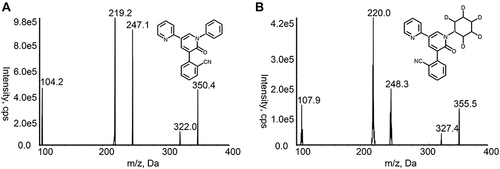
Abstract
Purpose
The present study aimed to establish and validate an isotope-dilution-UHPLC-MS/MS method for the determination of perampanel (PER) concentration and investigate the effect of eslicarbazepine acetate (ESL) on the pharmacokinetics of PER in rats.
Methods
The rats were randomly divided into the control (0.5% CMC-Na) and experimental groups (ESL, 72 mg/kg), with six rats in each group. A single dose of PER (1 mg/kg) was administered after a week of repetitive 0.5% CMC-Na or ESL dosing (72 mg/kg); then, plasma samples were collected. Perampanel-d5 (PER-d5) was used as the internal standard (IS), liquid-liquid extraction of plasma samples was carried out using ethyl acetate, and chromatographic separation was carried out on a Titank C18 column (2.1 mm × 50 mm, 3.0 μm) using a gradient mobile phase consisting of 0.1% formic acid and acetonitrile at a flow rate of 0.3 mL/min.
Results
PER had good linearity (0.3–600 ng/mL, r >0.999), and the accuracy, precision, recovery rate, and matrix effects (ME) met the Food and Drug Administration (FDA) guidelines. Compared to the control group, the area under the curve AUC0→t, AUC0→∞, and Cmax of PER in the experimental group decreased by 30.28%, 30.34%, and 46.94%, respectively, and CL increased by 32.08%.
Conclusion
ESL could induce the metabolism of PER in rats and decreases plasma exposure to PER. Thus, the concomitant treatment with ESL may require a high dose of PER to achieve the same efficacy.
Introduction
Epilepsy is a neurological disorder that affects more than 7000 people worldwide.Citation1 About one-third of the individuals progressed to drug-resistant epilepsy, which was defined as a failure of adequate trials.Citation2 Subsequently, antiepileptic drug schedules (whether as monotherapies or in combination) were selected appropriately to achieve sustained freedom from seizures.Citation3 Drug resistance is a key challenge in treating epilepsy.Citation4 A small number of patients with drug-resistant epilepsy can achieve seizure freedom through surgery.Citation5 Most drug-resistant epilepsies require alternative treatments due to the inability of the traditional methods to accurately locate the epileptic foci.Citation5 The third generation of new antiepileptic drugs (AEDs), such as perampanel (PER) and eslicarbazepine acetate (ESL), offer new hope for decreasing resistance in treatment-resistant epilepsy.Citation6,Citation7
PER is a highly selective, noncompetitive α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonist and a novel antiepileptic agent approved in Europe and the United States for the treatment of partial-onset seizures (POS) and primary generalized tonic-clonic (PGTC) seizures.Citation8–10 In addition, PER is metabolized in the liver mainly through cytochrome P450 (CYP) 3A4, and its clearance can be achieved by an enzyme (CYP3A4)-inducer antiepileptic agents.Citation11 Population pharmacokinetic analysis showed that concomitant use of CYP3A4-inducing AEDs (carbamazepine, oxcarbazepine, or phenytoin) with perampanel resulted in a 2- to 3-fold increase in the oral clearance of PER and a 50–67% reduction in AUC.Citation12,Citation13
ESL, an antiepileptic agent that acts on the slow inactivated state of sodium channels, is approved for the treatment of POS in patients ≥4-years-old.Citation14,Citation15 It is also an inducer of CYP3A4 enzyme.Citation15 In vivo studies showed that ESL reduces the plasma concentration of simvastatin metabolized by CYP3A4 and the Cmax and AUC of b-hydroxyacid simvastatin, the main active metabolite of simvastatin by 41% and 49%, respectively.Citation16
The combinations of AEDs with different mechanisms of action might be more effective than monotherapy in the treatment of drug-resistant epilepsy.Citation17,Citation18 However, polytherapy AEDs have potentially adverse pharmacokinetic interactions.Citation19 Currently, there is no relevant study to show the effect of ESL on the pharmacokinetics of PER. Therefore, based on previous studies, we established an isotope-dilution Ultra-High Performance liquid chromatography-tandem mass spectrometry (UHPLC–MS/MS) with perampanel-d5 (PER-d5) as an internal standard (IS) to detect the plasma concentration of PER in SD rats and successfully verified the pharmacokinetic interaction between ESL and PER.Citation20–23
Materials and Methods
Chemicals and Reagents
PER (purity >98%) was purchased from Panphy Chemicals Corporation (Los Angeles, CA, USA), and PER-d5 (IS, purity >99%) was purchased from TLC Pharmaceutical Standards Ltd (Newmarket, Ontario, Canada). ESL (purity >98%) was purchased from TCI Chemicals Ltd (Shanghai, China). Acetonitrile, ethyl acetate and formic acid were chromatographic grade and obtained from Fisher Scientific (Pittsburgh, PA, USA). Ultrapure water was purchased from Wahaha Group Ltd (Hangzhou, Zhejiang, China).
