Abstract
Heart failure is a global problem with elevated prevalence, and it is associated with substantial cardiovascular morbidity and mortality. Treating heart-failure patients has been a very challenging task. This review highlights the main pharmacological developments in the field of heart failure with reduced ejection fraction, giving emphasis to a drug that has a dual-acting inhibition of the neprilysin and renin–angiotensin–aldosterone system. Neprilysin is an enzyme that participates in the breakdown of biologically active natriuretic peptides and several other vasoactive compounds. The inhibition of neprilysin has been a therapeutic target for several drugs tested in cardiovascular disease, mainly for heart failure and/or hypertension. However, side effects and a lack of efficacy led to discontinuation of their development. LCZ696 is a first-in-class neprilysin- and angiotensin-receptor inhibitor that has been developed for use in heart failure. This drug is composed of two molecular moieties in a single crystalline complex: a neprilysin-inhibitor prodrug (sacubitril) and the angiotensin-receptor blocker (valsartan). The PARADIGM-HF trial demonstrated that this drug was superior to an angiotensin-converting enzyme inhibitor (enalapril) in reducing mortality in patients with heart failure with reduced ejection fraction. The ability to block the angiotensin receptor and augment the endogenous natriuretic peptide system provides a distinctive mechanism of action in cardiovascular disease.
Introduction
The impact of heart failure in the global context
Cardiovascular disease (CVD) is the major cause of mortality in developed and many developing countries, accounting for about 30% of the overall mortality.Citation1 Early mortality rates associated with CVD, including those related to acute coronary syndromes, valvular and congenital heart disease, stroke, and hypertension, have decreased substantially.Citation2,Citation3 A study of the decrease in US deaths attributable to coronary heart disease from 1980 to 2000 suggests that ~47% of the decrease was attributable to increased use of evidence-based medical therapies for secondary prevention and 44% to changes in risk factors in the population attributable to lifestyle and environmental changes.Citation2 However, a great number of patients with these disorders progress with myocardial damage and consequently chronic heart disease, in spite of their lives having been prolonged. Hypertension, which is highly prevalent in the population, is one of the main factors associated with the elevated number of cardiovascular events. Therefore, an increasing number of individuals are exposed to greater risk of subsequently developing heart failure (HF).
HF is a global problem, with an estimated 38 million patients diagnosed worldwide.Citation1,Citation3–Citation6 The Global Burden of Disease 2010 study reported that from 1990 to 2010, ischemic heart disease, one source of myocardial damage, was the most common cause of death worldwide.Citation3,Citation4 Other very common conditions associated with HF are hypertension and diabetes. HF is now becoming more common, even in low-income and medium-income countries, because a high proportion of the population has a lifestyle that leads to obesity, diabetes mellitus, and in particular hypertension (75% of HF cases have antecedent hypertension). These are well-known risk factors for the development of HF.Citation5,Citation6
On the basis of data from the National Health and Nutrition Examination Survey of 2009–2012, an estimated 5.7 million Americans over 20 years old have HF. Projections show that the prevalence of HF will increase in the US to 46% by 2030, with more than 8 million people aged 18 years and over with HF.Citation7 There are 915,000 new HF cases annually in the US, with African-Americans having the highest risk of developing the disease because of the greater prevalence of hypertension, diabetes mellitus, and low socioeconomic status in this ethnic group.Citation1,Citation8 A 50% increase in the number of new cases of HF is also estimated, mainly due to the aging population.Citation9–Citation11
HF occurs most commonly in elderly people: it is the most common diagnosis at hospital admission in patients aged 65 years and older. Every year, about 1 million hospital admissions occur for HF in the US, with a similar number occurring in Europe.Citation1,Citation9,Citation10 In patients aged over 65 years in the US, the 30-day mean hospital-readmission rate is around 30%,Citation9 with 83% of patients hospitalized at least once and 43% hospitalized at least four times.Citation12 In developing countries, such as Brazil, HF is responsible for 20% of the total patients admitted to hospital with CVD.Citation13
Treating HF individuals is a very challenging task. The art of forming a diagnosis, staging the disease, and establishing an adequate drug association for the patient is crucial for clinical benefits. From the 1990s to the beginning of the 21st century, neurohormonal blockade has comprised the mainstay of therapy.Citation14–Citation19 The activation of neurohormonal pathways, such as the renin–angiotensin–aldosterone system (RAAS) and the sympathetic nervous system (SNS), is very important in the pathophysiology of HF. The inhibition of these pathways was a breakthrough in the treatment of HF. The importance of the RAAS is shown by the results of its blockade using ACE inhibitors (ACEIs), angiotensin-receptor blockers (ARBs) and mineralocorticoid-receptor antagonists.Citation14–Citation17 In turn, the beneficial effects of β-blockers suggest that the SNS has a role in HF.Citation18–Citation21 Although survival after the diagnosis and treatment of HF has improved over time, especially in patients with reduced ejection fraction (EF), many patients now experience a more prolonged course, resulting in increases in the prevalence of the problem in the population and the economic burden on the health care system.Citation1,Citation22–Citation24 Even so, the death rate remains high: >50% of people diagnosed with HF will die within 5 years.Citation1,Citation24 The number of deaths by any cause attributable to HF was approximately as high in 1995 (287,000) as it was in 2011 (284,000).Citation1 In patients aged over 65 years in the US, the 30-day inpatient-mortality rate for patients admitted to hospital with HF is fairly constant at about 11%, with similar results in Europe.Citation25,Citation26
The 5-year survival rate for HF is worse than it is for most cancers, and the annual cost of care for HF in the US has been estimated to exceed $30 billion.Citation7,Citation27 Projections show that by 2030, the total cost of HF will be almost 127% higher than 2012: at $69.7 billion. This equals ~$244 for every American adult.Citation7 HF is a particular threat in middle-income and low-income countries, where the adjusted hazard ratios for case fatality are 2.61 and 3.72, using high-income countries as the referent.Citation28
Evidence shows that the use of β-blockers and mineralocorticoid-receptor antagonists added to ACEIs results in incremental decreases in the risk of death of 30%–35% and 22%–30%, respectively.Citation18–Citation20 Despite this, much study is still needed to improve our understanding of the pathophysiology of HF and to develop new approaches to prevent or improve the care of patients with this lethal condition. Research on HF is now quite dynamic worldwide, and many areas are being explored with the discovery of new pharmacological options. Therefore, this article reviews the physiology of the natriuretic peptide (NP) system. It also considers how novel therapeutic agents that enhance NP levels, in particular those that simultaneously suppress the RAAS, may represent a new opportunity in the treatment of HF. Finally, it also discusses the PARADIGM-HF trialCitation29 and the implications it may have on the care of patients with chronic HF.
