Abstract
Golimumab (Simponi®) is a fully human tumor necrosis factor α inhibitor (TNFi) antibody administered subcutaneously. In the European Union, golimumab is indicated for the treatment of adults with severe, active axial spondyloarthritis (axSpA), which includes both ankylosing spondylitis (AS) and nonradiographic axSpA (nr-axSpA). In the US, it is indicated for the treatment of adults with active AS only. This article reviews the efficacy and tolerability of golimumab in nr-axSpA patients compared to other TNFi agents (adalimumab, infliximab, etanercept, and certolizumab pegol). In one ongoing, well-designed controlled study (GO-AHEAD), data at 16 weeks showed that treatment with golimumab (50 mg every 4 weeks) was effective in improving the clinical signs and symptoms of disease in nr-axSpA patients. In addition, 16 weeks of treatment with golimumab reduced inflammation in the sacroiliac joints and spine in patients with nr-axSpA. Moreover, objective evidence of active inflammation at baseline, such as a positive magnetic resonance imaging scan and/or an elevated CRP level, was a good predictor of treatment response to golimumab. Golimumab was generally well tolerated in this study, with a tolerability profile consistent with that seen in previous clinical trials for other indications. Although additional long-term data are needed, current evidence indicates that golimumab is an effective option for the treatment of nr-axSpA. However, in the absence of comparative head-to-head trials, there is no recommended hierarchy for the first prescription of a TNFi agent for the treatment of either nr-axSpA or AS.
Introduction
The Assessment in Spondyloarthritis International Society (ASAS) has recently developed new criteria for the classification of spondyloarthritis (SpA) with the aim of achieving earlier diagnosis and in the process has introduced the concept of predominantly axial versus peripheral disease.Citation1,Citation2 Patients presenting with persisting back pain for >3 months and an age of onset <45 years are classified as having axial SpA (axSpA) in the presence of either sacroiliitis (on radiographs or magnetic resonance imaging [MRI] scans) and at least one additional typical SpA feature (imaging arm) or HLA-B27 positivity and two additional SpA characteristics (clinical arm).Citation1 Depending on radiographic evidence indicating the presence or absence of permanent structural sacroiliac joint changes, patients are further classified as having either ankylosing spondylitis (AS) or nonradiographic axSpA (nr-axSpA).Citation3
More recently, the American College of Rheumatology, the Spondylitis Association of America, and the Spondyloarthritis Research and Treatment Network teamed up to develop recommendations for the treatment of axSpA, which includes both AS and nr-axSpA.Citation4 In adults with active AS and nr-axSpA, despite treatment with non-steroidal anti-inflammatory drugs, they recommend treatment with tumor necrosis factor α inhibitors (TNFis) over no treatment with TNFi.Citation4 However, in the USA, TNFis are indicated for the treatment of adults with active AS only, in accordance with the Food and Drug Administration decision not to approve TNFis for patients with nr-axSpA.Citation5 Indeed, the Food and Drug Administration expressed doubts about the specificity of the ASAS criteria and the natural history of nr-axSpA, hinting that these patients may remit spontaneously and thus would not require treatment with TNFis.Citation5 On the other hand, in the European Union (EU), TNFis are indicated for the treatment of adults with severe, active axSpA, which includes both AS and nr-axSpA. Importantly, TNFis, such as golimumab, are indicated for the treatment of adults with severe, active nr-axSpA but with objective signs of inflammation as evidenced by MRI scans and/or elevated CRP levels.Citation6
Many randomized, placebo-controlled clinical trials (RCTs)Citation7–Citation9 and retrospective studiesCitation10,Citation11 have demonstrated the efficacy of TNFis in the treatment of AS. Five TNFis are currently used in the treatment of AS, ie, adalimumab, certolizumab, etanercept, golimumab, and infliximab. Moreover, biosimilars of infliximab and etan-ercept are now available for treatment of AS.Citation12 The efficacy of these therapies has also been demonstrated in nr-axSpA patients in many RCTsCitation13–Citation18 and retrospective studies.Citation19,Citation20 In the EU, four TNFis are currently used in the treatment of nr-axSpA, ie, adalimumab, etanercept, certolizumab, and golimumab. TNFi biosimilar of etanercept is now available for treatment of nr-axSpA.
