Abstract
Aim
The purpose of this study was to investigate the protective effect of honokiol on experimental ischemia/reperfusion injury of rat ovary.
Materials and methods
A total of 40 female Wistar albino rats were used in this study. The rats were divided into five groups as follows: sham (Group I), torsion (Group II), torsion + detorsion (Group III), torsion + detorsion + saline (Group IV), and torsion + detorsion + honokiol (Group V). Bilateral adnexa in all the rats except for those in the sham group were exposed to torsion for 3 hours. The rats in Group IV were administered saline, whereas the rats in Group V were administered honokiol by intraperitoneal route 30 minutes before detorsion. Tissue and plasma concentrations of malondialdehyde and nitric oxide were determined. Ovarian tissue was histologically evaluated. Data analyses were performed by means of Kruskal–Wallis test and Mann–Whitney U-test (Bonferroni correction) in SPSS 15.0 (Statistical Package for Social Sciences; SPSS Inc., Chicago, IL, USA).
Results
The torsion and detorsion groups had higher scores in vascular congestion, hemorrhage, and inflammatory cell infiltration compared with the sham group (P<0.005). In addition, total histopathological scores were significantly higher in the torsion and detorsion groups compared with the sham group (P<0.005). A significant reduction was observed in hemorrhage, inflammatory cell infiltration, and cellular degeneration scores, of all histopathological scores, in the honokiol group (P<0.005). Ovarian tissue concentrations of malondialdehyde were significantly higher in the torsion and detorsion groups compared with the sham and honokiol groups (P<0.005). Ovarian tissue concentrations of nitric oxide, on the other hand, were significantly higher in the torsion group compared with the sham, saline, and honokiol groups (P<0.005).
Conclusion
Honokiol has a beneficial effect on ovarian torsion-related ischemia/reperfusion injury.
Introduction
Ovarian torsion is a rare but important gynecologic surgical emergency which most commonly occurs in the first three decades of life.Citation1 It might well occur in normal ovaries; however, it is usually associated with the (known) already existent tubal/ovarian pathologies.Citation2 Detorsion might be considered as soon as the diagnosis of ovarian torsion is established in order to restore the ovarian blood supply with a surgical intervention. This way, potential necrosis can be avoided.Citation3,Citation4 However, the recovery of ovarian circulation after detorsion might cause a pathologic process called ischemia/reperfusion (I/R) injury characterized by oxidative stress, leading to deteriorated tissue damage.Citation5 The causes of I/R injury of the ovary include elevated concentrations of nitric oxide (NO), apoptosis, neutrophil and thrombocyte activation, and free radical and cytokine release.Citation6–Citation9
In the post-I/R period, reactive oxygen species (ROS) are produced in large amounts, and antioxidant defense mechanisms of the body are undermined.Citation10 Increased production of ROS impairs the DNA chain, causes denaturation of proteins containing enzymes and ion channels, and does cellular damage through lipid membrane peroxidation.Citation11 Malondialdehyde (MDA) is the basic product of polyunsaturated fatty acid peroxidation and is quite a toxic molecule. Therefore, it is used to determine in vivo and in vitro oxidative stress levels.Citation12 NO, which is produced by normal endothelium, is the main determinant of normal endothelial and vascular functions. In case of inflammation, overproduction of NO, together with ROS, causes oxidative stress.Citation13 Honokiol is a natural biphenolic compound that is isolated from magnolia bark and has been used in the traditional Chinese and Japanese medicine as an antibacterial, antiemetic, antidepressant, antithrombotic, and anxiolytic agent. Studies have shown therapeutic effects including antioxidative and anti-inflammatory activities for honokiol.Citation14–Citation19 The effect of honokiol on ovarian torsion-related I/R injury has not been investigated earlier. The purpose of this study was to investigate the protective effect of honokiol on I/R injury in an experimental rat ad nexal torsion model.
Materials and methods
The protocol of this study was approved by the Dicle University Ethics Committee of Animal Rights (DÜHADEK). All animal-handling procedures were performed according to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and followed the guidelines of the Animal Welfare Act. A total of 40 female Wistar albino rats weighing between 220 g and 250 g were used in this study. The rats, which were given sufficient amount of water and food, were maintained at proper humidity and temperature in 12-hour light–dark cycle for at least 7 days. They were randomized into five groups, each consisting of eight rats as follows:
Group I: sham (n=8)
Group II: torsion (n=8)
Group III: torsion + detorsion (n=8)
Group IV: torsion + detorsion + saline (n=8)
Group V: torsion + detorsion + honokiol (n=8)
Numbers of rats and groups were noted by one of the researchers. The researchers who performed biochemical and histopathological analyses were not provided information about randomization until the end of the study.
