Abstract
Resistance to chemotherapeutic drugs is a major obstacle in non-small cell lung cancer (NSCLC) therapy. The molecular determinants of NSCLC resistance to doxorubicin are unknown. In the present study, we investigated whether topoisomerase IIβ binding protein 1 (TopBP1) was involved in the chemoresistance to doxorubicin in NSCLC cancer. We found that p53-deficient lung cancer cells (NCI-H1299) displayed the greatest resistance to doxorubicin compared with NCI-H358, A549, and HCC827 cells with p53 expression. The expression of TopBP1 was significantly higher in NCI-H1299 cells than the other three tumor cell lines. In addition, TopBP1 knockdown with specific small interfering RNA in NCI-H1299 cells enhanced the doxorubicin chemosensitivity and decreased the expression of p53 in the presence of doxorubicin. After doxorubicin administration, co-immunoprecipitation assay showed that TopBP1 promoted the expression of p53 in NCI-H1299 cells. These results for the first time demonstrated that TopBP1 plays an important role in NSCLC chemoresistance via upregulation of p53. Therefore, inhibition of TopBP1, in combination with chemotherapy, may represent a novel strategy for the treatment of chemotherapy-resistant NSCLC.
Introduction
Lung cancer is the leading cause of cancer-related death worldwide; 80% of lung cancers are non-small cell lung cancer (NSCLC) with poor therapeutic efficacy when diagnosed.Citation1,Citation2 It is estimated that approximately 40% of patients with NSCLC present with advanced-stage disease for which 5-year survival rates are in the region of 2%.Citation3 Currently, platinum-based regimens are the mainstay of lung cancer therapy, and chemotherapy serves as one of the important adjuvant therapies for its treatment.Citation4 However, drug resistance to conventional chemotherapeutics has become a major handicap in the success of NSCLC chemotherapy.Citation5,Citation6 Thus, it is imperative to develop novel therapeutic strategies that may enhance tumor cell response to anticancer drugs. Recently, studies have begun to investigate the molecular mechanism of NSCLC and identified various novel targeted agents, such as epidermal growth factor receptor tyrosine kinase inhibitors which exhibit greater efficacy than chemotherapy in patients with epidermal growth factor receptor-mutated tumors.Citation7 Despite the great progresses achieved in cancer therapy, the molecular mechanism of lung cancer pathogenesis and chemoresistance still remains elusive.
Topoisomerase IIβ binding protein 1 (TopBP1) was identified as an interacting partner for topoisomerase IIβ.Citation8,Citation9 It contains nine BRCA1 carboxyl-terminal domains and functions in DNA damage response, DNA checkpoint activation, replication, and regulation of transcription.Citation10–Citation13 TopBP1 also interacts several transcriptional factors, such as p53,Citation14 E2F1,Citation15,Citation16 Miz1,Citation17 and 53BP1.Citation18 Regulation of p53 by TopBP1 plays an important role in the regulation of proapoptotic activity and cell cycle transition.Citation19 It is reported that TopBP1 is frequently overexpressed in cancer and inactivates p53 functions.Citation19 In the current study, we aimed to explore the biological role of TopBP1 in chemoresistance along with the molecular mechanism underling these effects.
Materials and methods
Cell culture and reagents
Human lung cancer cell lines NCI-H358, A549, NCI-H1299, and HCC827 were purchased from the American Type Culture Collection (ATCC) (Manassas, VA, USA) and cultured in Dulbecco’s Modified Eagle’s Medium (Gibco, Carlsbad, CA, USA) supplemented with 10% FBS and 1% penicillin/streptomycin. All cells were maintained at 37°C in 5% CO2 incubator. Doxorubicin was purchased from Sigma-Aldrich (St Louis, MO, USA). The TopBP1 small interfering RNA (siRNA) and negative control siRNA were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). As clinical samples or animals were not used in this study ethical approval was not required by the institutional review board.
CCK-8 assay
Cancer cells or siRNA-transfected cancer cells were seeded onto 96-well plates at 3,000 cells/well. The medium was replaced with the corresponding serum-free medium for 24 hours to synchronize the cell cycle, and then the serum-free medium was replaced with complete medium containing the drugs at the indicated concentrations. Then, 10 μL/well CCK-8 solution (Dojindo, Kumamoto, Japan) was added, the plates incubated for 3 hours, and absorbance was measured at 450 nm using an MRX II microplate reader (Dynex, Chantilly, VA, USA).