Golimumab – a human monoclonal antibody to TNF, which is administered subcutaneously at a dose of 50 mg every 4 weeks – is approved for treating active AS. The results of the double-blind, randomized, placebo-controlled, Phase III GO-RAISE study have been previously reported.Citation21 In that study, golimumab was evaluated in patients with active AS at week 24Citation21 and week 104Citation22 and on completion of the 5-year GO-RAISE trial.Citation23 Regarding nr-axSpA, the results of the double-blind, randomized, placebo-controlled, Phase III GO-AHEAD study have been recently reported.Citation18 In the EU, golimumab is the latest TNFi to have been approved for the treatment of nr-axSpA.Citation6
Objectives
The aim of this study was to highlight the benefits and risks of golimumab compared to adalimumab, infliximab, etanercept, and certolizumab, in the treatment of severe, active nr-axSpA.
Methods
Types of studies
We included RCTs.
Types of participants
We included studies of patients satisfying the ASAS classification criteria for nr-axSpA.Citation1 We did not apply any additional restrictions in studies with regard to past medication comprising other TNFis.
Types of interventions
The following interventions were selected for inclusion:
adalimumab versus placebo,
infliximab versus placebo,
etanercept versus placebo,
certolizumab versus placebo,
golimumab versus placebo.
The following interventions were excluded:
etanercept versus sulfasalazine (ESTHER study),Citation24
infliximab plus naproxen versus naproxen alone (INFAST study).Citation25
Selected outcome measures
The following criteria were selected as the major outcome measures for this review:
ASAS20,
ASAS40,
ASAS partial remission,
BASDAI50,
MRI evidence of sacroiliitis,
improvement in BASDAI,
improvement in Bath Ankylosing Spondylitis Functional Index (BASFI).
An ASAS40 response was defined as a ≥40% improvement together with an absolute improvement from baseline of ≥2 units (range 0–10) in ≥3 of the following four domains: patient global assessment of disease activity (0–10 cm visual analogue scale [VAS]), pain (total back pain, 0–10 cm VAS), function (BASFI, 0–10 cm VAS16), and inflammation/morning stiffness (mean score of items 5 and 6 of the BASDAI, 0–10 cm VAS), without any worsening in the remaining domain.
Other measures
Patients with and without objective signs of inflammation were also compared. Finally, the safety measurements reported in the selected studies were analyzed.
Results
Included studies
Six trials with seven eligible treatment arms were available for patients with nr-axSpA.Citation13–Citation18 Further details on the included studies are provided in . One hundred thirteen patients received adalimumab across two studies, which included the ABILITY-1 study.Citation13,Citation15 Twelve patients received infliximab in one study.Citation14 One hundred six patients received etanercept in one study.Citation16 A study on certolizumab included both AS and nr-axSpA patients. Only the data from the nr-axSpA patients were used for this review. One hundred six nr-axSpA patients received certolizumab in the two arms of the study (200 mg and 400 mg).Citation17 Ninety-eight patients received golimumab in the GO-AHEAD study.Citation18 Previous exposure to TNFi was not permitted in three studies,Citation15,Citation16,Citation18 whereas patients could have had TNFi prior to baseline in two studies.Citation14,Citation17 This information was not provided in the study performed by Haibel et al.Citation13
Table 1 Summary of all studies including patients with nr-axSpA treated with a TNFi
Participants
Most participants were Caucasian in their thirties. Further details on the included studies are provided in . The percentage of female participants ranged from 25% to 62.2% in the treatment groups and from 25% to 57% in the control groups (37.8% and 48%, respectively in the GO-AHEAD study). The mean age ranged from 29.5 years to 38 years in the treatment groups and from 28.2 years to 39.9 years in the control groups (30.7 years and 31.7 years, respectively in the GO-AHEAD study). Between 59% and 100% of the participants in the treatment groups were HLA-B27 positive, and a similar distribution was observed in the control groups (74%–100%) (82.7% and 82%, respectively in the GO-AHEAD study). The mean disease duration ranged from 1.4 years to 10.1 years in the treatment groups and from 1.1 years to 10.1 years in the control groups. No data were provided on mean disease duration in the GO-AHEAD study, but 68.4% of the participants in the golimumab group and 65% of the participants in the control group had a disease duration <1 year from diagnosis. The mean CRP ranged from 5 mg/L to 15 mg/L in the treatment groups and from 6.4 mg/L to 13 mg/L in the control groups (15 mg/L and 13 mg/L, respectively, in the GO-AHEAD study).