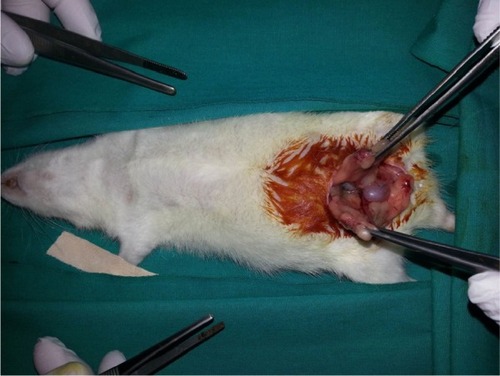
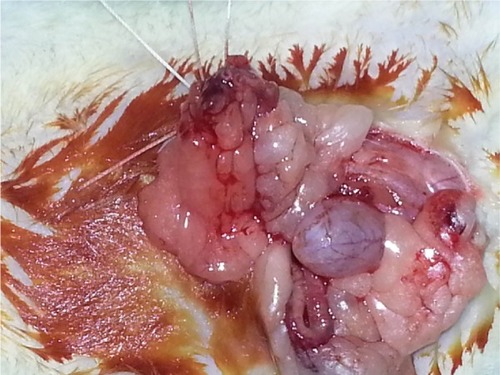
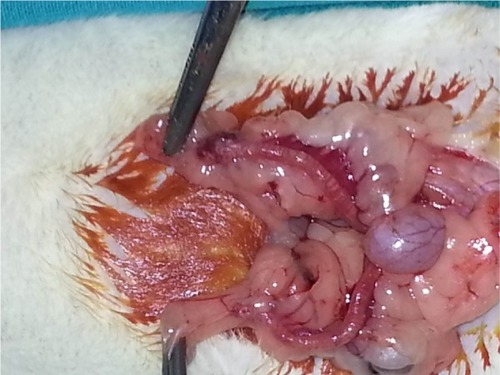
Each rat was weighed and anesthetized with intramuscular ketamine hydrochloride (50 mg/kg Ketalar; Eczacibasi, Istanbul, Turkey) and xylazine hydrochloride (10 mg/kg Rompun; Bayer AG, Leverkusen, Germany). Then, laparotomy was performed with a 2.5 cm midline abdominal incision after sufficient sterile conditions were ensured. In the sham group (Group I), uterine horns and ad nexa were exposed to nearly 1 minute and abdominal wall was closed with 3/0 silk sutures (sham operation). Three hours later, relaparotomy was performed in this group, and both ovaries were surgically removed. Adnexal torsion was performed as follows: adnexa containing tuba and ovarian tissues were twisted 360° clockwise and fixed to abdominal wall.Citation20 In the torsion group (Group II), relaparotomy was performed and both ovaries were surgically removed after 3 hours of ischemia. In the torsion + detorsion group (Group III), bilateral adnexal torsion (3 hours of ischemia) and bilateral detorsion (3 hours of reperfusion) were performed. Both ovaries were surgically removed after a total of 6 hours. In the torsion + detorsion + saline group (Group IV), 2 mL of saline was injected by intraperitoneal route 30 minutes before detorsion. Then, relaparotomy was performed for the purpose of detorsion in bilateral adnexa, and the incision was sutured. Both ovaries were surgically removed after 3 hours of reperfusion ( and ).
In the torsion + detorsion + honokiol group (Group V), honokiol (25 mg, Honokiol; Sigma-Aldrich Co., St Louis, MO, USA; honokiol dose: 2 mg of honokiol was dissolved in 2 mL of dimethylsulfoxide) was injected by intraperitoneal route 30 minutes before detorsion. Then, relaparotomy was performed for the purpose of detorsion in bilateral adnexa, and the incision was sutured. Both ovaries were surgically removed after 3 hours of detorsion (). Intracardiac blood was taken from all the groups after completion of surgical procedures, and the rats were sacrificed. One of the ovaries that was removed was stored in a deep freezer at −80°C for biochemical analysis. The other ovary, on the other hand, was transferred to the laboratory in enumerated vessels containing 10% formaldehyde for histopathological examination. Blood specimens were sent to the biochemistry laboratory under proper conditions for biochemical analysis.