Neutral endopeptidase: a new target for cardiovascular disease treatment
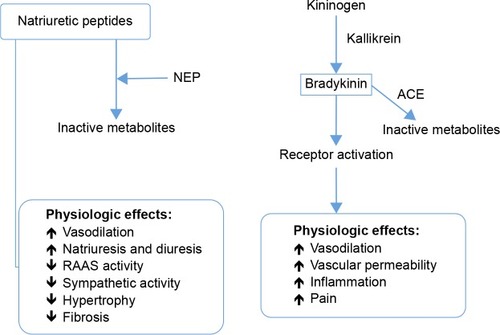
NPs constitute a family of similar, but genetically distinct peptides, including atrial NP (ANP), brain NP (BNP), and C-type NP (CNP).Citation30–Citation33 ANP and BNP exert their physiological actions through NP receptors type A (NPR-A) and type B (NPR-B), which are coupled to and activate guanylyl cyclase A. This increases the intracellular concentrations of the second messenger, cyclic guanosine 3′,5′-monophosphate (cGMP), known to be the active mediator of biologic effects that include vasodilation, natriuresis, diuresis, inhibition of the RAAS, endothelin, and vasopressin, and lipid mobilization.Citation34–Citation36 Moreover, it reduces sympathetic drive and has antiproliferative and antihypertrophic effects.Citation34–Citation36 Distension of the atria and ventricles, as occurs in ventricular dysfunction and HF, results in significant increases in the expression of NPs, particularly of ANP and BNP, as a compensatory response.
On the other hand, NPs have a short half-life in the circulation, with neprilysin (neutral endopeptidase 24.11 [NEP]) being the main enzyme that degrades them.Citation37 Therefore, NEP inhibitors would augment active NPs, increasing the generation of myocardial cGMP, which would improve myocardial relaxation and reduce hypertrophy. Moreover, neprilysin inhibitions should protect NPs and protect against the bradykinin catabolic process, and thus would shift the balance of endogenous hormonal factors from a vasoconstrictive, sodium-retaining, and hypertrophic state toward a more vasodilator, natriuretic, and cardioprotective condition, and hence they were expected to be effective in the management of hypertension and HF.Citation38 However, the beneficial effects in patients were modest, and NEP inhibition alone did not cause clinically meaningful reductions in blood pressure.Citation39 In addition to increasing the concentration of circulating NPs, NEP inhibitors were found to elevate the concentrations of two other circulating vasoconstrictor agents – angiotensin II and endothelin 1 – whose breakdown is dependent on NEP.Citation40–Citation42 These two opposing actions, ie, inhibition of the degradation of both vasoconstrictor and vasodilator peptides, neutralized the effects of each other, and as a consequence NEP inhibitors alone had little effect on blood pressure or HF.Citation43,Citation44 shows a schematic of NPs and bradykinin and the physiological effects of both.
Vasopeptidase inhibition
As hitherto described, elevations of circulating angiotensin II due to NEP inhibitors neutralize vasodilator and natriuretic actions. Therefore, it would be interesting to ascertain whether the clinical benefits of NEP inhibition might be enhanced by the concomitant blockade of the RAAS. This concept was the basis for a new drug class known as vasopeptidase inhibitors, which simultaneously inhibit two key enzymes involved in the regulation of cardiovascular function: NEP and ACE. These inhibitors reduce vasoconstriction and enhance vasodilatation, reducing vascular tone and lowering blood pressure.Citation45 Therefore, the initial vasopeptidase inhibitors had a dual mechanism of action, acting as neprilysin inhibitors and ACEIs. shows a schematic of the mechanism of action of neprilysin and an ACEI (omapatrilat).
Figure 2 Schematic of action mechanism of NEP and ACE inhibitors (omapatrilat).
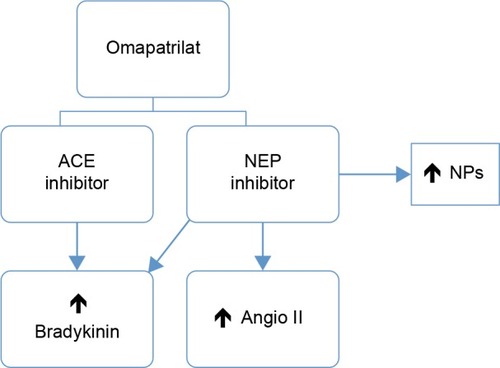
Omapatrilat, the most extensively evaluated of such agents, demonstrated its capacity to inhibit the pressor response to angiotensin I in rats and monkeys. Moreover, omapatrilat potentiated the natriuretic, cGMP, and ANP excretory responses to exogenous atrial NP in monkeys.Citation46 Omapatrilat also lowered mean arterial blood pressure at 24 hours in some kinds of experimental hypertension (low renin, normal renin, and high renin).Citation47 In healthy individuals, omapatrilat increased urinary ANP and cGMP, indicating NEP inhibition, and increased plasma renin activity, indicating a response to ACE inhibition.Citation48 In hypertensive patients, blood pressure-lowering and vasculoprotective effects were greater than for other therapeutic classes, including ACEIs and calcium-channel blockers.Citation49–Citation53 However, some studies showed that angioedema occurred more frequently with omapatrilat than with comparators.Citation50,Citation51 The increased incidence of this potentially life-threatening complication is likely due to concomitant inhibition of three enzymes (ACE, aminopeptidase P, and NEP) that participate in the breakdown of bradykinin (the putative mediator of angioedema induced by ACEIs).Citation53 Bradykinin is not only a vasodilator but also enhances prostaglandin concentrations and increases vascular permeability and fluid extravasation.Citation54 The initial question that made omapatrilat an attractive drug became a “double-edged sword”.Citation55,Citation56 Due to increased angioedema, the approval of omapatrilat and of further clinical research on the class of vasopeptidase inhibitors was halted.
Novel dual-acting inhibitor of the neprilysin and angiotensin II receptor
Nowadays, LCZ696, a novel dual-acting inhibitor of neprilysin (sacubitril) and the angiotensin II receptor (valsartan) was designed to minimize the risk of serious angioedema.Citation57,Citation58 ARBs have a lower risk of angioedema than ACEIs, probably because of their neutral effect on metallopeptidases involved in the breakdown of bradykinin.Citation59 This new drug class concurrently inhibits NEP and blocks angiotensin II receptors, thereby offering the cardioprotective benefits of vasopeptidase inhibitors without the increased risk of angioedema ().
Figure 3 Schematic of action mechanism of NEP (sacubitril) and ARB (valsartan) inhibitors in heart failure.
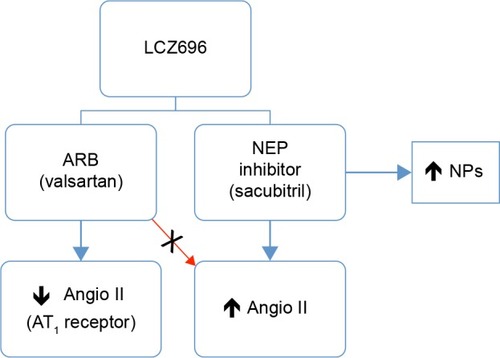
LCZ696 (Novartis International AG, Basel, Switzerland) is the first drug with a dual-acting ARB and neprilysin inhibitor in a single molecule – angiotensin II-receptor blockade via its valsartan molecular moiety,Citation60 and neprilysin inhibition via its prodrug AHU377 molecular moiety – which is metabolized to the active NEP inhibitor LBQ657 by enzymatic cleavage of its ethyl ester.Citation61 The molecular structure of LCZ696, consisting of trisodium (3-[{1S,3R}-1-biphenyl-4-ylmethyl-3-ethoxycarbonyl-1-butylcarbamoyl] propionate-[S]-3′-methyl-2′-[pentanoyl{2′′-(tetrazol-5-ylate) biphenyl 4′ylmethyl}amino]butyrate) hemipentahydrate, was established by X-ray crystallographic techniques.Citation57 This drug is also known as angiotensin-receptor and neprilysin inhibitor (ARNI).
