S, et al. Hepatic fatty acid translocase CD36 upregulation is associated with insulin resistance, Hyperinsulinaemia and increased Steatosis in non-alcoholic steatohepatitis and chronic hepatitis C. Gut. 2011;60(10):1394–1402. doi:10.1136/gut.2010.222844
- Heebøll S, Poulsen MK, Ornstrup MJ, et al. Circulating sCD36 levels in patients with non-alcoholic fatty liver disease and controls. Int J Obes. 2017;41(2):262–267. doi:10.1038/ijo.2016.223
- Sumida Y, Yoneda M. Current and future pharmacological therapies for NAFLD/NASH. J Gastroenterol. 2018;53(3):362–376. doi:10.1007/s00535-017-1415-1
- Mundi MS, Velapati S, Patel J, Kellogg TA, Abu Dayyeh BK, Hurt RT. Evolution of NAFLD and Its Management. Nutr Clin Pract. 2020;35(1):72–84. doi:10.1002/ncp.10449
- Glatz JF, Luiken JJ. From fat to FAT (CD36/SR-B2): understanding the regulation of cellular fatty acid uptake. Biochimie. 2017;136:21–26. doi:10.1016/j.biochi.2016.12.007
- Luiken JJ, Chanda D, Nabben M, Neumann D, Glatz JF. Post-translational modifications of CD36 (SR-B2): expression of glycoprotein expression in the expression of anthill fatty acid uptake. Biochim Biophys Acta. 2016;1862(12):2253–2258. doi:10.1016/j.bbadis.2016.09.004
- Tao N, Wagner SJ, Lublin DM. CD36 is palmitoylated on both N-and C-terminal cytoplasmic tails. J Biol Chem. 1996;271(37):22315–22320. doi:10.1074/JBC271.37.22315
- Zeng S, Wu F, Chen M. Inhibition of fatty acid translocase (FAT/CD36) palmitoylation enhances hepatic fatty acid β-oxidation increasing its localization to mitochondria and interaction with long-chain acyl-CoA synthetase 1. Antioxidants & Redox Signaling. 2022;36(16–18):1081–1100. doi:10.1089/ars.2021.0157
- Pohl J, Ring A, Korkmaz U, Ehehalt R, Stremmel W. FAT/CD36-mediated long-chain fatty acid uptake in adipocytes requires plasma membrane. Mol Biol Cell. 2005;16(1):24–31. doi:10.1091/MBC.E04-07-0616
- Zhao L, Zhang C, Luo X, et al. CD36 palmitoylation disrupts free fatty acid metabolism and promotes tissue inflammation in non-alcoholic Steatohepatitis. J Hepatol. 2018;69(3):705–717. doi:10.1016/j.jhep.2018.04.006
- Mota M, Banini BA, Cazanave SC, Sanyal AJ. Molecular mechanisms of Lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism: Clinical and Experimental. 2016;65(8):1049–1061. doi:10.1016/j.metabol.2016.02.014
- Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184(10):2537–2564. doi:10.1016/j.cell.2021.04.015
- Marra F, Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol. 2018;68(2):280–295. doi:10.1016/j.jhep.2017.11.014
- Ekstedt M, Hagstrom H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of Follow - up. Hepatology. 2015;61(5):1547–1554. doi:10.1002/hep.27368
- Geng Y, Faber KN, de Meijer VE, Blokzijl H, Moshage H. How does hepatic lipid accumulation lead to lipotoxicity in non-alcoholic fatty liver disease? J Hepatol Int. 2021;15(1):21–35. doi:10.1007/s12072-020-10121-2
- Gaemers IC, Stallen JM, Kunne C, et al. Lipotoxicity and steatohepatitis in an overfed mouse model for non-alcoholic fatty liver disease. Biochim Biophys Acta. 2011;1812(4):447–458. doi:10.1016/j.bbadis.2011.01.003
- Singh R, Kaushik S, Wang Y, et al. Autophagy regulates lipid metabolism. Nature. 2009;458(7242):1131–1135. doi:10.1038/nature07976
