Abstract
Background
Atopic dermatitis (AD) is one of the most common inflammatory cutaneous diseases. Thymic stromal lymphopoietin (TSLP) has been demonstrated to be an important immunologic factor in the pathogenesis of AD. The production of TSLP can be induced by a high level of intracellular calcium concentration and activation of the receptor-interacting protein 2/caspase-1/NF-κB pathway. Andrographolide (ANDRO), a natural bicyclic diterpenoid lactone, has been found to exert anti-inflammatory effects in gastrointestinal inflammatory disorders through suppressing the NF-κB pathway.
Objective
To explore the effect of ANDRO on the production of TSLP in human mast cells and AD mice model.
Methods
We utilized enzyme-linked immunosorbent assay, real-time reverse transcription polymerase chain reaction analysis, Western blot analysis, and immunofluorescence staining assay to investigate the effects of ANDRO on AD.
Results
ANDRO ameliorated the increase in the intracellular calcium, protein, and messenger RNA levels of TSLP induced by phorbol myristate acetate/calcium ionophore A23187, through the blocking of the receptor-interacting protein 2/caspase-1/NF-κB pathway in human mast cell line 1 cells. ANDRO, via oral or local administration, also attenuated clinical symptoms in 2,4-dinitrofluorobenzene-induced AD mice model and suppressed the levels of TSLP in lesional skin.
Conclusion
Taken together, ANDRO may be a potential therapeutic agent for AD through suppressing the expression of TSLP.
Introduction
Atopic dermatitis (AD) is one of the most common inflammatory cutaneous diseases, characterized by chronic and relapsing eczematous skin inflammation.Citation1 The pathophysiological mechanism of AD is not fully understood; however, increasingly literature has indicated that this disease is mediated by interactions between various cells and molecular mediators.Citation2–Citation4 Thymic stromal lymphopoietin (TSLP) was found to be an important immunologic factor in the pathogenesis of AD via enhancing the maturation of myeloid dendritic cells, which promoted the differentiation of naïve CD4+ T cells into inflammatory Th2 cells.Citation5 Acute and chronic AD patients had an enhanced expression of TSLP in skin lesions.Citation6 Previous studies reported that mast cells were activated and infiltrated in the skin lesions of AD, suggesting the important role of mast cells in the pathogenesis of AD.Citation7,Citation8 TSLP was proven to be expressed at higher levels in activated mast cells and triggered allergic inflammation.Citation9
Caspase-1 is the best characterized inflammatory caspase.Citation10 Quite unlike the role that most caspases play in apoptosis, caspase-1 is responsible for activating the inactivate precursor of interleukin (IL)-1β, which is vital for inflammation.Citation11 Receptor-interacting protein 2 (Rip2) is a caspase recruitment domain-containing kinase and interacts with caspase-1, thus activating the NF-κB signaling pathway, which has been identified to promote the transcription of TSLP gene in mast cells.Citation12,Citation13
Andrographolide (ANDRO), a natural bicyclic diterpenoid lactone, has been extracted and purified as the principal bioactive chemical ingredient from the herb Andrographis paniculata.Citation14 ANDRO is known to have various effects, such as antitumor,Citation15 anti-inflammatory,Citation16 and antidiabetic effects,Citation17 both in vitro and in vivo. In addition, ANDRO has been demonstrated to improve the efficacy of conventional therapy in the treatment of severe hand, foot, and mouth disease.Citation18 However, the effect of ANDRO on AD remains to be explored. Therefore, we studied the effects of ANDRO on phorbol myristate acetate/calcium ionophore A23187 (PMACI)-stimulated human mast cell line 1 (HMC-1 cells) in vitro and 2,4-dinitrofluorobenzene (DNFB)-induced AD-like lesional skin of mice in vivo.
Materials and methods
HMC-1 cell culture
HMC-1 cells (Cell Bank of the Chinese Academy of Science, Shanghai, People’s Republic of China) were cultured in Iscove’s Modified Dulbecco’s Medium (Thermo Fisher Scientific, Waltham, MA, USA) containing 10% fetal calf serum (FBS, HyClone) as well as 100 U/mL penicillin and 100 μg/mL streptomycin (Thermo Fisher Scientific). Cells were maintained in a humidified incubator at 37°C in the presence of 5% CO2.