Spotlight on sacubitril–valsartan for heart failure in the PARADIGM-HF trial
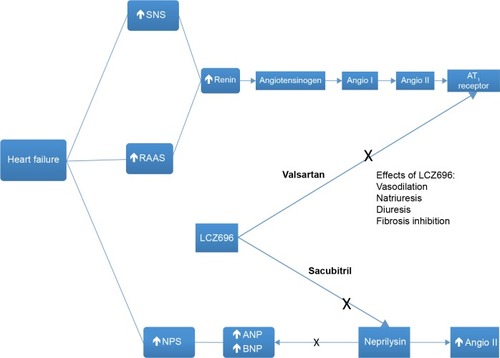
As has been described for more than two decades, RAAS blockers (particularly ACEIs and mineralocorticoid-receptor antagonists) and β-blockers have been the pharmacologic treatment that changed the natural history of HF with reduced EF (HFrEF).Citation14–Citation21 Now, the new product combination of the neprilysin inhibitor sacubitril with the ARB valsartan represents an attempt to further improve the bad prognosis of HF, reducing the risk of cardiovascular death and hospitalization rate linked to HF. Sacubitril was the first neprilysin inhibitor to become available in the US, and the sacubitril–valsartan (sacubitril 97 mg, valsartan 103 mg) association was approved by the US Food and Drug Administration in July 2015 for the treatment of patients with New York Heart Association class II–IV HF and reduced EF based on a double-blind trial: the PARADIGM-HF study.Citation29 shows the central role of LCZ696 (sacubitril–valsartan) in the dual inhibition of the RAAS and of NEP.
Figure 4 The central role of LCZ696 in the dual inhibition of the RAAS and of neprilysin.
Abbreviations: SNS, sympathetic nervous system; RAAS, renin–angiotensin–aldosterone system; NPS, natriuretic peptide system; Angio I, angiotensin I; Angio II, angiotensin II; ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide.
The PARADIGM-HF trial was a high-quality randomized clinical Phase III study of the sacubitril–valsartan combination (LCZ696) that focused on the key outcomes of mortality and HF-related hospitalization. This trial evaluated a total of 8,442 patients (mean age 63.8 years, 78.2% male) – 4,187 patients taking the combination and 4,212 taking enalapril – with reduced EF (≥35%) and New York Heart Association class II–IV HF symptoms. Participants were randomly assigned to sacubitril–valsartan (200 mg twice daily) or enalapril (10 mg twice daily), in addition to other recommended therapy. Prior to randomization, patients underwent single-blind run-in treatment with enalapril (median duration of treatment 15 days) followed by sacubitril–valsartan (median duration of treatment 29 days) to ensure tolerability. Of the 10,513 patients initially enrolled, 2,079 (19.8%) withdrew from the study during the run-in phase, 1,138 of whom (10.8%) withdrew due to intolerance to treatment; similar proportions withdrew while on run-in treatment with enalapril and sacubitril–valsartan. More than 80% of the patients in each arm were taking diuretics as background therapy, and 93% were taking β-blockers. In the sacubitril–valsartan arm, 54.2% of patients received a mineralocorticoid antagonist, as did 57% of the controls. About 30% of all patients were also taking digitalis.Citation29
After a median duration of 27 months of follow-up, the trial was interrupted, because the prespecified boundary for benefit (one-sided P<0.001 for reduction in both cardiovascular death and the composite of cardiovascular death and first hospitalization for worsening HF) had been crossed. In the PARADIGM-HF trial, the primary composite end point of death from cardiovascular causes or first hospitalization for worsening HF occurred in 21.8% of the sacubitril–valsartan group and 26.5% of the enalapril group (hazard ratio 0.80, 95% confidence interval 0.73–0.87; P<0.001). In this study, the numbers needed to treat to prevent one primary composite event (cardiovascular death or first hospitalization for chronic HF) and one cardiovascular death were 21 and 32, respectively. The combination significantly reduced the risk of first hospitalization for worsening HF (12.8% vs 15.6%, P<0.001), death from cardiovascular causes (13.3% vs 16.5%, P<0.001), and all-cause mortality (17.0% vs 19.8%, P<0.001).Citation29
Sacubitril–valsartan had higher reported rates of hypotension and lower rates of cough and renal impairment relative to enalapril. Discontinuation of study medication owing to an adverse event was less frequent among patients in the sacubitril–valsartan group (10.7% vs 12.3%, P=0.03). Nineteen patients (0.5%) in the sacubitril–valsartan group and ten patients (0.2%) in the enalapril group experienced angioedema (P=0.13).Citation29
Is PARADIGM-HF exempt from criticism?
In PARADIGM-HF, the authors declared that their results were applicable to a broad spectrum of patients with HF, including those currently taking an ACEI or ARB or who were likely to be able to take such an agent without having unacceptable side effects, but we have some doubts and considerations about this conclusion.
Firstly, this study had a run-in phase, ie, before patients were randomized, both treatments were tried, and only patients that tolerated the treatment participated in the study. Therefore, the results presented were from the patients selected for tolerating the drug, a condition that may reduce the external validity of the study’s safety. In relation to safety, what would the result be in a representative sample of a real-world population?
Secondly, which stable patients should be switched from using RAAS inhibitors to the sacubitril–valsartan combination? Unlike clinical practice, every trial patient underwent a controlled run-in period during which tolerability was carefully assessed. Despite the exclusion of a large number of potential study candidates, symptomatic hypotension remained higher in the sacubitril–valsartan group. This suggests that patients with borderline blood pressure, hypotensive individuals, or those tolerating less than recommended doses of vasodilators may encounter difficulties with the sacubitril–valsartan association. The authors stated that because of its greater vasodilator effects, treatment with LCZ696 was associated with a higher rate of symptomatic hypotension, but there was no increase in the rate of discontinuation due to possible hypotension-related adverse effects. Although the greater hypotensive effect of LCZ696 might impair renal perfusion, clinically important increases in the serum creatinine level and discontinuation of the study drug because of renal impairment were less frequent in the LCZ696 group than in the enalapril group.Citation29 In addition, the authors said that the effects of LCZ696 on renal function were consistent with the effects observed in experimental studiesCitation62 and with findings in earlier trials of omapatrilat.Citation51,Citation52
Finally, the target dose of enalapril in the control group was 20 mg/day. This dose was proportionally lower than the valsartan dose. The European and American guidelines recommend that the target enalapril dosage for treating HF is 10–20 mg twice daily, and the dose of enalapril in PARADIGM-HF was half the maximal approved dosage.Citation63,Citation64 On the other hand, in the PARADIGM-HF trial, sacubitril–valsartan twice daily reduced the incidence of cardiovascular death by 19% compared with enalapril 10 mg twice daily (the rates were 16.5% and 13.3%, respectively).Citation29 Besides, the sacubitril–valsartan combination lowered mean systolic blood pressure 3.2±0.4 mmHg more than enalapril,Citation29,Citation65 a fact that may account for much of this benefit. Previous data showed that a 2 mmHg decrease in systolic blood pressure reduced the risk of cardiovascular death by 7% in middle-aged adults.Citation66 That study did not involve HF patients, but if its results are remotely applicable to PARADIGM-HF, a 3.2 mmHg reduction in systolic blood pressure might be expected to reduce the rate of cardiovascular deaths by 10%–11%.Citation67
Therefore, would a sacubitril–valsartan combination be superior to enalapril if the maximal dose of enalapril were compared to the maximal dose of sacubitril–valsartan? Would sacubitril–valsartan be superior to enalapril if blood pressure were lowered comparably between the two groups?