- Ueno T, Komatsu M. Autophagy in liver: functions in health and disease. Nat Rev Gastroenterol Hepatol. 2017;14(3):170–184. doi:10.1038/nrgastro.2016.185
- Qian H, Chao X, Williams J, et al. Autophagy in liver diseases: a review. Mol Aspects Med. 2021;82:100973. doi:10.1016/j.mam.2021.100973
- Schulze RJ, Krueger EW, Weller SG, et al. Direct Lysosome-based autophagy of lipid caustic in hepatocytes. Proc Natl Acad Sci U S A. 2020;117(51):32443–32452. doi:10.1073/pnas.2011442117
- Laker RC, Drake JC, Wilson RJ, et al. Ampk phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat Commun. 2017;8(1):548. doi:10.1038/s41467-017-00520-9
- Kim J, Kim YC, Fang C, et al. Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell. 2013;152(1–2):290–303. doi:10.1016/j.cell.2012.12.016
- Li Y, Yang P, Zhao L, et al. CD36 plays a negative role in the regulation of lipophagy in the hepatocyte through an AMPK-dependent pathway. J Lipid Res. 2019;60(4):844–855. doi:10.1194/JLRM090969
- Ameer F, Scandiuzzi L, Hasnain S, Kalbacher H, Zaidi N. De Novo lipogenesis in Health and Disease. Metabolism: Clinical and Experimental. 2014;63(7):895–902. doi:10.1016/j.metabol.2014.04.003
- Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver Diseases. Gastroenterology. 2014;146(3):726–735. doi:10.1053/j.g.astro.2013.11.049
- Yang P, Zeng H, Tan W, et al. Loss of CD36 impairs hepatic insulin signaling by enhancing the interaction of PTP1B with IR. FASEB J. 2020;34(4):5658–5672. doi:10.1096/fj.201902777RR
- Xu D, Wang Z, Xia Y, et al. The gluconeogenic enzyme PCK1 phosphorylates INSIG1/2 for lipogenesis. Nature. 2020;580(7804):530–535. doi:10.1038/s41586-020-2183-2
- Zeng H, Qin H, Liao M, et al. CD36 Promotes de Novo Lipogenesis in the hepatocyte through Insig2-dependent SREBP1 Processing. Mol Metab. 2022;57. doi:10.1016/j.molmet.2021.101428
- Horton JD, Cohen JC, Hobbs HH. Molecular biology of PCSK9: its Role in LDL metabolism. Trends Biochem Sci. 2007;32(2):71–77. doi:10.1016/j.t.bs2006.12.008
- Lambert G. Unravelling the Functional Significance of PCSK9. Curr Opin Lipidol. 2007;18(3):304–309. doi:10.1097/MOL.0b013e3281338531
- Demers A, Samami S, Lauzier B, et al. PCSK9 Induces CD36 Degradation and Affects Long-Chain Fatty Acid Uptake and Triglyceride Metabolism in Adipocytes. Arterioscler Thromb Vasc Biol. 2015;35(12):2517–2525. doi:10.1161/ATVBAHA.115.306032
- Lebeau PF, Byun JH, Platko K, et al. Pcsk9 Knockout Counterfeiting of diet-induced non-alcoholic steatohepatitis, Fibrosis and liver injury in mice. JHEP Rep. 2019;1(6):418–429. doi:10.1016/j.jhepr.2019.10.009
- Palumbo M, Giammanco A, Purrello F, et al. Effects of PCSK9 inhibitors on HDL cholesterol efflux and serum cholesterol loading capacity in familial hypercholesterolemia subjects: a multi-lipid-center real-world evaluation. Front Mol Biosci. 2022;9:925587. doi:10.3389/fmolb.2022.925587
- Seidah NG, Garçon D. Expanding Biology of PCSK9: roles in Atherosclerosis and Beyond. Curr Atheroscler Rep. 2022;24(1):1–10. doi:10.1007/s11883-022-01057-z
- Repa JJ, Liang G, Ou J, et al. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and lxrbeta. Genes Dev. 2000;14(22):2819–2830. doi:10.1101/gad.844900
- Łtowski J. Liver X Receptors (LXRs). Part I: structure, function, regulation of activity and role in lipid metabolism. Postepy Hig Med Dosw. 2007;61:736–759.