Fluorescent measurements of the intracellular calcium level
The intracellular calcium level was determined as described previously.Citation19 Briefly, the HMC-1 cells (1×105) were incubated in a culture medium containing Fura-2/AM (Sigma-Aldrich Co., St Louis, MO, USA) for 30 minutes. Then the cells were washed twice with a calcium free medium containing 0.5 mM ethylene glycol tetraacetic acid (EGTA) (Sigma-Aldrich Co.). After being placed into a 96-well plate, the cells were pretreated with ANDRO (Sigma-Aldrich Co.) of various concentrations (5, 25, 50 μM) or 1,2-Bis(2-amino-5-methylphenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl) ester (BAPTA-AM) (10 μM) (Sigma-Aldrich Co.) for 20 minutes and then stimulated with PMACI (50 μM Phorbol myristate acetate +1 μg/mL calcium ionophore A23187) (Sigma-Aldrich Co.). The concentration of PMACI was used in subsequent experiments. The level of the intracellular calcium was measured every 10 seconds at 440 nm (excitation at 360 nm) in a spectrofluorometer. Experiments were independently repeated in triplicate.
Cytokines assay
The levels of TSLP and IL-1β were determined using a sandwich enzyme-linked immunosorbent assay method according to the manufacturer’s instructions (R&D Systems Inc., Minneapolis, MN, USA; Pharmingen, San Diego, CA, USA).
RNA preparation, reverse transcription, and quantitative real-time-PCR analysis
Total RNA from tissues and cells was extracted using Trizol reagent (Thermo Fisher Scientific). RNA was reversed transcribed into cDNAs using the Primer-Script™ one step reverse transcription-polymerase chain reaction (RT-PCR) kit (TaKaRa, Dalian, People’s Republic of China). The cDNA template was amplified by real-time RT-PCR using the SYBR® Premix Dimmer Eraser kit (TaKaRa). Gene expression in each sample was normalized to GADPH expression. The primer sequences used were as follows: for GADPH, 5′-TGGGGAAGGTGAAGGTCGG-3′ (forward) and 5′-CTGGAAGATGGTGATGGGA-3′ (reverse); TSLP, 5′-TATGAGTGGGACCAAAAGTACCG-3′ (forward) and 5′-GGGATTGAAGGTTAGGCTCTGG-3′ (reverse); and IL-1β, 5′-AAACAGATGAAGTGCTCCTT-3′ (forward) and 5′-TGGAGAACACCACTTGTTGC-3′ (reverse). The quantitative real time-PCR reaction was conducted under the following conditions: 95°C for 30 seconds, 40 cycles of 95°C for 5 seconds, and 60°C for 60 seconds. Real-time RT-PCR reactions were performed on the ABI7500 system (Thermo Fisher Scientific). The real-time PCRs were performed in triplicate. The relative expression fold change of messenger RNAs (mRNAs) was calculated by the 2−ΔΔCt method.
Cell viability assay
Cell proliferation assays were conducted using the MTT assay kits (R&D Systems Inc.) as described by the manufacturer. Briefly, HMC-1 cells were seeded into 96-well plates at a density of 1×104 cells/well. HMC-1 cells were pretreated with ANDRO for 2 hours and stimulated with PMACI for 8 hours. The cells were incubated in 0.1 mg/mL MTT solution at 37°C for 4 hours and lysed in dimethyl sulfoxide at room temperature for 10 minutes. The absorbance at 540 nm in each well was measured by a microplate reader (Bio-Rad Laboratories Inc., Hercules, CA, USA).
Caspase-1 activity
Caspase-1 activity was performed as described previously.Citation20
Western blot analysis
For harvested cells, nuclear and cytosolic proteins were prepared using the NE-PER Nuclear and Cytoplasmic Fractions Kit (Thermo Fisher Scientific). Protein content was determined by Bradford assay. Equal amounts (30–50 μg) of proteins were applied to an 8%–12% SDS-polyacrylamide separating gel and transferred to a PVDF Immobilon-P membrane (Millipore, Billerica, MA, USA). The membrane was blocked with 5% skim milk in Tris-Buffered Saline and Tween 20 and then probed with the indicated primary antibodies, with gentle shaking at 4°C overnight. Primary antibodies against NF-κB (p65), IκB (1:1,000), Rip2 (1:1,000), caspase-1 (1:700), and GADPH (1:3,000) (Abcam, Cambridge, UK) were used in this study. After washing the membranes three times, the immunoblots were incubated with the appropriated goat antirabbit IgG immunoglobulins-HRP secondary antibodies (Abcam) for 1 hour. Antibody-bound proteins were detected by BeyoECL Plus kit, Haimen, People’s Republic of China.