In relation to first question, the authors said that the choice of an active comparator was based upon the SOLVD trial, which was a pivotal ACEI mortality/morbidity study in a broad spectrum of patients with HFrEF.Citation15 In the SOLVD trial, the target dose of enalapril was 10 mg twice daily and the mean daily prescribed dose in patients taking enalapril was 16.6 mg/day. The same target dose of enalapril was used in several other HF trials, in which the mean daily dose of enalapril was between 15 and 18 mg.Citation14,Citation68–Citation73 Two trials, which had higher target doses (20 mg twice daily and 30 mg twice daily), achieved only slightly greater average doses (18.4 and 19.3 mg, respectively), with less than 50% of patients titrated to target.Citation14,Citation71 Therefore, the authors considered that “… from a regulatory perspective, the ‘gold standard’ comparator for LCZ696 is enalapril 10 mg bid. [twice daily], the most tested ACE inhibitor in HFrEF”, and they anticipated that achieving a similar average dose to that attained in the SOLVD trial would be required.Citation74 Moreover, this dosage of enalapril was chosen based on its survival benefit in previous trials. However, this still raises the question of whether the benefit seen in the sacubitril–valsartan group was due to greater inhibition of the RAAS rather than to the new drug.Citation75 Another important point is that the fixed dose of 20 mg a day of enalapril used in the study is not the same as a mean dose of 20 mg a day, which results from individualization according to the patient, using higher doses in some and lower doses in others. A mean dose of 20 mg a day is probably more effective than a fixed dose of 20 mg a day.Citation76
To address the second point, it seems that the sacubitril–valsartan group had better treatment than the control group. This can be suggested by a lower blood pressure level in the LCZ696 group compared to the control group, maybe evidencing a greater blockade of the RAAS (greater dose of valsartan vs enalapril), a fact previously highlighted.Citation77 On the other hand, the addition of the NEP inhibition in this group resulted in greater vasodilator effects, a situation evidenced by a higher chance of symptomatic hypotension in the sacubitril–valsartan group. This could be a limitation of the study, although the authors wrote that the benefit of LCZ696 over enalapril was not explained by the small difference in blood pressure, because when the difference in the blood pressure between the two groups was examined as a time-dependent covariate, it was not a significant predictor of the benefit of sacubitril–valsartan.Citation29
Progression of heart failure and evaluation of subgroups in PARADIGM-HF
The PARADIGM-HF investigators reported that among the survivors in the study, those who received sacubitril–valsartan presented better outcomes in terms of a number of markers of progression of HF, with lower rates of: 1) intensification of medical treatment for HF (16% risk reduction); 2) emergency department visits for worsening HF (34% risk reduction); and 3) hospitalizations for worsening HF (23% fewer), need for intensive care (18% rate reduction), need for intravenous inotropic agents (31% risk reduction), and need for cardiac devices or heart transplants (22% risk reduction). Besides, the patients also had lower rates of worsening symptom scores and elevation of biomarkers of myocardial injury.Citation78 In relation to evaluation of subgroups, a brief description of the effects of sacubitril–valsartan association in these situations follows.
Age
Drug tolerance and outcomes in patients with HF vary by age. The prespecified efficacy and safety outcomes were examined in PARADIGM-HF according to age-group (years): <55 (n=1,624), 55–64 (n=2,655), 65–74 (n=2,557), and ≥75 (n=1,563). A larger number of patients with a broader range of ages were included in PARADIGM-HF than in any previous trial in HFrEF. The sacubitril–valsartan:enalapril hazard ratio was <1 in all categories, with an overall hazard ratio of 0.8 (0.73, 0.87; P<0.001). Although the rate of death and HF hospitalization increased with age in both groups, the sacubitril–valsartan association was more beneficial than enalapril across the broad spectrum of age and intolerance of association leading to treatment withdrawal was uncommon, even in elderly individuals.Citation79 In addition, the enrolled population was in fact not very old (median age was 63.8 years).Citation80
Ethnicity
The majority of patients in PARADIGM-HF were white (66%) or Asian (18%). It is well known that black Americans have an increased risk of angioedema,Citation81 but their presence in the PARADIGM-HF population was low (~5%). Therefore, it is difficult to draw definitive conclusions regarding the safety of this medication in this racial group.Citation80
Ejection fraction
The influence of EF on clinical outcomes and the effectiveness of sacubitril–valsartan compared with enalapril was evaluated. The primary study end point was cardiovascular death or HF hospitalization. The mean left ventricular EF (LVEF) in PARADIGM-HF was 29.5 (interquartile range 25–34). The risk of all outcomes increased with decreasing LVEF. Each 5-point reduction in LVEF was associated with a 9% increased risk of cardiovascular death or HF hospitalization and a 7% increased risk in all-cause mortality in adjusted analyses. In this post hoc analysis of patients with HFrEF, LVEF was a powerful independent predictor of all outcomes. The sacubitril–valsartan association was effective at reducing cardiovascular outcomes and all-cause mortality across the LVEF spectrum, with no evidence of effect modification for any end point.Citation82
Biomarkers
In patients with HFrEF, BNP measurement contributes to better accuracy in diagnosis, reduces the rate of hospitalizations, and decreases mortality rates.Citation83 Moreover, NP level represents a useful marker to monitor the course of the disease in relation to the benefits of therapeutic strategies.Citation84 Therefore, the important clinical implications of NPs in HF indicate that NP-level measurement is one of the most effective tools for HF hormone-guided therapy.Citation85 These clinical findings were confirmed by the effects on biomarkers measured in surviving patients in the trial.Citation78 The levels of both urinary cyclic GMP and plasma BNP were higher during treatment with sacubitril–valsartan than with enalapril,Citation78 which is expected when NEP inhibition occurs.Citation86 In contrast, patients receiving LCZ696 had consistently lower levels of N-terminal (NT)-proBNP (reflecting reduced cardiac wall stress) and troponin (reflecting reduced cardiac injury) compared to the enalapril group. As BNP (but not NT-proBNP) is a substrate for NEP, levels of BNP reflect the action of the drug, whereas levels of NT-proBNP reflect the effects of the drug on the heart.Citation78,Citation87