- Wang B, Tontonoz P. Liver X receptors in lipid signalling and envelope homeostasis. Nat Rev Endocrinol. 2018;14(8):452–463. doi:10.1038/s41574-018-0037-x
- Lee JH, Xie W. PXR and LXR in hepatic steatosis: a new dog and an old dog with new tricks. Mol Pharm. 2008;5(1):60–66. doi:10.1021/mp700121u
- Zhou J, Febbraio M, Wada T, et al. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR. Gastroenterology. 2008;134(2):556. doi:10.1053/j.gastro.2007.11.037
- Zhou J, Zhai Y, Mu Y, et al. A novel pregnane X receptor-mediated and sterol regulatory element-binding protein-independent lipogenic Pathway. J Biol Chem. 2006;281(21):15013–15020. doi:10.1074/JBCM511116200
- Hajri T, Zaiou M, Fungwe TV, Ouguerram K, Besong S. Epigenetic Regulation of Peroxisome Proliferator-Activated Receptor Gamma Mediates High-Fat Diet-Induced Non-alcoholic Fatty Liver Disease. Cells. 2021;10(6):548. doi:10.3390/cells10061355
- Hoffman EC, Reyes H, Chu FF. Cloning of a factor required for activity of the Ah (Dioxin) receptor. Science. 1991;252(5008):954–958. doi:10.1126/science.1852076
- Lee JH, Wada T, Febbraio M. A novel role of dioxin receptor in fatty acid metabolism and hepatic steatosis. Gastroenterology. 2010;139(2):653–663. doi:10.1053/j.gastro.2010.03.033
- Yao L, Wang C, Zhang X, et al. Hyperhomocysteinemia activates the aryl hydrocarbon receptor/CD36 pathway to promote hepatic steatosis in Mice. Hepatology. 2016;64(1):92–105. doi:10.1002/hep.28518
- Kawano Y, Nishiumi S, Tanaka S, et al. Activation of the aryl hydrocarbon receptor induces hepatic steatosis via the upregulation of fatty acid. Arch Biochem Biophys. 2010;504(2):221–227. doi:10.1016/j.abb2010.09.001
- Yuan P, Dong M, Lei H, et al. Targeted metabolomics reveals that 2,3,7,8-tetrachlorodibenzofuran exposure induces hepatic steatosis in male Mice. Environ Pollut. 2020;259:113820. doi:10.1016/j.nvpol.2019.113820
- Jin J, Wahlang B, Thapa M, et al. Proteomics and metabolic phenotyping define principal roles for the aryl hydrocarbon receptor in mouse Liver. Acta Pharm Sin B. 2021;11(12):3806–3819. doi:10.1016/j.aPSB2021.10.014
- Suto T, Karonitsch T. The immunobiology of mTOR in autoimmunity. J Autoimmun. 2020;110:102373. doi:10.1016/j.jaut.2019.102373
- Takashima M, Ogawa W, Emi A, Kasuga M. Regulation of SREBP1c expression by mTOR signaling in the hepatocyte. Kobe J Med Sci. 2009;55(2):E45–E52.
- Duvel K, Yecies JL, Menon S, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39(2):171–183. doi:10.1016/j.molcel.2010.06.022
- Zhang Y, Ma KL, Ruan XZ, Liu BC. Dysregulation of the Low-Density Lipoprotein Receptor Pathway Is Involved in Lipid Disorder-Mediated Organ Injury. Int J Biol Sci. 2016;12(5):569–579. doi:10.7150/ijbs.14027
- Tan SH, Shui G, Zhou J, et al. Critical role of SCD1 in autophagy regulation via lipogenesis and lipid rafts-coupled AKT-FOXO1 signaling Pathway. Autophagy. 2014;10(2):226–242. doi:10.4161/auto.27003
- Han J, Wang Y. mTORC1 signaling in hepatic lipid metabolism. Protein Cell. 2018;9(2):145–151. doi:10.1007/s13238-017-0409-3
- Wang C, Yan Y, Hu L, et al. Rapamycin-mediated CD36 translational suppression contributes to Worsting of hepatic steatosis. Biochem Biophys Res Commun. 2014;447(1):57–63. doi:10.1016/j.bBRC.2014.03.103
- Wang C, Hu L, Zhao L, et al. Inflammatory stress increases hepatic CD36 translational efficiency via activation of the mTOR signalling Pathway. PLoS One. 2014;9(7):e103071. doi:10.1371/journalpone.0103071
- Barroso E, Rodriguez-Rodriguez R, Zarei M, et al. SIRT3 deficiency ates fatty liver by attenuating the HIF1α -lipin 1 pathway and increasing CD36 through Nrf2. Cell Commun Signal. 2020;18(1). doi:10.1186/s12964-020-00640-8
- Zhou SS, Zhang YL, Chang YS. Sheng Li Xue Bao. Sheng Li Xue Bao: [Acta Physiologica Sinica]. 2021;73(5):772–780.