Instrumentation and Analytical Conditions
UHPLC-MS/MS method was conducted using a Shimadzu LC-30 UHPLC system (Shimadzu Corporation; Kyoto, Japan) and an AB Sciex 5500 triple quadrupole tandem mass spectrometer equipped with electrospray ionization source (Framingham, MA, USA). The chromatographic separation was performed on a Titank C18 column (2.1 mm×50 mm, 3.0 μm), and the column temperature was 40 °C. The mobile phase was composed of acetonitrile (B) and 0.1% formic acid-water (A), and the gradient elution procedure was as follows: 0–0.5 min, 30% B; 0.5–2.0 min, 30–90% B; 2.0–3.0 min, 90% B; 3.0–3.5 min, 90–30% B; 3.5–5.0 min, 30% B. The injection volume was 5 µL, and the flow rate was 0.3 mL/min. The positive ion multi-response monitoring mode was used, and the target of the parent and daughter ions of PER and IS were m/z 350.2 → 219.2 and m/z 355.3 → 220.0, respectively (). The declustering potential and collision energy for PER and IS were 195 V and 50 eV, respectively. The other optimized mass spectrum parameters were as follows: temperature (TEM), 600 °C; ion spray voltage, 5500 V; ion source gas 1, 60 psi; ion source gas 2, 60 psi; curtain gas, 30 psi.
Preparation of Standard Solutions and Quality Control (QC) Samples
PER and IS were solubilized in acetonitrile to prepare the standard stock solution of 1 mg/mL. The stock solution of PER was diluted with acetonitrile to obtain two groups of working solutions. An appropriate volume of working solutions was added to the blank rat plasma to obtain a series of calibration standards (0.3, 3, 6, 30, 60, 300, and 600 ng/mL) and quality control (QC) samples (0.6, 45, and 450 ng/mL). The stock solution of IS was diluted to the working solution of 30 ng/mL acetonitrile.
Plasma Sample Preparation
Plasma samples were pretreated by liquid-liquid extraction. A volume of 4 µL of IS working solution was added to 40 µL of plasma samples and vortexed for 1 min. Then, 200 µL ethyl acetate was added to the mixture and vortexed for 2 min, followed by centrifugation (4 °C) at 13,000 rpm for 10 min. The upper organic layer was transferred to a centrifugation tube and evaporated to dryness using a stream of nitrogen at room temperature. The residue was dissolved in 150 µL of 50% acetonitrile. The supernatant, collected by centrifugation (4 °C) at 12,000 rpm for 2 min, was injected (5 µL) into the column for analysis.
Method Validation
The bioanalytical assay method was verified following the Guidelines for Industry Bioanalytical Method Validation (UFDA), and the parameters evaluated were selectivity, linearity, precision, accuracy, matrix effect, extraction recovery, and stability.Citation24
The selectivity of the method reflected that the interfering substances to the target analyte was within the acceptable range in the biological matrix. The interference was evaluated by comparing the chromatograms of blank plasma samples from six different rats; the rat plasma samples and the blank plasma spiked with 0.3 ng/mL of PER and IS, respectively.
The standard curve of the method was the correlation between the analyte concentration and the instrument response value within a specified concentration range. A weighted (1/X2) least-squares linear regression method was used via Analyst Software. The independent variable was the concentration of the analyte, and the dependent variable was the peak area ratio of the analyte to IS. LLOQ, the lowest point of the standard curve, can be quantified with precision and accuracy.
Six replicates of QC samples at four concentration levels, LLOQ, low, mid, and high (repeated for three consecutive days), were selected to inspect the inter- and intraday precision and accuracy. The acceptance criteria for precision and accuracy were within 15% RSD and 15% RE, respectively, except at LLOQ, wherein these were within 20% of the minimum calibration concentration.