Disease activity at baseline
Further details on the included studies are provided in . All of the studies reported BASFI, BASDAI, and MRI evidence of sacroiliitis at baseline. The mean BASDAI ranged from 5.9 to 6.7 in the treatment groups and from 5.8 to 6.5 in the control groups (6.6 and 6.4, respectively, in the GO-AHEAD study). Between 50.5% and 100% of the participants in the treatment groups were MRI positive for sacroiliitis, and a similar distribution was observed in the control groups (45.7%–100%) (67.3% and 66%, respectively in the GO-AHEAD study).
Table 2 Summary of all studies including patients with axSpA treated with a TNFi
Major outcomes
Further details on the included studies are provided in . The primary outcome was the ASAS20 response in two studies,Citation17,Citation18 which included the GO-AHEAD study, and the ASAS40 response in three studies.Citation13,Citation15,Citation16 In Barkham et al,Citation14 the primary outcome was the change in the total MRI score for sacroiliitis between baseline and week 16.
Table 3 Summary of all studies including patients with nonradiographic axial spondyloarthritis treated with TNFi: ASAS20, ASAS40, ASAS PR, and BASDAI50 responses in patients with nonradiographic axial spondyloarthritis
Five studiesCitation13,Citation15–Citation18 reported an ASAS20 response after 12 weeks or 16 weeks of treatment with TNFi. In all studies, when TNFis were compared with placebo, statistically significant improvements in ASAS20 response were found (71.1% versus 40% in the GO-AHEAD study). In the golimumab versus placebo intervention, the percentage of patients in the placebo group who achieved an ASAS20 response was relatively high (40% at 16 weeks), and the reason for this is unclear.
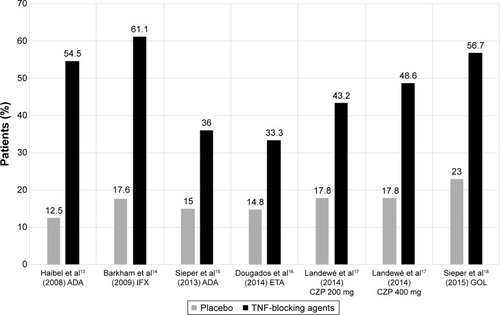
All studiesCitation13–Citation18 reported an ASAS40 response after 12 weeks or 16 weeks of treatment with TNFi. In all studies, when TNFis were compared with placebo, statistically significant improvements in ASAS40 response were found () (56.7% versus 23% in the GO-AHEAD study).
Figure 1 ASAS40 response in nr-axSpA patients after 12 (or 16) weeks of treatment with TNF-blocking agents.
Abbreviations: ADA, adalimumab; ASAS, Assessment in Spondyloarthritis International Society; CZP, certolizumab; ETA, etanercept; GOL, golimumab; IFX, infliximab; nr-axSpA, nonradiographic axial spondyloarthritis; TNF, tumor necrosis factor.
Five studiesCitation13,Citation15–Citation18 reported an ASAS partial remission after 12 weeks or 16 weeks of treatment with TNFi. In all studies, when TNFis were compared with placebo, statistically significant improvements in ASAS partial remission were found (33% versus 18% in the GO-AHEAD study).
Five studiesCitation13,Citation15–Citation18 reported a BASDAI50 response after 12 weeks or 16 weeks of treatment with TNFi. In all studies, when TNFis were compared with placebo, statistically significant improvements in BASDAI50 response were found (57.7% versus 30% in the GO-AHEAD study).
Four studiesCitation14–Citation16,Citation18 reported a change in the total MRI score for sacroiliitis between baseline and week 12 or week 16. In all studies, when TNFis were compared with placebo, statistically significant improvements in total MRI score for sacroiliitis were found. The mean change in the SPARCC (Spondyloarthritis Research Consortium of Canada) MRI SI joint score (0–72) revealed significant improvement in patients treated with golimumab (difference, golimumab versus placebo: −4.3; P<0.0001), adalimumab (difference, adalimumab versus placebo: −2.6; P=0.003), and etanercept (difference, etanercept versus placebo: −3.0; P<0.001). Moreover, the mean reduction in the total MRI score (0–24) from week 0 to week 16 was significantly greater in infliximab-treated patients compared with placebo-treated patients (P=0.033).