EdU incorporation assay
Cell proliferation was calculated using EdU incorporation assay. Measurement of the inhibitive rate of cell proliferation was carried out using a Click-iTEdU Imaging Kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacture’s protocol.
Flow cytometry analysis
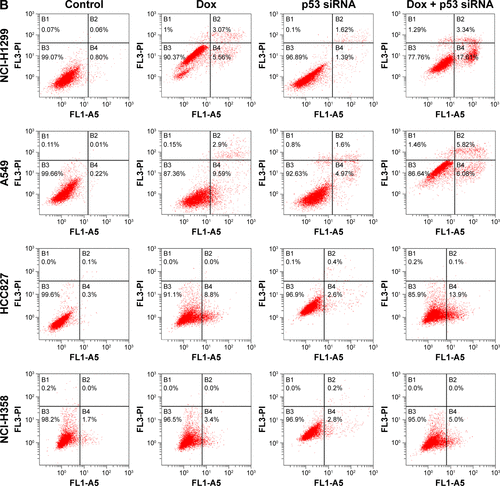
Tumor cells were exposed to doxorubicin and p53 siRNA alone or in combination. After treatment for 48 hours, cells were trypsinized and centrifuged rpm for 5 minutes and the pellet washed twice with phosphate-buffered saline (PBS). Cells were resuspended and then washed with PBS three times. Apoptosis cells were detected with annexin V-FITC/PI according to the protocol of Annexin V-FITC cell Apoptosis Detection Kit (Sigma-Aldrich Co., St Louis, MO, USA).
siRNA transfection
Lung cancer cells were seeded to achieve 30%–50% confluency on the day of transfection. Cells were transfected with TopBP1 siRNA, p53 siRNA, or negative control siRNA using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instruction. The transfection medium (Opti-MEM; Gibco) was replaced with complete medium 12 hours after transfection for further analysis.
Western blot analysis
Cells were lysed in 50 μL cell lysis buffer (Cell Signaling, Danvers, MA, USA). The protein concentration was quantified using the BCA Protein Kit (Thermo, Rockford, IL, USA). Cell lysates were separated by 10% SDS-PAGE and proteins were transferred to polyvinylidenedifluoride membranes (Merck Millipore, Billerica, MA, USA). The membranes were then incubated with anti-TopBP1 or anti-p53 antibodies (Abcam, Cambridge, MA, USA) at 4°C overnight. The membranes were washed three times with TBS/T and then incubated with the appropriate HRP-conjugated secondary antibodies (Abcam) for 1 hour at room temperature. Protein expression was detected by chemiluminescence (GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA).
Co-immunoprecipitation
Cells were harvested and lysed in 50 μL cell lysis buffer (Cell Signaling) after treatment with doxorubicin for 48 hours. An aliquot of the cell lysates was lysed with SDS lysis buffer, and the rest of the cell lysates were incubated with appropriate antibodies or beads for 24 hours at 4°C. Immunoprecipitates were fractionated by SDS-PAGE and electrotransferred to the polyvinylidenedifluoride membranes (Merck Millipore). The specific signals were detected with anti-TopBP1 or anti-p53 antibodies (Abcam). Protein expression was detected using chemiluminescence (GE Healthcare Bio-Sciences Corp.).
Statistical analysis
Each experiment was performed in triplicate and repeated at least three times. All the data were presented as mean ± standard deviation and treated for statistical analysis by Statistical Product and Service Solutions (SPSS) 16.0 (IBM, Armonk, NY, USA). Comparison between groups was made using one-way ANOVA and a statistically significant difference was defined as P<0.05.