- Shen C, Chen JH, Oh HR, Park JH. Transcription factor SOX2 contributes to nonalcoholic fatty liver disease development by regulating the Expression of the fatty acid transporter CD36. FEBS Lett. 2021;595(19):2493–2503. doi:10.1002/1873-3468.14193
- Lin HY, Wang FS, Yang YL, Huang YH. MicroRNA-29a Suppresses CD36 to Ameliorate High Fat Diet-Induced Steatohepatitis and Liver Fibrosis in Mice. Cells. 2019;8(10):1298. doi:10.3390/cells8101298
- Wang X, Ma Y, Yang LY, Zhao D. 20A-5P Ameliorates non-alcoholic Fatty Liver Disease via Inhibiting the Expression of CD36. Front Cell Dev Biol. 2020;8:59 6329. doi:10.3389/fcell.2020.596329
- Smolka C, Schlosser D, Hohnloser C, et al. Mir-100 overexpression attenuates high fat diet induced weight gain, Liver steatosis, hypertriglyceridemia and development of metabolic syndrome in mice. Mol Med. 2021;27(1):101. doi:10.1186/S10020-021-00364-6
- Wang K, Feng X, Chai L, Cao S, Qiu F. The metabolism of berberine and its contribution to the pharmacological effects. Drug Metab Rev. 2017;49(2):139–157. doi:10.1080/03602532.2017.1306544
- Yu M, Alimujiang M, Hu L, Liu F, Bao Y, Yin J. Berberine alleviates lipid metabolism disorders via inhibition of mitochondrial complex I in gut and liver. Int J Biol Sci. 2021;17(7):1693–1707. doi:10.7150/iJbs.54604
- Choi YJ, Lee KY, Jung SH, et al. Activation of AMPK by berberine induces hepatic lipid accumulation by upregulation of fatty acid translocase CD36 In mice. Toxicology and Applied Pharmacology. 2017;316:74–82. doi:10.1016/j.t.aap2016.12.019
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of Curcumin: problems and Promises. Mol Pharm. 2007;4(6):807–818. doi:10.1021/mp700113r
- Nelson KM, Dahlin JL, Bisson J, Graham J, Pauli GF, Walters MA. The Essential Medicinal Chemistry of Curcumin. J Med Chem. 2017;1(5):1620–1637. doi:10.1021/acs.Jmedchem.6b00975
- Tsuda T. Curcumin as a functional food-derived factor: metabolites, bioactivity, and Future Perspectives. Food Funct. 2018;9(2):705–714. doi:10.1039/c7fo01242j
- Yan C, Zhang Y, Zhang X, Aa J, Wang G, Xie Y. Curcumin regulates endogenous and Exogenous metabolism via NRF2-FXR-LXR Pathway in NAFLD mice. Biomed Pharmacother. 2018;105:274–281. doi:10.1016/j.biopha.2018.05.135
- Takaki A, Kawai D, Yamamoto K. Multiple hits, including oxidative stress, Pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH). Int J Mol Sci. 2013;14(10):20704–20728. doi:10.3390/ijms141020704
- Ortiz-masia D, Diez I, Calatayud S, et al. Induction of CD36 and thrombospondin-1 in macrophages by hypoxia-inducible factor 1 and its relevance. PLoS One. 2012;7(10):e48535. doi:10.1371/journalpone.0048535
- Liu Y, Wu J, Chen L, et al. β-patchoulene simultaneously ameliorated dextran sulfate sodius-induced colitis and secondary liver injury in rats Mice via suppressing colonic odors and flora imbalance. Biochem Pharmacol. 2020;182:114260.