Diabetes
The patients of PARADIGM-HF were also examined according to history of diabetes mellitus and glycemic status (baseline HbA1c <6%, 6%–6.4% [prediabetes], and ≥6.5% [diabetes mellitus]). Patients with a history of diabetes mellitus (n=2,907 [35%]) had a higher risk of the primary composite outcome of HF hospitalization or cardiovascular mortality compared with those without a history of diabetes mellitus (adjusted hazard ratio 1.38, 95% confidence interval 1.25–1.52; P<0.001). Patients with prediabetes were also at higher risk (hazard ratio 1.27, 95% confidence interval 1.10–1.47; P<0.001) compared with those with HbA1c <6%. LCZ696 (sacubitril–valsartan) was beneficial compared with enalapril, irrespective of glycemic status.Citation88
Concerns in relation to sacubitril–valsartan
Two potential long-term side effects and safety concerns related to the inhibition of neprilysin warrant consideration. Initially, there is evidence that NEP has protective activity against Alzheimer’s disease by degrading the amyloid-β (Aβ) peptide.Citation89 Inhibition of NEP in mice has resulted in increases in the levels of Aβ and plaque-like deposits in the brain to levels that are 30–50 times higher than normal levels; these increases might lead to cognitive impairment.Citation89 However, neprilysin is one of more than 20 enzymes that modulate the removal of Aβ peptides, some of which are implicated in the pathogenesis of Alzheimer-type dementia.Citation90 On the other hand, the results on this condition are controversial. Cognition-, memory-, and dementia-related adverse events did not increase in the LCZ696 group of the PARADIGM-HF trial. Therefore, NEP inhibition may block the breakdown of the key Aβ peptide that has been implicated in the pathogenesis and progression of Alzheimer’s disease. Cognitive function must be assessed during long-term treatment with sacubitril–valsartan, especially in elderly individuals. A trial of sacubitril–valsartan vs valsartan that includes repeated measurements of cognitive function in patients who have HF and preserved EF (HFpEF) is ongoing.Citation91
Another concern about neprilysin and its inhibition is related to cancer. NEP seems to have a protector role in some kinds of cancer. Protection derives from the inactivation of mitogenic peptides, including endothelin 1 and bradykinin. NEP inhibits prostate cancer-cell invasion in vitro,Citation92 and neprilysin overexpression has been associated with improved disease-free survival among women with breast cancer.Citation93 However, in PARADIGM-HF no increase in the risk of cancer was associated with LCZ696. Moreover, 2-year carcinogenicity studies involving rodents that received the NEP inhibitor (sacubitril) component of LCZ696 did not show any increase in the incidence of tumors. Furthermore, there was no evidence of genotoxic potential in genetic toxicity studies of LCZ696, sacubitril, or the active metabolite LBQ657.Citation94
Other uses for sacubitril–valsartan
Hypertension
In one of the first studies in the area of hypertension, Ruilope et al assessed 1,328 hypertensive subjects with uncomplicated mild-to-moderate essential hypertension to establish whether the dual actions of LCZ696 were superior to valsartan in lowering of blood pressure.Citation95 Systolic, diastolic, and pulse pressures, both sitting and ambulatory, presented greater reductions with LCZ696 than with either valsartan or an NEP-inhibitor prodrug (AHU377) administered separately. The dual inhibition of the angiotensin II receptor and neprilysin was well tolerated at all doses, without excessive coughing and with no instances of angioedema, possibly because neprilysin is a minor enzyme in the metabolic pathway for bradykinin degradation. This shows that the dual inhibition provided by this drug has complementary effects, and suggests that the effects related to kinins from ACEIs are not needed for these beneficial effects. Similar findings were reported in an Asian population of hypertensive subjects.Citation96
The safety of LCZ696 was also evaluated in 35 Japanese patients with severe hypertension (systolic blood pressure ≥180 mmHg or diastolic blood pressure ≥110 mmHg), who initially received LCZ696 200 mg. If necessary, the LCZ696 dose was increased to 400 mg after 2 weeks followed by an optional addition of another antihypertensive drug (except ARBs or ACEIs) after 4 weeks (n=21). There was clinically meaningful blood pressure reduction in patients with severe hypertension. The LCZ696-based regimen was well tolerated, and cases of angioedema were not registered.Citation97 The use of LCZ696 is justified, because it enhances NP levels and may be an attractive treatment option, particularly in Asian patients who generally have high salt sensitivity and salt intake.
Heart failure with preserved ejection fraction
Individuals with HFpEF were evaluated in the PARAMOUNT trial, which compared LCZ696 with valsartan.Citation87 Compared to valsartan alone, the LCZ696 group had significantly lower NT-proBNP levels, and at 36 weeks decreased left atrial size and a trend toward improvements in New York Heart Association functional class.Citation87 Another study to confirm the beneficial effects of dual inhibition in individuals with HFpEF, the PARAGON trial has begun.Citation91 It intends to enroll 4,300 patients with LVEF >45%.
Renal disease
Blockade of the RAAS by either ACEIs or ARBs slows the progression of chronic kidney disease, with and without diabetes.Citation98–Citation100 The NEP inhibitor candoxatrilat has been shown to be associated with natriuresis in patients with moderate impairment of renal function.Citation101 In trials with humans, omapatrilat was associated with greater slowing of renal function impairment than ACEIs.Citation50,Citation51 Similar findings were observed with ARNI (neprilysin–valsartan) in the PARADIGM-HF trial.Citation29
These data suggest that neprilysin–valsartan may be superior to blockers of the RAAS in respect to renal function. Another study is comparing neprilysin–valsartan with the ARB irbesartan in subjects with renal disease and an estimated glomerular filtration rate between 20 and 60 mL/min/1.73 m2.Citation102 As HF and renal dysfunction coexist, the question is whether ARNI might retard the development and/or progression of both conditions.