The extraction recovery was accessed by comparing the intended peak area of the samples spiked with analyte pre-extraction to the samples spiked with analyte post-extraction. The matrix effect was evaluated by comparing the intended peak area of samples of the blank plasma post-extraction spiked with the analyte to the samples of the corresponding standard solution spiked with the analyte. The extraction recovery and matrix effect were determined by analysis of three QC concentration samples (0.6, 45, and 450 ng/mL) from six individual sources.
The stability of PER in rat plasma was investigated by analyzing the three concentrations of QC. The following four kinds of stability were examined: autosampler stability (in an autosampler for 8 h), freeze-thaw stability (three freeze-thaw cycles), long-term stability (at −20 °C for one month), and room-temperature stability (at room temperature for 4 h).
Pharmacokinetic Studies in Rat
Specific pathogen-free (SPF) grade male Sprague–Dawley (SD) rats (weight: 230±10 g) were provided by the Laboratory Animal Resources Institute of the National Institutes for Food and Drug Control (Beijing, China). The license number is SCXK (Beijing) 2017–0005. The animal experiment protocols were approved by the Experimental Animal Welfare Ethics Committee of Hebei Medical University (Shijiazhuang, China) and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals. Rats were fed twice a day in an appropriate environment at 18–24 °C and 40–60% humidity. Before the experiment, the rats were fasted overnight without lack of water. A total of 12 rats were randomly and equally divided into two groups: experimental and control. The two groups were administered 72 mg/kg ESL and the corresponding volume of solvent (0.5% sodium carboxymethyl cellulose) intragastrically for 7 consecutive days, respectively. On day 7, rats in both groups were given 1 mg/kg PER by intragastric administration 30 min after administration of ESL or solvent. At 0.083, 0.25, 0.5, 0.75, 1, 1.5, 2, 4, 6, 8, 10, 12, and 24 h after the last administration, a volume of 150 µL blood sample was collected from venous plexus of the inner canthus into heparinized tubes. The supernatant obtained by centrifugation at 3000 rpm for 10 min was stored at −20 °C until analysis.
Statistical Analysis
The pharmacokinetic parameters of PER were calculated in the non-compartmental model using DAS 2.1.1 software (Mathematical Pharmacology Professional Committee of China, Shanghai, China). SPSS 25.0 software package (SPSS Inc., Chicago, IL, USA) was used to compare the pharmacokinetic parameters of each group. t-test or nonparametric test was used according to whether the data were normally distributed; P<0.05 indicated a statistically significant difference. The mean concentration-time curves of PER were drawn using GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA).
Results
Method Validation
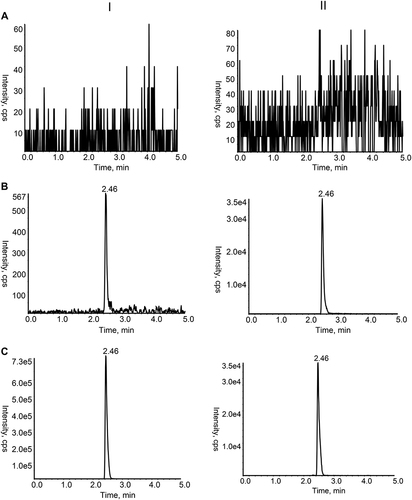
The chromatograms of blank plasma, rat plasma samples after gavaging PER administration, and the blank plasma spiked with 0.3 ng/mL of PER and IS revealed non-interfering peaks from endogenous substances (). The retention time of both PER and IS was 2.46 min.
Figure 2 Chromatograms of PER (I) and IS (II) in rat plasma samples. (A) Blank rat plasma sample; (B) Rat plasma sample spiked with PER at the LLOQ level and IS; (C) Rat plasma sample after oral administration.
A satisfactory linear range of PER was 0.3–600 ng/mL in rat plasma. A typical regression equation of PER was y=0.0436x+0.00128 (r>0.999). Based on a signal-to-noise ratio of at least 5:1, the lower limit of quantification (LLOQ) of PER was established to be 0.3 ng/mL.
Inter- and intraday precision and accuracy data are shown in . The %RSD and %RE values were in full compliance with acceptable FDA standards <15% for the QCs and <20% for LLOQs.
Table 1 Precision and Accuracy of per in Rat Plasma
The extraction recovery and matrix effect for QC samples of PER are summarized in . The extraction recovery for PER at LQC, MQC, and HQC was 75.99 ± 8.34%, 80.28 ± 4.81%, and 75.93 ± 3.69%, respectively. The range of matrix effect was 96.03–100.64%, indicating that the co-elution of matrix components did not enhance or suppress the ion concentrations.