- Wu J, Gan Y, Luo H, et al. β-Patchoulene Ameliorates Water Transport and the Mucus Barrier in 5-Fluorouracil-Induced Intestinal Mucositis Rats via the cAMP/PKA/CREB Signaling Pathway. Front Pharmacol. 2021;12:689491. doi:10.3389/fphar.2021.689491
- Luo H, Xu N, Wu J, et al. β-patchoulene protects against non-alcoholic steatohepatitis via interrupting the vicious circle among oxidative. Int Immunopharmacol. 2021;98:107915. doi:10.1016/j.intimp.2021.107915
- Shirani K, Yousefsani BS, Shirani M, Karimi G. Protective effects of naringin against drugs and chemical toxins induced hepatotoxicity: a Review. Phytother Res. 2020;34(8):1734–1744. doi:10.1002/PTR.6641
- Akamo AJ, Rotimi SO, Akinloye DI, et al. Naringin prevents cyclophosphamide-induced hepatotoxicity in rats by attenuating oxidative stress, fibrosis, And Inflammation. Food Chem Toxicol. 2021;153:112266. doi:10.1016/j.fct.2021.112266
- Zhang X, Zhang Y, Gao W, et al. Naringin improves lipid metabolism in a tissue-engineered liver model of NAFLD and the underlying Mechanisms. Life Sci. 2021;277:119487. doi:10.1016/j.lfs.2021.119487
- Yen CH, Chang HS, Yang TH, et al. High-content Screening of a Taiwanese Indigenous Plant Extract Library Identifies Syzygium Simile leaf Extract as. Int J Mol Sci. 2018. doi:10.3390/IJMS19072130
- Zheng J, Li Z, Manabe Y, et al. Siphonaxanthin, a Carotenoid from Green Algae. Lipids. 2018;53(1):41–52. doi:10.1002/lipd.12002
- Im AR, Kim YH, Lee HW, Song KH. Water Extract of Dolichos lablab Attenuates Hepatic Lipid Accumulation in a Cellular Nonalcoholic Fatty Liver Disease Model. J Med Food. 2016;19(5):495–503. doi:10.1089/JMF.2015.3623
- Adisakwattana S. Cinnamic Acid and Its Derivatives: mechanisms for Prevention and Management of Diabetes and Its Complications. Nutrients. 2017;9(2):163. doi:10.3390/NU9020163
- Wang Y, Sun Y, Wang J, Zhou M, Wang M. Fungal Activity and Action Mechanism of Natural Product gingic Acid Against Sclerotinia l Sclerotiorum. Plant the Dis. 2019;103(5):944–950. doi:10.1094/PDIS-08-18-1355-RE
- Ruwizhi N. Cinnamic Acid Derivatives and Their Biological Efficacy. Int J Mol Sci. 2020;1:548. doi:10.3390/IJMS21165712
- Wu Y, Wang M, Yang T, et al. Cinnamic Acid Ameliorates Nonalcoholic Fatty Liver Disease by Suppressing Hepatic Lipogenesis and Promoting Fatty Acid Oxidation. Evid Based Complement Alternat Med. 2021;2021:1–13. doi:10.1155/201/9561613
- Zhao Z, Deng ZT, Huang S, et al. Alisol B Alleviates Hepatocyte Lipid Accumulation and Lipotoxicity via Regulating RARα-PPARγ-CD36 Cascade and Attenuates Non-Alcoholic Steatohepatitis in Mice. Nutrients. 2022;14(12):2411. doi:10.3390/nu14122411
- Liu L, Gao C, Yao P, Gong Z. Quercetin Alleviates High-Fat Diet-Induced Oxidized Low-Density Lipoprotein Accumulation in the Liver: implication for Autophagy Regulation. Biomed Res Int. 2015;2015:607531. doi:10.1155/2015/607531
- Huang X, Chen W, Yan C, et al. Gypenosides improve the intestinal microbiota of non-alcoholic fatty liver in mice and alleviate its Progression. Biomed Pharmacother. 2019;118:109258. doi:10.1016/j.biopha.2019.109258
- Liu B, Zhang J, Sun P, Yi R, Han X, Zhao X. Raw Bowl Tea (Tuocha) Polyphenol Prevention of Nonalcoholic Fatty Liver Disease by Regulating Intestinal Function in Mice. Biomolecules. 2019;9(9):435. doi:10.3390/biom9090435