Histologic evaluation
Ovarian tissues were fixed in 10% neutral buffered formalin solution for 48 hours and were dehydrated, cleared, and embedded in paraffin, as usual. Serial tissue sections, which were 4 μm thick, were cut using a microtome (Leica RM2125RTS) and stained with hematoxylin and eosin for general morphological observation. All the sections were examined with a light microscope (Olympus BX43), and the pieces were photographed. At least five microscopic regions were examined to score the specimens semiquantitatively. Criteria for ovarian injury were as follows: follicular cell degeneration (granulosa cells), vascular congestion, hemorrhage, and inflammation (neutrophil infiltration). Each specimen was scored for each criterion using a scale ranging from 0 to 3 (0, none; 1, mild; 2, moderate; 3, severe).Citation21 Ovary sections were analyzed in a blinded fashion by the same pathologist.
Biochemical analyses
Blood specimens were centrifuged at 3,000 rpm for nearly 10 minutes at +4°C to obtain plasma. Plasma specimens that were obtained were analyzed for MDA and NO. Ovarian tissue homogenates were prepared at +4°C to measure MDA and NO concentrations. Tissues were weighed and sliced into small pieces. Then, they were homogenized with a homogenizer (İKA®Ultra-Turrax® T8 dispersing homogenizator; IKA-Werke GmbH, Staufen, Germany) using an ice cold solution of 10× phosphate-buffered saline (50 mM/L, Ph 7.0). Homogenates were centrifuged at 15,000 rpm for nearly 10 minutes at +4°C to obtain supernatant. Supernatant specimens were used to measure MDA and NO concentrations.
MDA measurements
MDA concentrations in the specimens were determined by means of a predefined methodCitation22 as follows: 0.5 mL specimen (plasma/homogenate) was taken by pipette to a 10 mL centrifuge tube containing a solution of 1.0 mL of thiobarbituric acid (0.6%) and 2.5 mL of trichloroacetic acid (20%). Tubes were heated in boiling water bath for 30 minutes. Then, the reaction mix was cooled down in ten ice baths following the addition of 4.0 mL of n-butanol. Tubes were vortex mixed and centrifuged at 3,000 rpm for 10 minutes. Absorbance of the organic layer was measured at 535 nm.
NO measurements
The production of NO was determined by indirectly measuring nitrite concentrations based on the Griess reaction.Citation23 At the beginning, specimens were deproteinized with 75 mmol/L ZnSO4. Following cleanup, equal volumes of specimens were treated with a copper–cadmium mixture in glycine buffer at pH 9.7 in an effort to reduce nitrate to nitrite. This way, nitrite concentrations in the specimens showed total nitrate + nitrite. In Griess reaction, a chromophore with a strong absorbance at 545 nm was formed by the reaction of nitrite with a mixture of sulphanilamide and naphthalenediamine.
Statistical analyses
Data analyses were performed by SPSS 15.0 (SPSS Inc.), and mean and standard deviations were calculated. Whether there were differences between the groups or not was determined by means of Kruskal–Wallis test. Pairwise comparisons were made using Mann–Whitney U-test. For measures between the five different groups, Mann–Whitney U-test (Bonferroni correction) was used (as a post hoc test). Results were considered statistically significant at P-value <0.005. Bonferroni correction was used for repeated measures, and the predefined significance level (P<0.05) was reset at P<0.005 after correction.
Results
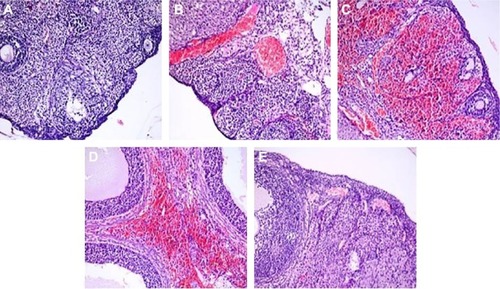
Histopathologic scores for all five groups are demonstrated in . The torsion and detorsion groups had higher scores in vascular congestion, hemorrhage, and inflammatory cell infiltration compared with the sham group (P<0.005). The mean cellular degeneration score, which indicated acute tissue damage, was significantly higher in the detorsion group compared with the sham group (). The total histologic evaluation score, which was obtained when all four ovarian damage parameters were added together, was significantly higher in the torsion and detorsion groups compared with the sham group (). The histologic ovarian morphologies for all the groups are demonstrated in .