Vascular stiffness
In the elderly, vascular stiffness raises systolic blood pressure, pulse pressure, and pulse-wave velocity, which are independent predictors of cardiovascular events and of the progression of renal disease.Citation103–Citation105 In turn, noninvasively determined aortic blood pressure (central) is superior to brachial blood pressure (peripheral) in estimating cardiovascular risk.Citation106,Citation107 It is well known that central systolic blood pressure is a potent predictor of LV hypertrophy, whereas pulse pressure is a predictor of vascular hypertrophy.Citation108 Omapatrilat presents a better response than enalapril in the reduction of both central and peripheral blood pressures, reflecting a reduction of vascular stiffness. This effect may be caused by the elevation of circulating ANP consequent to NEP inhibitors.Citation49
LCZ696 also reduced both ambulatory systolic and pulse pressures more than valsartan.Citation95 Further research could help to elucidate the role of the sacubitril–valsartan combination on vascular stiffness, and the PARAMETER study will compare LCZ696 with an ARB (olmesartan) in elderly hypertensive patients with a pulse pressure >60 mmHg.Citation109
Mechanisms involved in the therapeutic answer to sacubitril–valsartan association in PARADIGM-HF
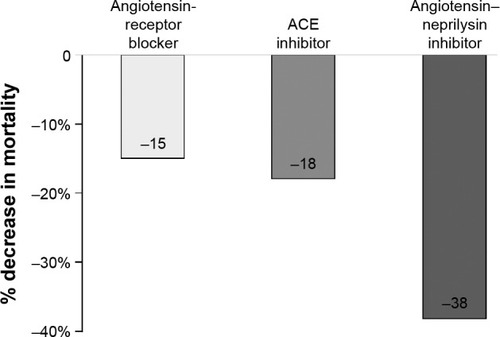
When we compared the two RAAS blockers (ACEIs and ARBs) already standard in the treatment of HFrEF, with a new class of ARNI, we observed a significant additional reduction in cardiovascular mortality with ARNI ().Citation15,Citation29,Citation36,Citation110 In summary, the effects of sacubitril–valsartan on the NP system and RAAS in HF could explain the clinical benefits, but more studies will be necessary to investigate these findings. It seems unlikely that the benefits of sacubitril–valsartan are due to the valsartan component of the ARNI by itself, because the studies with ARBs were never superior to studies with ACEIs.Citation36,Citation110–Citation113 The elevation of NP levels in the blood by itself does not seem to justify the benefits either, in view of the negative results on outcomes of previous studies with NEP inhibitors and vasopeptidase inhibitors, although a higher quantity of NPs can bind to receptors and produce the biological response. This possibility can be observed in a subpopulation of PARADIGM-HF, in which urinary cGMP was elevated in the group treated with sacubitril–valsartan.Citation114 This can occur because NEP inhibitors augment the active NPs and consequently increase the generation of myocardial cGMP. On the other hand, the concomitant blockade of RAAS by the ARB (valsartan) may have partially antagonized the NPR-A downregulation, given the interactions between the angiotensin II and NP intracellular signaling pathways.Citation36,Citation115,Citation116 Finally, two important roles can be attributed to the blood pressure-lowering effect of LCZ696 and the blockade of the AT1 receptor, which counteracts the increase in angiotensin II due to NEP inhibition.
Figure 5 Additional benefit of an angiotensin–neprilysin inhibitor on cardiovascular death compared to an angiotensin-receptor blocker and an ACE inhibitor.
Abbreviation: ACE, angiotensin-converting enzyme.
Conclusion
The better clinical outcomes of the sacubitril–valsartan combination over enalapril in patients with HFrEF in the PARADIGM-HF trial represent a significant success with important clinical implications, despite all the doubts and concerns about this association. Although the results of PARADIGM-HF are noteworthy and promising, further research in other trials on HF and hypertension is necessary. It is important to ascertain how the drug will be tolerated when it is used in clinical practice.
Disclosure
The author reports no conflicts of interest in this work.
References
- MozaffarianDBenjaminEJGoASHeart disease and stroke statistics – 2016 update: a report from the American Heart AssociationCirculation20161334e38e36026673558
- FordESAjaniUACroftJBExplaining the decrease in U.S. deaths from coronary disease, 1980–2000N Engl J Med2007356232388239817554120
- LozanoRNaghaviMForemanKGlobal and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010Lancet201238098592095212823245604
- ErratumLancet2013381986762823439101
- MoranAEForouzanfarMHRothGAThe global burden of ischemic heart disease in 1990 and 2010: the Global Burden of Disease 2010 studyCirculation2014129141493150124573351
- NicholsMTownsendNScarboroughPRaynerMTrends in age-specific coronary heart disease mortality in the European Union over three decades: 1980–2009Eur Heart J201334393017302723801825
- AmbrosyAPFonarowGCButlerJThe global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registriesJ Am Coll Cardiol201463121123113324491689
- HeidenreichPAAlbertNMAllenLAForecasting the impact of heart failure in the United States: a policy statement from the American Heart AssociationCirc Heart Fail20136360661923616602
- BahramiHKronmalRBluemkeDADifferences in the incidence of congestive heart failure by ethnicity: the Multi-ethnic Study of AtherosclerosisArch Intern Med2008168192138214518955644
- BraunwaldEThe war against heart failure: the Lancet lectureLancet2015385997081282425467564
- GoASMozaffarianDRogerVLHeart disease and stroke statistics – 2014 update: a report from the American Heart AssociationCirculation20141293e28e29224352519
- VigenRMaddoxTMAllenLAAging of the United States population: impact on heart failureCurr Heart Fail Rep20129436937422940871
- DunlaySMRedfieldMMWestonSAHospitalizations after heart failure diagnosis: a community perspectiveJ Am Coll Cardiol200954181695170219850209
- Ministério da SaúdeInformation about morbidity: hospital admission2014 Available from: http://tabnet.datasus.gov.br/cgi/tabcgi.exe?sih/cnv/niuf.defAccessed December 24, 2015
- No authors listedEffects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS)N Engl J Med198731623142914352883575
- No authors listedEffect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failureN Engl J Med199132552933022057034
- PittBZannadFRemmeWJThe effect of spironolactone on morbidity and mortality in patients with severe heart failureN Engl J Med19993411070971710471456
- McMurrayJJCONSENSUS to EMPHASIS: the overwhelming evidence which makes blockade of the renin angiotensin aldosterone system the cornerstone of therapy for systolic heart failureEur J Heart Fail201113992993621816763
- No authors listedEffect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF)Lancet199935391692001200710376614
- No authors listedThe Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trialLancet1999353914691310023943
- PackerMCoatsAJFowlerMBEffect of carvedilol on survival in severe chronic heart failureN Engl J Med2001344221651165811386263
- ShibataMCFlatherMDWangDSystematic review of the impact of beta blockers on mortality and hospital admissions in heart failureEur J Heart Fail20013335135711378007
- LevyDKenchaiahSLarsonMGLong-term trends in the incidence of and survival with heart failureN Engl J Med2002347181397140212409541
- RogerVLWestonSARedfieldMMTrends in heart failure incidence and survival in a community-based populationJAMA2004292334435015265849
- BarkerWHMulloolyJPGetchellWChanging incidence and survival for heart failure in a well-defined older population, 1970–1974 and 1990–1994Circulation2006113679980516461823