Table 2 Matrix Effect and Extraction Recovery of per in Rat Plasma (n = 6)
Strikingly, LQC, MQC, and HQC were within the acceptable standard of ±15% after being placed in an autosampler for 8 h, three freeze-thaw cycles, being placed at −20 °C for one month, or being placed at room temperature for 4 h (). PER was stable under all conditions, as assessed by the bioassay method.
Table 3 Stability of per in Rat Plasma Under Various Storage Conditions (n = 6)
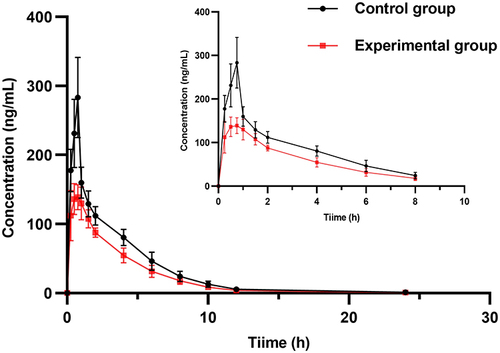
Effect of ESL on the Pharmacokinetics of per
The mean concentration-time curves of the experimental and control groups are shown in . The pharmacokinetic parameters of PER in the two groups are shown in . The results showed that AUC0→t, AUC0→∞, and Cmax of PER decreased by 30.28%, 30.34%, and 46.94%, respectively, while CL increased by 42.74% when PER was used in combination with ESL compared to PER alone. The significant changes in the pharmacokinetic parameters indicated that ESL obviously induced the metabolism of PER in rats.
Table 4 Pharmacokinetic Parameters of per with or Without ESL
Discussion
Based on previous studies, a new assay was developed.Citation20–23 We selected m/z transitions of 350.2 → 219.2 and 355.3 → 220.0 for PER and PER-d5, respectively, to obtain the best peak intensity. The other parameters of mass spectrometry were optimized to improve the reproducibility of analytes. Since PER-d5 and PER have similar physicochemical properties and chromatographic behavior, the use of stable isotopes as IS can improve the sensitivity and stability of the method by reducing the matrix effect. After trying different volumes and types of extractants, 150 µL ethyl acetate effectuated a high extraction yield, which could purify and enrich the analytes in 40 µL of rat plasma, suitable for small sample aliquots. The optimized mobile phase and gradient elution procedures can reduce the interference of endogenous substances in the plasma to improve the peak shape and obtain the best peak resolution.
ESL is derived from traditional dibenzodiazepine AEDs (carbamazepine and oxcarbazepine) by altering the substituents at 10.11-position on the dibenzazepine nucleus bearing the 5-carboxamide substitute.Citation16,Citation25,Citation26 This change in the chemical structure leads to metabolic differences and enzyme-induced changes in vivo.Citation16,Citation25,Citation27 Compared to carbamazepine, ESL reduces the incidence of dizziness and sleepiness because it does not produce the toxic metabolite carbamazepine-10,11-epoxide.Citation16,Citation25 Moreover, ESL reduced the dose of administration compared to oxcarbazepine because ESL is stereoselectively metabolized to the active metabolite (S)-licazepine (94%).Citation16,Citation25 Also, it has a lower risk of enzyme-induced drug interactions than carbamazepine and oxcarbazepine.Citation16,Citation25 However, the weak induction of CYP3A4 by ESL can significantly reduce the statins exposure by approximately 50%, thus influencing the effect of lowered blood lipids.Citation28
Accumulating evidence indicated that the combination of PER with other AEDs in the treatment of epilepsy significantly reduces seizures.Citation29 In a post-hoc analysis of three phase-III clinical trials, Gidal et al demonstrated that treatment responses were higher at PER doses of 8 and 12 mg/day in patients who did not receive an antiepileptic agent with CYP3A4 inducers.Citation12 In patients receiving enzyme-inducer antiepileptic drugs (EIAEDs), PER plasma concentrations are decreased; hence, a high PER dose may be required to achieve the same efficacy.Citation12 Since carbamazepine, oxcarbazepine, or phenytoin can significantly reduce exposure to PER, FDA recommends increasing the initial dose of PER and shortening the titration period when PER is used in combination with EIAEDs.Citation9,Citation30 The starting dose of PER was increased from 2–4 mg, and the titration frequency was shortened from 2 mg every two weeks to 2 mg every week.Citation9,Citation30 The maintenance dose of PER was determined according to the patient’s clinical response and tolerability.Citation9,Citation30 EMA recommends that patients receive a 2 mg increase or a 2 mg decrease in the dose of PER when the concomitant inducer and non-inducer AEDs are switched among each other.Citation10 When EIAED is removed from the prescription, the dose of PER should be adjusted accordingly to prevent adverse events caused by its high concentrations in the plasma.Citation10
ESL and PER are two AEDs with different mechanisms of action that can produce synergistic pharmacodynamic effects in combination. The current results showed that ESL significantly enhances the oral clearance rate of PER and reduces the plasma exposure of PER in rats. Thus, increasing the dose of PER when combined with ESL is recommended. When ESL is withdrawn from the treatment regimen, the dose of PER should be appropriately reduced to prevent adverse events caused by the sudden increase in the plasma concentration of PER. This preclinical study provides a reference for the rational use of PER in clinical practice. The effect of ESL on the pharmacokinetics of PER needs further clinical study.