Table 1 Histopathologic evaluation scores of rat ovarian tissues in all groups (n=8)
Figure 4 H&E-stained ovarian sections (×200).
Abbreviation: H&E, hematoxylin and eosin.
Plasma and ovarian tissue concentrations of MDA and NO are demonstrated in . Ovarian tissue concentrations of MDA were significantly higher in the torsion and detorsion groups compared with the sham and honokiol groups (P<0.005). On the one hand, ovarian tissue concentrations of NO were significantly higher in the torsion group compared with the sham, saline, and honokiol groups (). There was no significant difference between the groups in plasma concentrations of MDA. On the other hand, plasma concentrations of NO were significantly higher in the honokiol group compared with the detorsion group (P<0.005).
Table 2 MDA and NO concentrations in all groups (n=8)
Discussion
The purpose of this study was to investigate the changes in plasma and ovarian tissue concentrations of MDA and NO as well as histopathologic changes occurring in response to the honokiol treatment in a rat ovarian torsion/detorsion model. Ovarian torsion/detorsion leads to morphological and biochemical changes in the ovarian tissue caused by I/R injury. Ischemia is a restriction in blood supply to organs which results in elevated concentrations of lactic acid and hypoxanthine caused by lipid peroxide accumulation and adenosine triphosphate degradation.Citation25 Free oxygen radicals, which occur as a result of reperfusion injury, react with lipids, causing the formation of lipid peroxides. Lipid peroxidation impairs membrane functions and creates toxic substances such as aldehydes. Restriction in blood supply causes tissue hypoxia, resulting in the formation of lipid peroxides. MDA is a stable metabolite of the lipid peroxidation cascade. It is considerably elevated in I/R injury, which is believed to cause damages to the integrity and permeability of the cell wall.Citation25–Citation27 In their study on a rat ovarian I/R model, Ergun et al found that plasma and ovarian tissue concentrations of MDA were significantly higher in the torsion and detorsion groups compared with the sham group.Citation28 In this study, as well, MDA concentrations were measured in an effort to determine and verify the degree of reperfusion injury in plasma and ovarian tissue, and significantly higher ovarian tissue concentrations of MDA were found in the torsion and detorsion groups compared with the sham group. This finding shows that I/R causes lipid peroxidation in the ovarian tissue, leading to oxidative damage.
One of the most important findings of this study was the significant reduction in ovarian tissue concentrations of MDA in the honokiol group, which shows that honokiol might prevent oxidative damage in I/R injury of the ovary. On the other hand, the absence of a significant difference between the groups in plasma concentrations of MDA might have been caused by the short torsion duration.
During the reperfusion of ischemic tissue, increased production of peroxynitrite, NO, and ROS including hydrogen peroxide, hydroxyl radicals, and superoxide anions is observed. These free radicals cause cellular damage via lipid peroxidation in the mitochondrial and cellular membranes.Citation24–Citation28 In previous studies, significantly increased tissue and plasma concentrations of NO were shown after I/R injury.Citation27,Citation28 In this study, as well, ovarian tissue concentrations of NO were significantly higher in the torsion group compared with the sham, saline, and honokiol groups. However, plasma concentrations of NO were significantly lower in the detorsion group compared with the honokiol group. This paradoxical result might be explained by the prevention of NO consumption by ROS in the ovary after the administration of honokiol. The increase in NO concentrations might be of protective importance in ovarian torsion. In addition, the absence of a significant difference in the other groups in respect of plasma concentrations of NO might be explained by the short torsion duration.
The basic functions of the human ovary include the nurture, development, and release of a mature oocyte that is ready for fertilization and successful propagation of the species. In this process, the ovary assumes the following roles: 1) secreting steroid hormones that induce the development and growth of secondary sexual characteristics; 2) playing a key role in the orderly, repetitive cyclic ovulation; and 3) providing support for the successful uterine implantation and the early phase of pregnancy.Citation29 These functions are achieved by means of complex mechanisms under stable oxygen supply and consumption conditions requiring normal microcirculation. Oxidative damage induced by I/R might impair the ovarian functions. In a healthy individual, there is a balance between ROS and antioxidants. A shift in this balance in favor of ROS causes oxidative stress, which might affect the entire female reproductive life, even thereafter. ROS might affect a number of physiological processes, including oocyte maturation, fertilization, embryo development, and pregnancy.