- FonarowGCAbrahamWTAlbertNMFactors identified as precipitating hospital admissions for heart failure and clinical outcomes: findings from OPTIMIZE-HFArch Intern Med2008168884785418443260
- GuhaKMcDonaghTHeart failure epidemiology: European perspectiveCurr Cardiol Rev20139212312723597298
- RogerVLEpidemiology of heart failureCirc Res2013113664665923989710
- YusufSRangarajanSTeoKCardiovascular risk and events in 17 low-, middle-, and high-income countriesN Engl J Med2014371981882725162888
- McMurrayJJPackerMDesaiASAngiotensin-neprilysin inhibition versus enalapril in heart failureN Engl J Med201437111993100425176015
- LevinERGardnerDGSamsonWKNatriuretic peptidesN Engl J Med199833953213289682046
- BaxterGFThe natriuretic peptidesBasic Res Cardiol2004992717514963664
- PandeyKNBiology of natriuretic peptides and their receptorsPeptides200526690193215911062
- VolpeMNatriuretic peptides and cardio-renal diseaseInt J Cardiol2014176363063925213572
- PotterLRAbbey-HoschSDickeyDMNatriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functionsEndocr Rev2006271477216291870
- RubattuSSciarrettaSValentiVStanzioneRVolpeMNatriuretic peptides: an update on bioactivity, potential therapeutic use, and implication in cardiovascular diseasesAm J Hypertens200821773374118464748
- VolpeMCarnovaliMMastromarinoVThe natriuretic peptides system in the pathophysiology of heart failure: from molecular basis to treatmentClin Sci (Lond)20161302577726637405
- SonnenbergJLSakaneYJengAYIdentification of protease 3.4.24.11 as the major atrial natriuretic factor degrading enzyme in the rat kidneyPeptides1988911731802966343
- SeguraJRuilopeLMDual-acting angiotensin receptor-neprilysin inhibitionCurr Hypertens Rep2011131747821046489
- BevanEGConnellJMDoyleJCandoxatril, a neutral endopeptidase inhibitor: efficacy and tolerability in essential hypertensionJ Hypertens19921076076131321186
- RichardsAMWittertGACrozierIGChronic inhibition of endopeptidase 24.11 in essential hypertension: evidence for enhanced atrial natriuretic peptide and angiotensin IIJ Hypertens19931144074168390508
- McDowellGCoutieWShawCBuchananKDStruthersADNichollsDPThe effect of the neutral endopeptidase inhibitor drug, candoxatril, on circulating levels of two of the most potent vasoactive peptidesBr J Clin Pharmacol19974333293329088591
- FerroCJSprattJCHaynesWGWebbDJInhibition of neutral endopeptidase causes vasoconstriction of human resistance vessels in vivoCirculation19989723232323309639376
- AndoSRahmanMAButlerGCSennBLFlorasJSComparison of candoxatril and atrial natriuretic factor in healthy men: effects on hemodynamics, sympathetic activity, heart rate variability, and endothelinHypertension1995266 Pt 2116011667498988
- ClelandJGSwedbergKLack of efficacy of neutral endopeptidase inhibitor ecadotril in heart failureLancet19983519116165716589620738
- BurnettJCJrVasopeptidase inhibition: a new concept in blood pressure managementJ Hypertens199917Suppl 1S37S43
- RoblJASunCQStevensonJDual metalloprotease inhibitors: mercaptoacetyl-based fused heterocyclic dipeptide mimetics as inhibitors of angiotensin-converting enzyme and neutral endopeptidaseJ Med Chem19974011157015779171867
- TrippodoNCRoblJAAsaadMMFoxMPanchalBCSchaefferTREffects of omapatrilat in low, normal, and high renin experimental hypertensionAm J Hypertens1998113 Pt 13633729544878
- LiaoWCVesterqvistODelaneyCPharmacokinetics and pharmacodynamics of the vasopeptidase inhibitor, omapatrilat in healthy subjectsBr J Clin Pharmacol200356439540612968984
- MitchellGFIzzoJLJrLacourcièreYOmapatrilat reduces pulse pressure and proximal aortic stiffness in patients with systolic hypertension: results of the Conduit Hemodynamics of Omapatrilat International Research StudyCirculation2002105252955296112081987
- RouleauJLPfefferMAStewartDJComparison of vasopeptidase inhibitor, omapatrilat, and lisinopril on exercise tolerance and morbidity in patients with heart failure: IMPRESS randomised trialLancet2000356923061562010968433
- PackerMCaliffRMKonstamMAComparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE)Circulation2002106892092612186794
- KostisJBPackerMBlackHRSchmiederRHenryDLevyEOmapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trialAm J Hypertens200417210311114751650
- FryerRMSegretiJBanforPNEffect of bradykinin metabolism inhibitors on evoked hypotension in rats: rank efficacy of enzymes associated with bradykinin-mediated angioedemaBr J Pharmacol2008153594795518084312
- BlumbergALDennySEMarshallGRNeedlemanPBlood vessel-hormone interactions: angiotensin, bradykinin, and prostaglandinsAm J Physiol19772323H305H310842686
- CampbellDJVasopeptidase inhibition: a double-edged sword?Hypertension200341338338912623931
- BraunwaldEThe path to an angiotensin receptor antagonist-neprilysin inhibitor in the treatment of heart failureJ Am Coll Cardiol201565101029104125766951
- GuJNoeAChandraPAl-FayoumiSLigueros-SaylanMPharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor(ARNi)J Clin Pharmacol201050440141419934029
- HegdeLGYuCRennerTConcomitant angiotensin AT1 receptor antagonism and neprilysin inhibition produces omapatrilat-like antihypertensive effects without promoting tracheal plasma extravasation in the ratJ Cardiovasc Pharmacol201157449550421297495
- CicardiMZingaleLCBergamaschiniLAgostoniAAngioedema associated with angiotensin-converting enzyme inhibitor use: outcome after switching to a different treatmentArch Intern Med2004164891091315111379
- MistryNBWestheimASKjeldsenSEThe angiotensin receptor antagonist valsartan: a review of the literature with a focus on clinical trialsExpert Opin Pharmacother20067557558116553573
- KsanderGMGhaiRDde JesusRDicarboxylic acid dipeptide neutral endopeptidase inhibitorsJ Med Chem19953810168917007752193
- RademakerMTCharlesCJEspinerEANichollsMGRichardsAMKosoglouTCombined neutral endopeptidase and angiotensin-converting enzyme inhibition in heart failure: role of natriuretic peptides and angiotensin IIJ Cardiovasc Pharmacol19983111161259456286
- McMurrayJJAdamopoulosSAnkerSDESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESCEur Heart J201233141787184722611136
- YancyCWJessupMBozkurtB2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice GuidelinesJ Am Coll Cardiol20136216e147e23923747642
- JessupMNeprilysin inhibition – a novel therapy for heart failureN Engl J Med2014371111062106425176014
- LewingtonSClarkeRQizilbashNPetoRCollinsRAge-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studiesLancet200236093491903191312493255
- BlankfieldRPTo the editor: the PARADIGM-HF trialCleve Clin J Med201683316726974982
- No authors listedEffect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractionsN Engl J Med1992327106856911463530
- CohnJNJohnsonGZiescheSA comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failureN Engl J Med199132553033102057035
- No authors listedClinical outcome with enalapril in symptomatic chronic heart failure; a dose comparison: the NETWORK investigatorsEur Heart J19981934814899568453
- NanasJNAlexopoulosGAnastasiou-NanaMIOutcome of patients with congestive heart failure treated with standard versus high doses of enalapril: a multicenter studyJ Am Coll Cardiol20003672090209511127445
- KomajdaMLutigerBMadeiraHTolerability of carvedilol and ACE-inhibition in mild heart failure: results of CARMEN (Carvedilol ACE-Inhibitor Remodelling Mild CHF EvaluatioN)Eur J Heart Fail20046446747515182773
- Messner PellencPRudnickiALeclercqFGrolleauREnalapril in the treatment of mild-to-moderate heart failure in general medical practice: a prospective and multicentre study concerning 17,546 patientsActa Cardiol19955031872017676758