Results
Different doxorubicin sensitivity and TopBP1 expression in lung cancer cells
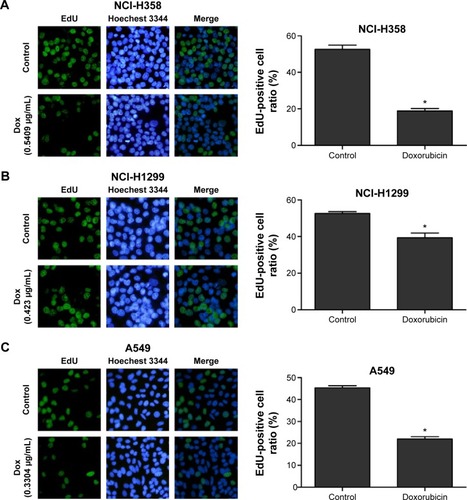
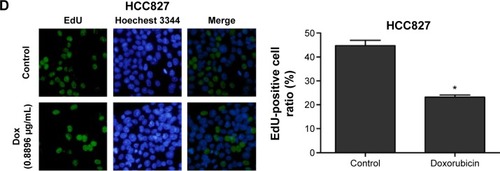
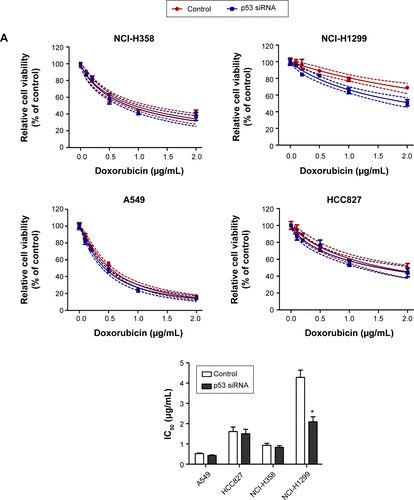
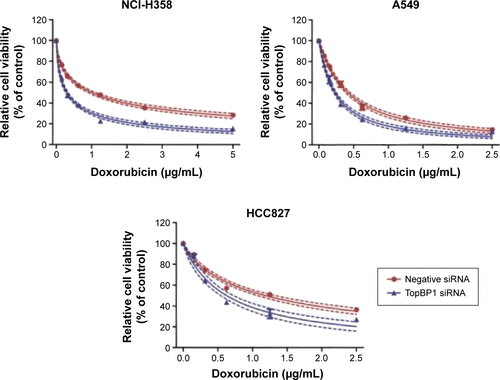
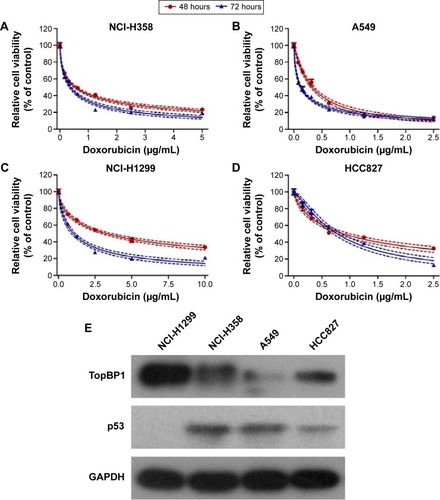
Firstly, CCK-8 assay we performed to examine cell viability of different lung cancer cell lines (NCI-H358, A549, NCI-H1299, and HCC827) after incubation with doxorubicin for 48 and 72 hours. It was found that the doxorubicin sensitivity varied among cell lines (). As shown in , the IC50 values at 48 or 72 hours were significantly higher in NCI-H1299 cells compared with the other three cell lines. In addition, the protein expression of TopBP1 was detected in different cell lines by Western blot. We found that TopBP1 expression was significantly higher in NCI-H1299 cells compared with three other cell lines (). Furthermore, EdU incorporation assay was carried out to measure the proliferation of lung cancer cells. Results showed that the inhibitive effect of doxorubicin was reduced in NCI-H1299 cells compared with that in NCI-H358, A549, and HCC827 cells (). These results implied that the TopBP1 might be associated with the drug resistance to doxorubicin in NSCLC cells.
Table 1 Determination of doxorubicin IC50 in different tumor cell lines
Figure 1 Different doxorubicin sensitivity and topoisomerase IIβ binding protein 1 (TopBP1) expression in lung cancer cells.