There are a number of experimental drug studies on the protection of ovary from I/R injury.Citation20,Citation21,Citation28 Anti-inflammatory and antioxidant effects of honokiol were investigated in a number of studies.Citation15–Citation17,Citation30,Citation31 In a molecular study, honokiol was reported to block lipopolysaccharide-induced cytotoxicity and the production of cytotoxic cytokines such as interleukin-1β and tumor necrosis factor-α, NO, and ROS.Citation32 In addition, honokiol was shown to have a scavenging effect on both superoxide and peroxyl radicals.Citation33 Besides, chronic treatment with honokiol in hypertensive rats was reported to decrease blood pressure due to its vasorelaxant and antioxidant effects.Citation34 Furthermore, honokiol with its antioxidant effects was shown to provide protection against cerebral I/R injuries and dermatological disorders.Citation31,Citation35 In our study, we demonstrated the protective effects of honokiol on I/R injury in rat ovary. This is the first study in the literature to show the protective effects of honokiol on ovarian I/R injury. Ischemia that occurs in case of ovarian torsion causes oxidative damage. Even if the torsion is corrected, effects of this damage may persist. Therefore, early administration of such agents that could prevent oxidative damage or reverse the effects of the damage might prevent a potential future damage to the ovarian reserve, which might amount to fertility. Given the available clinical and experimental data, we are of the opinion that honokiol is a promising agent and a promising topic of investigation for future clinical trials.
The results of this study are short term. Therefore, we believe that there is a need for further studies to demonstrate the long-term results of honokiol on ovarian I/R injury as well as its fertility-preserving effects. Maybe, there should be clinical studies in humans to demonstrate the effects of honokiol on human ovary. We believe that such studies will shed light on the long-term protective effects of honokiol on ovarian follicular reserve and fertility.
In conclusion, honokiol can be administered prior to the treatment of ovarian torsion in order to prevent oxidative damage in I/R injury of the ovary. However, clinical studies are needed to verify the results of this study.
Acknowledgments
This research was funded by Dicle University Scientific Research Commission (grant number: 13-TF-100). The authors are grateful to the Department of Obstetrics and Gynecology and Department of Pathology, School of Medicine, Dicle University, for their valuable support.
Disclosure
The authors report no conflicts of interest in this work.
References
- HouryDAbbottJTOvarian torsion: a fifteen-year reviewAnn Emerg Med20013815615911468611
- McWilliamsGDHillMJDietrichCSGynecologic emergenciesSurg Clin North Am20088826528318381113
- ChenMChenCDYangYSTorsion of the previously normal uterine adnexa. Evaluation of the correlation between the pathological changes and the clinical characteristicsActa Obstet Gynecol Scand200180586111167190
- OelsnerGShasharDAdnexal torsionClin Obstet Gynecol20064945946316885653
- LiCJacksonRMReactive species mechanisms of cellular hypoxia-reoxygenation injuryAm J Physiol Cell Physiol2002282227241
- SahinFKCosarEKokenGToyHBasaraliKBuyukbasSProtective effect of aprotinin on ischemia-reperfusion injury in rat ovaryJ Obstet Gynecol Res20083479480018834336
- KurodaSSiesjoBKReperfusion damage following focal ischemia: pathophysiology and therapeutic windowsClin Neurosci199741992129186042
- LandWGThe role of postischemic reperfusion injury and other non-antigen dependent inflammatory pathways in transplantationTransplantation20057950515753838
- BakerGLCorryRJAutorAPOxygen free radical induced damage in kidneys subjected to warm ischemia and reperfusion: protective effect of superoxide dismutaseAnn Surg19852026286413840348
- McCordJMOxygen-derived free radicals in postischemic tissue injuryN Engl J Med19853121591632981404