- Lee W, Koo HR, Choi YJ, et al. Z-ligustilide and n-Butylidenephthalide Isolated from the Aerial Parts of Angelica tenuissima Inhibit Lipid Accumulation In Vitro and In Vivo. Planta Med. 2019;85(9–10):719–728. doi:10.1055/a-0901-1307
- Yang JS, Tongson J, Kim KH, Park Y. Piceatannol attenuates fat accumulation and oxidative stress in steatosis-induced HepG2 cells. Curr Res Food Sci. 2020;3:92–99. doi:10.1016/j.crfs.2020.03.008
- Kuo NC, Huang SY, Yang CY, Shen HH, Lee YM. Involvement of HO-1 and Autophagy in the Protective Effect of Magnolol in Hepatic Steatosis-Induced NLRP3 Inflammasome Activation In Vivo and In Vitro. Antioxidants. 2020;9(10):924. doi:10.3390/antiox9100924
- Pfohl M, DaSilva NA, Marques E, et al. Hepatoprotective and anti-inflammatory effects of a standardized pomegranate (Punica granatum) fruit extract in High Fat dime-induced obesity C57BL/6 mice. Int J Food Sci Nutr. 2021;72(4):499–510. doi:10.1080/09637486.2020.1849041
- Gong X, Li T, Wan R, Sha L. Cordycepin attenuates high-fat diet-induced non-alcoholic fatty liver disease via down-regulation of lipid. Int Immunopharmacol. 2021;91:107173. doi:10.1016/j.intimp.2020.107173
- Kim MJ, Yoo YC, Sung NY, et al. Anti-Inflammatory Effects of Liriope platyphylla in LPS-Stimulated Macrophages and Endotoxemic Mice. Am J Chin Med. 2016;44(6):1127–1143. doi:10.1142/S0192415X16500634
- Le TNH, Choi HJ, Jun HS. Ethanol Extract of Liriope platyphylla Root Attenuates non-alcoholic Fatty Liver Disease in high-fat Diet-induced obesity Mice via Regulation of Lipogenesis and Lipid Uptake. Nutrients. 2021;13(10). doi:10.3390/NU13103338
- Yang Q, Shu F, Gong J, et al. Sweroside ameliorates NAFLD in high-fat diet induced obese mice through the regulation of lipid metabolism and inflammatory response. J Ethnopharmacol. 2020;255:112556. doi:10.1016/j.jep.2020.112556
- Gao Y, Zhang S, Li J, et al. Effect and mechanism of ginsenoside Rg1-regulating hepatic steatosis in HepG2 cells induced by free fatty acid. Biosci Biotechnol Biochem. 2020;84(11):2228–2240. doi:10.1080/09168451.2020.1793293
- Quan T, Zhou F, Chen H, et al. Ficus hirta Vahl. Ameliorates Nonalcoholic Fatty Liver Disease through Regulating Lipid Metabolism and Gut Microbiota. Oxid Med Cell Longev. 2022;2022:3474723. doi:10.1155/2022/3474723
- Li Y, Jiang W, Feng Y, Wu L, Jia Y, Zhao R. Betaine Alleviates High-Fat Diet-Induced Disruption of Hepatic Lipid and Iron Homeostasis in Mice. Int J Mol Sci. 2022;23(11):6263. doi:10.3390/ijms23116263
- Liao Z, Zhang J, Liu B, et al. Polysaccharide from Okra (Abelmoschus esculentus (L.) Moench) Improves Antioxidant Capacity via PI3K/AKT Pathways and Nrf2 Translocation in a Type 2 Diabetes Model. Molecules. 2019;24(10):1906. doi:10.3390/molecules24101906
- Peng CH, Ker YB, Li HH, Tsou SH, Lin CL, Huang CN. Abelmoschus esculentus subfractions ameliorate hepatic lipogenesis and lipid uptake via regulating dipeptidyl peptidase-4-With improving insulin resistance. PLoS One. 2022;17(3):e0265444. doi:10.1371/journal.pone.0265444
- Poornima MS, Sindhu G, Billu A, et al. Pretreatment of hydroethanolic extract of Dillenia indica L. attenuates oleic acid induced NAFLD in HepG2 cells via modulating SIRT-1/p-LKB-1/AMPK, HMGCR & PPAR-α signaling pathways. J Ethnopharmacol. 2022;292:115237. doi:10.1016/j.jep.2022.115237