Conclusion
In this study, we developed and validated an isotope dilutions UHPLC-MS/MS method to determine the plasma concentrations of PER in SD rats. The applicability of this method was confirmed in drug interaction studies between PER and the inducer ESL. Consequently, ESL significantly reduces the plasma exposure to PER, rendering it necessary to adjust the dose of PER when combined with ESL.
Disclosure
The authors report no conflicts of interest in this study.
Acknowledgments
This study did not receive specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Thijs RD, Surges R, O’Brien TJ, Sander JW. Epilepsy in adults. Lancet. 2019;393(10172):689–701. doi:10.1016/S0140-6736(18)32596-0
- Löscher W, Potschka H, Sisodiya SM, Vezzani A. Drug resistance in epilepsy: clinical impact, potential mechanisms, and new innovative treatment options. Pharmacol Rev. 2020;72(3):606–638. doi:10.1124/pr.120.019539
- Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies. Epilepsia. 2010;51(6):1069–1077. doi:10.1111/j.1528-1167.2009.02397.x
- Lerche H. Drug-resistant epilepsy - time to target mechanisms. Nat Rev Neurol. 2020;16(11):595–596. doi:10.1038/s41582-020-00419-y
- Solli E, Colwell NA, Say I, et al. Deciphering the surgical treatment gap for drug‐resistant epilepsy (DRE): a literature review. Epilepsia. 2020;61(7):1352–1364. doi:10.1111/epi.16572
- Chen Z, Brodie MJ, Kwan P. What has been the impact of new drug treatments on epilepsy? Curr Opin Neurol. 2020;33(2):185–190. doi:10.1097/WCO.0000000000000803
- Kanner AM, Ashman E, Gloss D, et al. Practice guideline update summary: efficacy and tolerability of the new antiepileptic drugs II: treatment-resistant epilepsy. Neurology. 2018;91(2):82–90. doi:10.1212/WNL.0000000000005756
- Yuan CL, Shi EY, Srinivasan J, Ptak CP, Oswald RE, Nowak LM. Modulation of AMPA receptor gating by the anticonvulsant drug, perampanel. Acs Med Chem Lett. 2019;10(3):237–242. doi:10.1021/acsmedchemlett.8b00322
- US Food and Drug Administration. FYCOMPA®(perampanel): highlights of prescribing information; 2022. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/202834s016,208277s004lblpdf. Accessed September 15, 2022.
- European Medicines Agency. FYCOMPA®(perampanel): summary of product characteristics; 2022. Available from: https://www.ema.europa.eu/en/documents/product-information/fycompa-epar-product-information_en.pdf. Accessed September 15, 2022.
- Patsalos PN. The clinical pharmacology profile of the new antiepileptic drug perampanel: a novel noncompetitive AMPA receptor antagonist. Epilepsia. 2015;56(1):12–27. doi:10.1111/epi.12865
- Gidal BE, Laurenza A, Hussein Z, et al. Perampanel efficacy and tolerability with enzyme-inducing AEDs in patients with epilepsy. Neurology. 2015;84(19):1972–1980. doi:10.1212/WNL.0000000000001558
- Patsalos PN. Drug interactions with the newer antiepileptic drugs (AEDs)—Part 1: pharmacokinetic and pharmacodynamic interactions between AEDs. Clin Pharmacokinet. 2013;52(11):927–966. doi:10.1007/s40262-013-0087-0
- Hebeisen S, Pires N, Loureiro AI, et al. Eslicarbazepine and the enhancement of slow inactivation of voltage-gated sodium channels: a comparison with carbamazepine, oxcarbazepine and lacosamide. Neuropharmacology. 2015;89:122–135. doi:10.1016/j.neuropharm.2014.09.008
- US Food and Drug Administration. APTIOM®(eslicarbazepine acetate): highlights of prescribing information; 2022. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022416s011lbl.pdf. Accessed September 15, 2022.