- GutteridgeJFree radicals in disease processes: a compilation of cause and consequenceFree Radic Res Commun1993191411488244084
- Del RioDStewartAJPellegriniNA review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress?Nutr Metab Cardiovasc Dis20051531632816054557
- LubosEHandyDELoscalzoJRole of oxidative stress and nitric oxide in atherothrombosisFront Biosci2008135323534418508590
- HayashiTSaitoAOkunoSFerrand-DrakeMDoddRLChanPHDamage to the endoplasmic reticulum and activation of apoptotic machinery by oxidative stress in ischemic neuronsJ Cereb Blood Flow Metab200525415315678111
- LiouKTLinSMHuangSSChihCLTsaiSKHonokiol ameliorates cerebral infarction from ischemia-reperfusion injury in ratsPlanta Med20036913013412624817
- LiouKTShenYCChenCFTsaoCMTsaiSKHonokiol protects rat brain from focal cerebral ischemia-reperfusion injury by inhibiting neutrophil infiltration and reactive oxygen species productionBrain Res200399215916614625055
- LiouKTShenYCChenCFTsaoCMTsaiSKThe anti-inflammatory effect of honokiol on neutrophils: mechanisms in the inhibition of reactive oxygen species productionEur J Pharmacol2003475192712954355
- SheuMLLiuSHLanKHHonokiol induces calpain-mediated glucose-regulated protein-94 cleavage and apoptosis in human gastric cancer cells and reduces tumor growthPLoS One2007210e109617971859
- TseAKWanCKShenXLYangMFongWFHonokiol inhibits TNF-alpha-stimulated NF-kappaB activation and NF-kappaB-regulated gene expression through suppression of IKK activationBiochem Pharmacol2005701443145716181613
- SakMESoydincHESakSThe protective effect of curcumin on ischemia-reperfusion injury in rat ovaryInt J Surg20131196797023796447
- OzlerATurgutAGorukNYAlabalikUBasaraliMKAkdemirFEvaluation of the protective effects of CoQ10 on ovarian I/R Injury: an experimental studyGynecol Obstet Invest20137610010623886769
- YoshiokaTKawadaKShimadaTMoriMLipid peroxidation in maternal and cord blood and protective mechanism against active-oxygen toxicity in the bloodAm J Obstet Gynecol1979135372376484629
- CortasNKWakidNWDetermination of inorganic nitrate in serum and urine by a kinetic cadmium-reduction methodClin Chem199036440443
- SomuncuSCakmakMDikmenGAkmanHKayaMIschemia-reperfusion injury of rabbit ovary and protective effect of trapidil: an experimental studyPediatr Surg Int20082431531818060415
- AkgürFMKılıncKAktuğTReperfusion injury after detorsion of unilateral testicular torsionUrol Res1993213953998171761
- VuralHAksoyNOzbilgeHAlterations of oxidative-antioxidative status in human cutaneous leishmaniasisCell Biochem Funct20042215315615124179
- CardenDLGrangerDNPathophysiology of ischemia-reperfusion injuryJ Pathol200019025526610685060
- ErgunYKocADolapciogluKThe protective effect of erythropoietin and dimethylsulfoxide on ischemia-reperfusion injury in rat ovaryEur J Obstet Gynecol Reprod Biol201015218619020576345
- KaseNThe normal human ovary, part I. Reproductive and endocrine functionsAltchekADeligdishLKaseNGDiagnosis and Management of Ovarian DisordersSan Diego, CAElsevier Science200311
- HuangKHWengTIHuangHYHonokiol attenuates torsion/detorsion-induced testicular injury in rat testis by way of suppressing endoplasmic reticulum stress-related apoptosisUrology2012794967.e5967.e1122285174
- ShenJLManKMHuangPHHonokiol and magnolol as multifunctional antioxidative molecules for dermatologic disordersMolecules20101596452646520877235
- LeeSYChoJYInhibitory effects of honokiol on LPS and PMA-induced cellular responses of macrophages and monocytesBMB Rep200942957457919788858
- DikalovSLosikTArbiserJLHonokiol is a potent scavenger of superoxide and peroxyl radicalsBiochem Pharmacol200876558959618640101
- ZhangGSWangRJZhangHNZhangGPLuoMSLuoJDEffects of chronic treatment with honokiol in spontaneously hypertensive ratsBiol Pharm Bull201033342743120190404
- ZhangPLiuXZhuYChenSZhouDWangYHonokiol inhibits the inflammatory reaction during cerebral ischemia reperfusion by suppressing NF-κB activation and cytokine production of glial cellsNeurosci Lett20138534123127