Data on improvement in BASDAI and BASFI were available in all the studies.Citation13–Citation18 In the GO-AHEAD study, the difference in golimumab versus placebo was −2.00 (−2.68, −1.35), with P<0.0001 for BASDAI, and −2.73 (−2.33, −1.13), with P<0.0001 for BASFI.
Comparison of patients with and without objective signs of inflammation
Patients with objective signs of inflammation (OSI) were defined by the presence of sacroiliitis as evidenced by MRI and/or an elevated CRP level. In patients with OSI, golimumab had a robust treatment effect: 76.9% of these patients achieved an ASAS20 response at week 16 compared with 37.5% in the placebo group. In patients without OSI, there were no differences in the efficacy end points (ASAS20 and ASAS40 responses), indicating that this subgroup of nr-axSpA patients may not be suitable candidates for treatment with golimumab, as was also recently shown for the treatment of nr-axSpA with adalimumab and etanercept.Citation15,Citation16
Safety
Overall, golimumab was well tolerated: the incidence of serious adverse effects and other significant AEs was comparable between patients treated with golimumab and those treated with placebo. No new safety signals were identified during the treatment of nr-axSpA with TNFi ().
Table 4 Summary of all studies including patients with non-radiographic axial spondyloarthritis treated with TNFi: AEs
Conclusion
These results demonstrated that golimumab, administered subcutaneously every 4 weeks, is efficacious and generally well tolerated in patients with nr-axSpA. Golimumab treatment led to a rapid reduction in the signs and symptoms of nr-axSpA, and this effect was sustained through week 16. Thus, objective evidence of active inflammation at baseline, such as a positive MRI scan and/or an elevated CRP level, seems to be a good predictor of treatment response to TNFis.
Although additional long-term data are needed, and current evidence indicates that golimumab is an effective option for the treatment of nr-axSpA. However, in the absence of comparative head-to-head trials, there is no recommended hierarchy for the first prescription of a TNFi agent for the treatment of either nr-axSpA or AS.
Perspectives
Recently, Deodhar et alCitation26 highlighted that AS and nr-axSpA represent the ends of the spectrum of axSpA, which should be considered as a single process, and proposed that RCTs designed to obtain regulatory approval for drugs be conducted in patients across the entire spectrum of axSpA. Furthermore, if the aim of a clinical trial is to treat patients with axSpA early in the course of their disease, the duration of symptoms should be more important inclusion criteria than the presence (AS) or absence of definite sacroiliitis (nr-axSpA).
Another challenge would be to investigate whether patients with nr-axSpA can achieve a state of drug-free remission. Indeed, the European Medical Agency has requested a “withdrawal” trial design, in which patients with nr-axSpA in remission for a predefined duration of time are randomized to receive either placebo or active study agent.
Disclosure
The authors report no conflicts of interest in this work.