- Bialer M, Soares-da-Silva P. Pharmacokinetics and drug interactions of eslicarbazepine acetate. Epilepsia. 2012;53(6):935–946. doi:10.1111/j.1528-1167.2012.03519.x
- Abou-Khalil B. Selecting rational drug combinations in epilepsy. Cns Drugs. 2017;31(10):835–844. doi:10.1007/s40263-017-0471-7
- Verrotti A, Lattanzi S, Brigo F, Zaccara G. Pharmacodynamic interactions of antiepileptic drugs: from bench to clinical practice. Epilepsy Behav. 2020;104:106939. doi:10.1016/j.yebeh.2020.106939
- Johannessen SI, Landmark CJ. Antiepileptic drug interactions - principles and clinical implications. Curr Neuropharmacol. 2010;8(3):254–267. doi:10.2174/157015910792246254
- Mano Y, Takenaka O, Kusano K. High-performance liquid chromatography–tandem mass spectrometry method for the determination of perampanel, a novel α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor antagonist in human plasma. J Pharmaceut Biomed. 2015;107:56–62. doi:10.1016/j.jpba.2014.12.018
- de Grazia U, D’Urso A, Ranzato F, et al. A liquid chromatography-mass spectrometry assay for determination of perampanel and concomitant antiepileptic drugs in the plasma of patients with epilepsy compared with a fluorescent HPLC assay. Ther Drug Monit. 2018;40(4):477–485. doi:10.1097/FTD.0000000000000531
- Paul D, Allakonda L, Sahu A, Surendran S, Satheeshkumar N. Pharmacokinetics and brain uptake study of novel AMPA receptor antagonist perampanel in SD rats using a validated UHPLC-QTOF-MS method. J Pharmaceut Biomed. 2018;149:234–241. doi:10.1016/j.jpba.2017.11.008
- Kim DY, Moon J, Shin YW, et al. Usefulness of saliva for perampanel therapeutic drug monitoring. Epilepsia. 2020;61(6):1120–1128. doi:10.1111/epi.16513
- US Food and Drug Administration. Guidance for industry: bioanalytical method validation; 2018. Available from: https://www.fda.gov/media/70858/download. Accessed October 3, 2022.
- Lawthom C, Peltola J, McMurray R, Dodd E, Villanueva V Dibenzazepine agents in epilepsy: how does eslicarbazepine acetate differ? Neurology and Therapy. 2018;7(2):195–206. doi:10.1007/s40120-018-0111-2.
- Gierbolini J, Giarratano M, Benbadis SR. Carbamazepine-related antiepileptic drugs for the treatment of epilepsy - a comparative review. Expert Opin Pharmaco. 2016;17(7):885–888. doi:10.1517/14656566.2016.1168399
- Zhang C, Zuo Z, Kwan P, Baum L. In vitro transport profile of carbamazepine, oxcarbazepine, eslicarbazepine acetate, and their active metabolites by human P-glycoprotein. Epilepsia. 2011;52(10):1894–1904. doi:10.1111/j.1528-1167.2011.03140.x
- Patsalos PN. Drug Interactions with the Newer Antiepileptic Drugs (AEDs)—Part 2: pharmacokinetic and pharmacodynamic interactions between AEDs and drugs used to treat non-epilepsy disorders. Clin Pharmacokinet. 2013;52(12):1045–1061. doi:10.1007/s40262-013-0088-z
- Hsu WWQ, Sing CW, He Y, Worsley AJ, Wong ICK, Chan EW. Systematic review and meta-analysis of the efficacy and safety of perampanel in the treatment of partial-onset epilepsy. Cns Drugs. 2013;27(10):817–827. doi:10.1007/s40263-013-0091-9
- de Biase S, Gigli GL, Nilo A, Romano G, Valente M. Pharmacokinetic and pharmacodynamic considerations for the clinical efficacy of perampanel in focal onset seizures. Expert Opin Drug Metab Toxicol. 2019;15(2):93–102. doi:10.1080/17425255.2019.1560420