- Poornima MS, Sindhu G, Billu A, et al. Pretreatment of hydroethanolic extract of Dillenia indica L. attenuates oleic acid induced NAFLD in HepG2 cells via modulating SIRT-1/p-LKB-1/AMPK, HMGCR & PPAR-α signaling pathways. J Ethnopharmacol. 2022;292:115237. doi:10.1016/j.jep.2022.115237
- Li CL, Liu B, Wang ZY, et al. Salvianolic acid B improves myocardial function in diabetic cardiomyopathy by suppressing IGFBP3. J Mol Cell Cardiol. 2020;139:98–112. doi:10.1016/j.yjmcc.2020.01.009
- Meng LC, Zheng JY, Qiu YH, et al. Salvianolic acid B ameliorates non-alcoholic fatty liver disease by inhibiting hepatic lipid accumulation and NLRP3 inflammasome in ob/ob mice. Int Immunopharmacol. 2022;111:109099. doi:10.1016/j.intimp.2022.109099
- Zhang L. Pharmacokinetics and drug delivery systems for puerarin, a bioactive flavone from traditional Chinese medicine. Drug Deliv. 2019;26(1):860–869. doi:10.1080/10717544.2019.1660732
- Zhou J, Zhang N, Aldhahrani A, Soliman MM, Zhang L, Zhou F. Puerarin ameliorates nonalcoholic fatty liver in rats by regulating hepatic lipid accumulation, oxidative stress, and inflammation. Front Immunol. 2022;13:956688. doi:10.3389/fimmu.2022.956688
- Bai J, Zhang Y, Tang C, et al. Gallic acid: pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed Pharmacother. 2021;133:110985. doi:10.1016/j.biopha.2020.110985
- Tanaka M, Sato A, Kishimoto Y, Mabashi-Asazuma H, Kondo K, Iida K. Gallic Acid Inhibits Lipid Accumulation via AMPK Pathway and Suppresses Apoptosis and Macrophage-Mediated Inflammation in Hepatocytes. Nutrients. 2020;12(5):1479. doi:10.3390/nu12051479
- Camps-Bossacoma M, Garcia-Aloy M, Saldaña-Ruiz S, et al. Role of Theobromine in Cocoa’s Metabolic Properties in Healthy Rats. J Agric Food Chem. 2019;67(13):3605–3614. doi:10.1021/acs.jafc.8b07248
- Lee HW, Choi IW, Ha SK. Immunostimulatory Activities of Theobromine on Macrophages via the Activation of MAPK and NF-κB Signaling Pathways. Curr Issues Mol Biol. 2022;44(9):4216–4228. doi:10.3390/cimb44090289
- Wei D, Wu S, Liu J, et al. Theobromine ameliorates nonalcoholic fatty liver disease by regulating hepatic lipid metabolism via mTOR signaling pathway in vivo and in vitro. Can J Physiol Pharmacol. 2021;99(8):775–785. doi:10.1139/cjpp-2020-0259
- Ye P, Xiang M, Liao H, et al. Dual-Specificity Phosphatase 9 Protects Against Nonalcoholic Fatty Liver Disease in Mice Through ASK1 Suppression. Hepatology. 2019;69(1):76–93. doi:10.1002/hep.30198
- Bou Khzam L, Son NH, Mullick AE, Abumrad NA, Goldberg IJ. Endothelial cell CD36 deficiency prevents normal angiogenesis and vascular repair. Am J Transl Res. 2020;12(12):7737–7761.
- Bizzarri M, Giuliani A, Monti N, Verna R, Pensotti A, Cucina A. Rediscovery of natural compounds acting via multitarget recognition and noncanonical pharmacodynamical actions. Drug Discov Today. 2020;25(5):920–927. doi:10.1016/j.drudis.2020.02.010
- Azab A, Nassar A, Azab AN. Anti-Inflammatory Activity of Natural Products. Molecules. 2016;21(10):1321. doi:10.3390/molecules21101321
- Caesar LK, Cech NB. Synergy and antagonism in natural product extracts: when 1 + 1 does not equal 2. Nat Prod Rep. 2019;36(6):869–888. doi:10.1039/c9np00011a