TopBP1 was involved in the chemoresistance of NSCLC cells
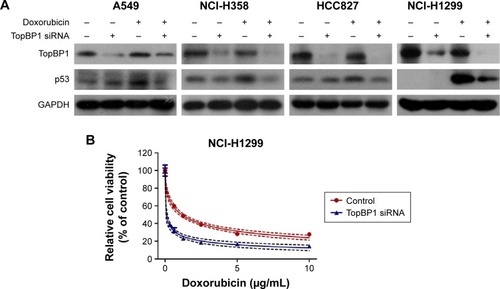
In order to confirm whether TopBP1 was involved in the tumor cells chemoresistance, TopBP1 siRNA was transfected into lung cancer cells to interfere with TopBP1 expression. Western blot showed that the TopBP1 expression was obviously decreased in four cancer cell lines after transfection with TopBP1 siRNA (). Addition of doxorubicin increased the p53 expression in NCI-H358, A549, and HCC827 cells and, interestingly, induced the expression of p53 in NCI-H1299 cells (). Moreover, we found that p53 siRNA-transfected NCI-H1299 cells became more sensitive to doxorubicin, while downregulation of p53 in other three cell lines led to no significant changes in their cellular responses to doxorubicin treatment (). CCK-8 assay demonstrated that TopBP1 knockdown increased the sensitivity of NCI-H1299 (), NCI-H358, A549, and HCC827 cells () to doxorubicin. These data suggested that TopBP1 was involved in NSCLC cell sensitivity to doxorubicin.
Figure 3 TopBP1 was involved in the chemoresistance of tumor cells.
Abbreviations: TopBP1, topoisomerase IIβ binding protein 1; siRNA, small interfering RNA.
Downregulation of TopBP1 sensitized NCI-H1299 cells to doxorubicin
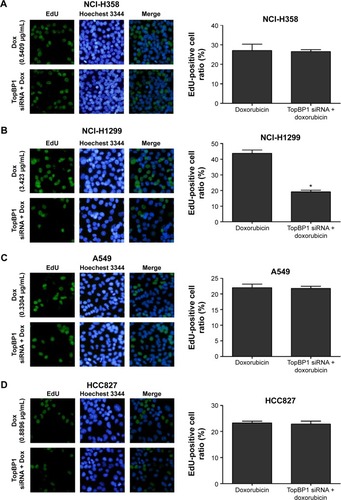
We further performed EdU incorporation assay to measure the proliferation of lung cancer cells after transfection with TopBP1 siRNA. Results showed that TopBP1 downregulation led to no significant differences in the proliferation of NCI-H358 (), A549 (), and HCC827 () cells in the presence of doxorubicin. However, the inhibitive effect of doxorubicin was increased in TopBP1 siRNA-transfected NCI-H1299 cells compared with doxorubicin treatment alone ().
Figure 4 Measurement of cell proliferation in TopBP1 siRNA-transfected lung cancer cells.
Abbreviations: TopBP1, topoisomerase IIβ binding protein 1; siRNA, small interfering RNA.
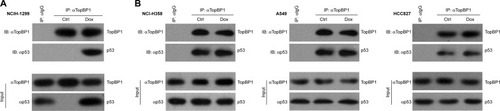
Doxorubicin-induced p53 was mediated by TopBP1 in NSCLC cells
Finally, co-immunoprecipitation was performed to investigate the relationship between TopBP1 and doxorubicin-induced p53. It was demonstrated that TopBP1 was involved in the p53 induction after doxorubicin administration in NCI-H1299 cells (). However, there are little changes in p53 levels in NCI-H358, A549, and HCC827 cells (). These results showed that the drug resistance was regulated by TopBP1-dependent p53 induction by doxorubicin in NCI-H1299 cells.
Figure 5 Upregulation of p53 by topoisomerase IIβ binding protein 1 (TopBP1) in NCI-H1299 cells.
Abbreviations: Ctrl, control; Dox, doxorubicin; TopBP1, topoisomerase IIβ binding protein 1.
Discussion
Currently, chemotherapy serves as an important component of postoperative treatment for NSCLC. However, development of drug resistance to the conventional chemotherapeutics has become a major challenge to successful chemotherapy.Citation20,Citation21 Thus, it is urgent that we find novel therapeutic targets involved in the acquisition of drug resistance. In this study, we demonstrated that TopBP1 modulated the chemoresistance to doxorubicin in NSCLC cells. It is noteworthy to mention that, to our knowledge, this study was the first to demonstrate the therapeutic potential of TopBP1 in lung cancer.