References
- RudwaleitMLandewéRvan der HeijdeDThe development of assessment of SpondyloArthritis International Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisalAnn Rheum Dis20096877077619297345
- RudwaleitMvan der HeijdeDLandewéRThe Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in generalAnn Rheum Dis201170253121109520
- SieperJvan der HeijdeDNon-radiographic axial spondyloarthritis: new definition of an old disease?Arthritis Rheum20136554355123233285
- WardMMDeodharAAklEAAmerican College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 Recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondy-loarthritisArthritis Rheumatol20166828229826401991
- DeodharAReveilleJDvan den BoschFThe concept of axial spondyloarthritis: joint statement of the spondyloarthritis research and treatment network and the assessment of SpondyloArthritis international society in response to the US Food and Drug Administration’s comments and concernsArthritis Rheumatol2014662649265625154344
- European Medicines Agency [webpage on the Internet]Committee for Medicinal Products for Human Use2015Summary of opinion (post authorisation): Simponi (golimumab); 2015 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion/human/000992/WC500187055.pdfAccessed May 21, 2015
- van der HeijdeDDijkmansBGeusensPAnkylosing spondylitis study for the evaluation of recombinant infliximab therapy study group. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT)Arthritis Rheum20055258259115692973
- van der HeijdeDKivitzASchiffMHATLAS Study GroupEfficacy and safety of adalimumab in patients with ankylosing spondyli-tis: results of a multicenter, randomized, double-blind, placebo-controlled trialArthritis Rheum2006542136214616802350
- CalinADijkmansBAEmeryPOutcomes of a multicentre randomised clinical trial of etanercept to treat ankylosing spondylitisAnn Rheum Dis2004631594160015345498
- LorenzinMOrtolanAFrallonardoPOlivieroFPunziLRamondaRPredictors of response and drug survival in ankylosing spondylitis patients treated with infliximabBMC Musculoskelet Disord20151616626205000
- SpadaroALubranoEMarchesoniARemission in ankylosing spondylitis treated with anti-TNF-α drugs: a national multicentre studyRheumatology (Oxford)2013521914191923878312
- ParkWYooDHJaworskiJComparable long-term efficacy, as assessed by patient-reported outcomes, safety and pharmacokinetics, of CT-P13 and reference infliximab in patients with ankylosing spondylitis: 54-week results from the randomized, parallel-group PLANETAS studyArthritis Res Ther2016182526795209
- HaibelHRudwaleitMListingJEfficacy of adalimumab in the treatment of axial spondylarthritis without radiographically defined sacroiliitis: results of a twelve-week randomized, double-blind, placebo-controlled trial followed by an open-label extension up to week fifty-twoArthritis Rheum2008581981199118576337
- BarkhamNKeenHICoatesLCClinical and imaging efficacy of infliximab in HLA-B27-positive patients with magnetic resonance imaging-determined early sacroiliitisArthritis Rheum20096094695419333933
- SieperJvan der HeijdeDDougadosMEfficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis: results of a randomised placebo-controlled trial (ABILITY-1)Ann Rheum Dis20137281582222772328
- DougadosMvan der HeijdeDSieperJSymptomatic efficacy of etanercept and its effects on objective signs of inflammation in early nonradiographic axial spondyloarthritis: a multicenter, randomized, double-blind, placebo-controlled trialArthritis Rheumatol2014662091210224891317
- LandewéRBraunJDeodharAEfficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled phase 3 studyAnn Rheum Dis201473394724013647
- SieperJvan der HeijdeDDougadosMA randomized, double-blind, placebo-controlled, sixteen-week study of subcutaneous golimumab in patients with active nonradiographic axial spondyloarthritisArthritis Rheumatol2015672702271226139307
- LubranoEPerrottaFMMarchesoniARemission in nonradiographic axial spondyloarthritis treated with anti-tumor necrosis factor-α drugs: an Italian multicenter studyJ Rheumatol20154225826325512483
- CorliJFlipoRMPhilippePTumor necrosis factor-α inhibition in ankylosing spondylitis and nonradiographic axial spondyloarthritis: treatment response, drug survival, and patient outcomeJ Rheumatol2015422376238226568593
- InmanRDDavisJCJrHeijdeDVEfficacy and safety of goli-mumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trialArthritis Rheum2008583402341218975305
- van der HeijdeDDeodharABraunJGO-RAISE InvestigatorsThe effect of golimumab therapy on disease activity and health-related quality of life in patients with ankylosing spondylitis: 2-year results of the GO-RAISE trialJ Rheumatol2014411095110324737912
- DeodharABraunJInmanRDGolimumab administered subcutaneously every 4 weeks in ankylosing spondylitis: 5-year results of the GO-RAISE studyAnn Rheum Dis20157475776125387477
- SongIHWeißAHermannKGSimilar response rates in patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis after 1 year of treatment with etanercept: results from the ESTHER trialAnn Rheum Dis20137282382523172749
- SieperJLenaertsJWollenhauptJEfficacy and safety of infliximab plus naproxen versus naproxen alone in patients with early, active axial spondyloarthritis: results from the double-blind, placebo-controlled INFAST study, part 1Ann Rheum Dis20147310110723696633
- DeodharAStrandVKayJBraunJThe term non-radiographic axial spondyloarthritis is much more important to classify to diagnose patient with axial spondyloarthritisAnn Rheum Dis20167579179426768406