- McMurrayJJPackerMDesaiASDual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure trial (PARADIGM-HF)Eur J Heart Fail20131591062107323563576
- SabeMAJacobMSTaylorDOIn reply: the PARADIGM-HF trialCleve Clin J Med201683316716826974983
- CorreiaLCRassiAJrParadigm-HF: a paradigm shift in heart failure treatment?Arq Bras Cardiol20161061777926815651
- SabeMAJacobMSTaylorDOA new class of drugs for systolic heart failure: the PARADIGM-HF studyCleve Clin J Med2015821069370126469827
- PackerMMcMurrayJJVDesaiASAngiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failureCirculation20151311546125403646
- JhundPSFuMBayramEEfficacy and safety of LCZ696 (sacubitril-valsartan) according to age: insights from PARADIGM-HFEur Heart J201536382576258426231885
- KaplinskyEPARADIGM-HF trial: will LCZ696 change the current treatment of systolic heart failure?J Geriatr Cardiol201512547047326512236
- BrownNJRayWASnowdenMGriffinMRBlack Americans have an increased rate of angiotensin converting enzyme inhibitor-associated angioedemaClin Pharmacol Ther19966018138689816
- SolomonSDClaggettBDesaiASInfluence of ejection fraction on outcomes and efficacy of sacubitril/valsartan (LCZ696) in heart failure with reduced ejection fraction: the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trialCirc Heart Fail201693e00274426915374
- BhardwajAJanuzziJLJrNatriuretic peptide-guided management of acutely destabilized heart failure: rationale and treatment algorithmCrit Pathw Cardiol20098414615019952548
- MotiwalaSRJanuzziJLJrThe role of natriuretic peptides as biomarkers for guiding the management of chronic heart failureClin Pharmacol Ther2013931576723187878
- VolpeMRubattuMBurnettJJrNatriuretic peptides in cardiovascular diseases: current use and perspectivesEur Heart J201435741942524227810
- CharlesCJEspinerEANichollsMGClearance receptors and endopeptidase 24.11: equal role in natriuretic peptide metabolism in conscious sheepAm J Physiol19962712 Pt 2R373R3808770137
- SolomonSDZileMPieskeBThe angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trialLancet201238098511387139522932717
- KristensenSLPreissDJhundPSRisk related to pre-diabetes mellitus and diabetes mellitus in heart failure with reduced ejection fraction: insights from Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure trialCirc Heart Fail201691e00256026754626
- MinersJSBaruaNKehoePGGillSLoveSAβ-degrading enzymes: potential for treatment of Alzheimer diseaseJ Neuropathol Exp Neurol2011701194495922002425
- NalivaevaNNBelyaevNDKerridgeCTurnerAJAmyloid-clearing proteins and their epigenetic regulation as a therapeutic target in Alzheimer’s diseaseFront Aging Neurosci2014623525278875
- Novartis PharmaceuticalsEfficacy and Safety of LCZ696 Compared to Valsartan, on Morbidity and Mortality in Heart Failure Patients with Preserved Ejection Fraction (PARAGON-HF) Available from: https://clinicaltrials.gov/ct2/show/NCT01920711. NLM identifier: NCT01920711Accessed January 4, 2016
- DawsonLAMaitlandNJTurnerAJUsmaniBAStromal-epithelial interactions influence prostate cancer cell invasion by altering the balance of metallopeptidase expressionBr J Cancer20049081577158215083188
- SmollichMGötteMYipGWOn the role of endothelin-converting enzyme-1 (ECE-1) and neprilysin in human breast cancerBreast Cancer Res Treat2007106336136917295044
- Center for Drug Evaluation and ResearchApplication number: 207620orig1s000 – summary review2015 Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207620Orig1s000SumR.pdfAccessed January 4, 2016
- RuilopeLMDukatABöhmMLacourcièreYGongJLefkowitzMPBlood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator studyLancet201037597221255126620236700
- KarioKSunNChiangFTEfficacy and safety of LCZ696, a first-in-class angiotensin receptor neprilysin inhibitor, in Asian patients with hypertension: a randomized, double-blind, placebo-controlled studyHypertension201463469870524446062
- KarioKTamakiYOkinoNGotouHZhuMZhangJLCZ696, a first-in-class angiotensin receptor-neprilysin inhibitor: the first clinical experience in patients with severe hypertensionJ Clin Hypertens (Greenwich)201618430831426402918
- LewisEJHunsickerLGBainRPThe effect of angiotensin-converting-enzyme inhibition on diabetic nephropathyN Engl J Med199332920145614628413456
- RuggenentiPPernaAGherardiGRenoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuriaLancet1999354917635936410437863
- BrennerBMCooperMEde ZeeuwDEffects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathyN Engl J Med20013451286186911565518
- LipkinGWDawnayABHarwoodSMCattellWRRaineAEEnhanced natriuretic response to neutral endopeptidase inhibition in patients with moderate chronic renal failureKidney Int19975237928019291201
- JudgePHaynesRLandrayMJBaigentCNeprilysin inhibition in chronic kidney diseaseNephrol Dial Transplant201530573874325140014
- Mattace-RasoFUvan der CammenTJHofmanAArterial stiffness and risk of coronary heart disease and stroke: the Rotterdam StudyCirculation2006113565766316461838
- WeberTAuerJO’RourkeMFArterial stiffness, wave reflections, and the risk of coronary artery diseaseCirculation2004109218418914662706
- FordMLTomlinsonLAChapmanTPRajkumarCHoltSGAortic stiffness is independently associated with rate of renal function decline in chronic kidney disease stages 3 and 4Hypertension20105551110111520212269
- RomanMJDevereuxRBKizerJRCentral pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart StudyHypertension200750119720317485598
- BooysenHLNortonGRMasekoMJAortic, but not brachial blood pressure category enhances the ability to identify target organ changes in normotensivesJ Hypertens20133161124113023552129
- RomanMJOkinPMKizerJRLeeETHowardBVDevereuxRBRelations of central and brachial blood pressure to left ventricular hypertrophy and geometry: the Strong Heart StudyJ Hypertens201028238438820051906
- WilliamsBCockcroftJRKarioKRationale and study design of the Prospective Comparison of Angiotensin Receptor Neprilysin Inhibitor with Angiotensin Receptor Blocker Measuring Arterial Stiffness in the Elderly (PARAMETER) studyBMJ Open201442e004254
- YoungJBDunlapMEPfefferMAMortality and morbidity reduction with Candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: results of the CHARM low-left ventricular ejection fraction trialsCirculation2004110172618262615492298
- PittBSegalRMartinezFARandomised trial of losartan versus captopril in patients over 65 with heart failure (Evaluation of Losartan in the Elderly study, ELITE)Lancet199734990547477529074572
- PfefferMAMcMurrayJJVelazquezEJValsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or bothN Engl J Med2003349201893190614610160
- CohnJNTognoniGA randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failureN Engl J Med2001345231667167511759645
- KlaiberMKruseMVölkerKNovel insights into the mechanisms mediating the local antihypertrophic effects of cardiac atrial natriuretic peptide: role of cGMP-dependent protein kinase and RGS2Basic Res Cardiol2010105558359520352235
- KimDAizawaTWeiHAngiotensin II increases phosphodi-esterase 5A expression in vascular smooth muscle cells: a mechanism by which angiotensin II antagonizes cGMP signalingJ Mol Cell Cardiol200538117518415623434
- FrantzSKlaiberMBabaHAStress-dependent dilated cardiomyopathy in mice with cardiomyocyte-restricted inactivation of cyclic GMP-dependent protein kinase IEur Heart J201334161233124422199120