TopBP1 has been shown to be involved in DNA damage response, chromosome replication, and transcription.Citation10–Citation13 Depletion of TopBP1 led to embryonic lethality at the peri-implantation stage and cellular senescence in primary cells.Citation22,Citation23 In breast cancer, Liu et al observed overexpression of TopBP1 in tumor tissues and demonstrated that those patients with higher expression of TopBP1 had shorter overall survival time than those without overexpression of TopBP1.Citation14 In a recent study, the expression of TopBP1 was found to be upregulated in cancer cells and depletion of TopBP1 enhanced the sensitivity to radiation in radio-resistant lung cancer cells.Citation24 Our study found that NCI-H1299 cells exhibited the lowest sensitivity to doxorubicin among four lung cancer cell lines. Interestingly, Western blot analysis showed that the protein level of TopBP1 was higher in NCI-H1299 cells compared with NCI-H358, A549, and HCC827 cells. Thus, it was hypothesized that enhanced expression of TopBP1 might be involved in the chemoresistance to doxorubicin. To test such a hypothesis, the TopBP1 expression was decreased via transfection of TopBP1 siRNA into NCI-H1299 cells. Consequently, TopBP1 knockdown in NCI-H1299 cells increased the sensitivity to doxorubicin. These results suggested that TopBP1 was a critical modulator in the acquired chemoresistance of lung cancer cells.
The tumor suppressor p53 is a transcription factor that responds to various types of cellular stress, such as oncogene activation and genotoxic drug-induced DNA damage.Citation25,Citation26 p53 regulates a variety of cellular functions, including DNA repair, cell cycle arrest, and apoptosis.Citation27–Citation29 Abnormal expression of p53 is found in nearly all types of cancers, and p53 mutations are associated with resistance to chemotherapeutics and poor patient prognosis.Citation30–Citation33 In our study, the p53-negative NCI-H1299 cells displayed more doxorubicin resistance than three other cell lines with p53 expression. In order to explore the role of p53 in TopBP1-modulated chemosensitivity, we examined TopBP1 and p53 expression with or without doxorubicin treatment. Our data showed that doxorubicin administration resulted in upregulation of p53 in NCI-H358, A549, and HCC827 cells. Interestingly, we found that the addition of doxorubicin led to p53 induction in NCI-H1299 cells. These results implied that, contrary to the tumor suppressive effect of p53 originally expressed in cells, doxorubicin-induced p53 probably contributed to drug resistance in lung cancer cells. In addition, co-immunoprecipitation assay showed that TopBP1 mediated the doxorubicin-induced p53 expression in NCI-H1299 cells, but not in NCI-H358, A549, and HCC827 cells.
Conclusion
In conclusion, our study for the first time demonstrated that TopBP1 is a target whose inhibition restores the sensitivity to doxorubicin in p53-null tumor cells. Furthermore, TopBP1 contributes to the drug resistance though regulation of doxorubicin-induced p53. Therefore, targeting TopBP1, in combination with chemotherapy, may represent a novel strategy for the treatment of chemotherapy-resistant tumors.
Acknowledgments
This research was supported by the Science Technology Department of Zhejiang Province Welfare projects: role and mechanism of TopBP1 on chemotherapy resistance in non-small cell lung cancer (2013C33100), the National 973 Basic Research Program of China (2013CB911303), Zhejiang Natural Science Foundation: identification and application of new molecular targets phlpp2 of lung cancer (Y15H160213), National Natural Science Foundation of China (81200662), and the Zhejiang Natural Science Foundation (LY14H120004).
Supplementary materials
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRMaJZouZJemalACancer statistics, 2014CA Cancer J Clin201464192924399786
- WangYYLinSXYangGQLiuHCSunDNWangYSClinical efficacy of CyberKnife combined with chemotherapy and hyperthermia for advanced non-small cell lung cancerMol Clin Oncol20131352753024649205
- ImaiHShukuyaTYoshinoREfficacy and safety of platinum combination chemotherapy re-challenge for relapsed patients with non-small-cell lung cancer after postoperative adjuvant chemotherapy of cisplatin plus vinorelbineChemotherapy201359430731324480845
- PerolMChouaidCPerolDRandomized, phase III study of gemcitabine or erlotinib maintenance therapy versus observation, with predefined second-line treatment, after cisplatin-gemcitabine induction chemotherapy in advanced non-small-cell lung cancerJ Clin Oncol201230283516352422949150
- OhashiRTakahashiFCuiRInteraction between CD44 and hyaluronate induces chemoresistance in non-small cell lung cancer cellCancer Lett2007252222523417276588
- YangHWangWZhangYThe role of NF-E2-related factor 2 in predicting chemoresistance and prognosis in advanced non-small-cell lung cancerClin Lung Cancer201112316617121663859
- PallisAGSyrigosKNEpidermal growth factor receptor tyrosine kinase inhibitors in the treatment of NSCLCLung Cancer201380212013023384674
- WrightRHDornanESDonaldsonMMMorganIMTopBP1 contains a transcriptional activation domain suppressed by two adjacent BRCT domainsBiochem J2006400357358216984230
- YamaneKKawabataMTsuruoTA DNA-topoisomerase-II-binding protein with eight repeating regions similar to DNA-repair enzymes and to a cell-cycle regulatorEur J Biochem199725037947999461304
- RappasMOliverAWPearlLHStructure and function of the Rad9-binding region of the DNA-damage checkpoint adaptor TopBP1Nucleic Acids Res201139131332420724438
- GarciaVFuruyaKCarrAMIdentification and functional analysis of TopBP1 and its homologsDNA Repair (Amst)20054111227123915897014
- KimJEMcAvoySASmithDIChenJHuman TopBP1 ensures genome integrity during normal S phaseMol Cell Biol20052524109071091516314514
- YamaneKTsuruoTConserved BRCT regions of TopBP1 and of the tumor suppressor BRCA1 bind strand breaks and termini of DNAOncogene199918375194520310498869
- LiuKBellamNLinHYRegulation of p53 by TopBP1: a potential mechanism for p53 inactivation in cancerMol Cell Biol200929102673269319289498
- LiuKLuoYLinFTLinWCTopBP1 recruits Brg1/Brm to repress E2F1-induced apoptosis, a novel pRb-independent and E2F1-specific control for cell survivalGenes Dev200418667368615075294
- LiuKLinFTRuppertJMLinWCRegulation of E2F1 by BRCT domain-containing protein TopBP1Mol Cell Biol20032393287330412697828
- HeroldSWanzelMBeugerVNegative regulation of the mammalian UV response by Myc through association with Miz-1Mol Cell200210350952112408820
- CescuttiRNegriniSKohzakiMHalazonetisTDTopBP1 functions with 53BP1 in the G1 DNA damage checkpointEMBO J201029213723373220871591
- ImbrianoCGnesuttaNMantovaniRThe NF-Y/p53 liaison: well beyond repressionBiochim Biophys Acta20111825213113922138487
- ZhangWFengMZhengGChemoresistance to 5-fluorouracil induces epithelial-mesenchymal transition via up-regulation of Snail in MCF7 human breast cancer cellsBiochem Biophys Res Commun2012417267968522166209
- WuFLiJJangCWangJXiongJThe role of Axl in drug resistance and epithelial-to-mesenchymal transition of non-small cell lung carcinomaInt J Clin Exp Pathol20147106653666125400744
- BangSWKoMJKangSHuman TopBP1 localization to the mitotic centrosome mediates mitotic progressionExp Cell Res20113177994100421291884
- JeonYKoELeeKYTopBP1 deficiency causes an early embryonic lethality and induces cellular senescence in primary cellsJ Biol Chem201128675414542221149450
- ChoiSHYangHLeeSHKiJHNamDHYooHYTopBP1 and Claspin contribute to the radioresistance of lung cancer brain metastasesMol Cancer20141321125216549
- ManfrediJJThe Mdm2-p53 relationship evolves: Mdm2 swings both ways as an oncogene and a tumor suppressorGenes Dev201024151580158920679392
- MarteBCancer: super p53Nature2002420691327912447427
- RileyTSontagEChenPLevineATranscriptional control of human p53-regulated genesNat Rev Mol Cell Biol20089540241218431400
- Garcia-CaoIGarcia-CaoMMartin-CaballeroJ“Super p53” mice exhibit enhanced DNA damage response, are tumor resistant and age normallyEMBO J200221226225623512426394
- VogelsteinBLaneDLevineAJSurfing the p53 networkNature2000408681030731011099028
- SoussiTBeroudCAssessing TP53 status in human tumours to evaluate clinical outcomeNat Rev Cancer20011323324011902578
- PapadogianniDSoulitzisNDelakasDSpandidosDAExpression of p53 family genes in urinary bladder cancer: correlation with disease aggressiveness and recurrenceTumour Biol20143532481248924213852
- ShetzerYSolomonHKoifmanGMolchadskyAHoreshSRotterVThe paradigm of mutant p53-expressing cancer stem cells and drug resistanceCarcinogenesis20143561196120824658181
- KimCWLuJNGoSIp53 restoration can overcome cisplatin resistance through inhibition of Akt as well as induction of BaxInt J Oncol20134351495150223970333