Immunofluorescence staining
HMC-1 cells were grown on glass chamber slides for 24 hours and pretreated with ANDRO (50 μM) for 2 hours, followed by PMACI stimulation for 20 minutes. Cells were then fixed with 4% paraformaldehyde in PBS for 30 minutes. The cells were then permeabilized in 0.1% Triton X-100 (Sigma-Aldrich Co.) for 30 minutes and blocked with 0.5% bovine serum albumin (Sigma-Aldrich Co.) in PBS for 30 minutes at room temperature. After washing with PBS, the cells were incubated with anti-p65 antibody for 1 hour at room temperature. After being washed with phosphate-buffered saline with Tween 20, the cells were incubated with appropriate fluorescein isothiocyanate-conjugated secondary antibodies (Abcam) and then stained with 4′,6-diamidino-2-phenylindole (Hoffman-La Roche Ltd., Basel, Switzerland). Images were later observed using a fluorescence microscope (Eclipse Ti, Nikon Corporation, Tokyo, Japan).
Sensitization with DNFB and administration of ANDRO
BALB/c mice were obtained from animal laboratory center of Xinhua Hospital. All animal experiments were performed in the animal laboratory center of Xinhua Hospital and in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no 85–23, revised 1996). The study protocol was approved by the Animal Care and Use committee of Xinhua Hospital (approval ID: 2014012). All mice were housed in specific pathogen-free rodent facilities, on sterilized, ventilated racks and supplied with commercial chow and sterile water, both previously autoclaved. Mice were sacrificed by CO2 inhalation.
The active sensitization procedure was performed as described previously.Citation20 Briefly, 100 μL 0.15% DNFB (Sigma-Aldrich Co.) dissolved in acetone was topically challenged to the shaved abdominal skins of mice on the first day. A week later, the shaved dorsal skins of mice were challenged with 100 μL 0.15% DNFB dissolved in acetone every 3 days until the 16th day. On the seventh day, ANDRO (50 mg/kg for oral, 30 mg/kg for local) or saline (control group) was administrated to DNFB-challenged mice on a daily basis until the end of the experiment. In the control group, the same volume of acetone was challenged to the shaved dorsal skin and saline was administrated. After anesthetization, dorsal skin samples were obtained 4 hours after the last DNFB challenge on the 16th day. The number of scratching behaviors was measured for 10 minutes, 4 hours after the last DNFB challenge.
Histological analysis
Dorsal skin samples were embedded in paraffin, cut into 4 μm serial sections. After dewaxing and dehydration, sections were stained with hematoxylin and eosin (H&E) or toluidine blue to estimate epidermal inflammation (hypertrophy and infiltration by inflammatory cells) and mast cell counts, respectively. Epithelial thickness and the number of mast cells were determined under the inverted microscope.
Statistical analysis
All statistical analyses were performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). The results shown are a summary of the data from at least three experiments and are presented as mean ± standard deviation. Statistical analysis of the results was performed with an independent t-test. P-values were two-sided and a value of less than 0.05 was considered to be statistically significant.
Results
ANDRO decreases the intracellular calcium level and downregulates the expression of TSLP in the PMACI-activated HMC-1 cells
The chemical structure of ANDRO is shown in . An increase in the intracellular calcium level has been shown to be a sufficient condition for the activation of mast cells and the production of a large number of cytokines.Citation21 The regulatory effect of ANDRO on the intracellular calcium level in the PMACI-activated HMC-1 cells was determined with a spectrofluorometer, and BAPTA-AM (a calcium chelator) was used as a positive control. The HMC-1 cells were pretreated with ANDRO (5, 25, 50 μM) or BAPTA-AM (10 μM) for 20 minutes and then activated with PMACI. The intracellular calcium level was measured every 10 seconds at 440 nm for 500 seconds. While PMACI increased the intracellular calcium level (in 0.5 mM EGTA containing media), ANDRO attenuated its effect in a dose-dependent manner (). As TSLP was demonstrated to be upregulated by a high intracellular calcium level in mast cells, we then examined the effects of ANDRO on the expression of TSLP. The HMC-1 cells were pretreated with ANDRO (5, 25, 50 μM) for 2 hours and then stimulated with PMACI. The ANDRO induced a significant reduction in TSLP production (P<0.05, ) and mRNA levels (P<0.05, ) from PMACI-activated HMC-1 cells in a dose-dependent manner. Furthermore, ANDRO exhibited no cytotoxic effects on HMC-1 cells at the above mentioned concentrations ().
Figure 1 Effects of ANDRO on the production and mRNA levels of TSLP in PMACI-stimulated HMC-1 cells.
Abbreviations: ANDRO, andrographolide; mRNA, messenger RNA; PMACI, phorbol myristate acetate/calcium ionophore A23187; HMC-1, human mast cell line 1; PCR, polymerase chain reaction; ELISA, enzyme-linked immunosorbent assay; TSLP, thymic stromal lymphopoietin; +, chemical is added; −, chemical is absent.
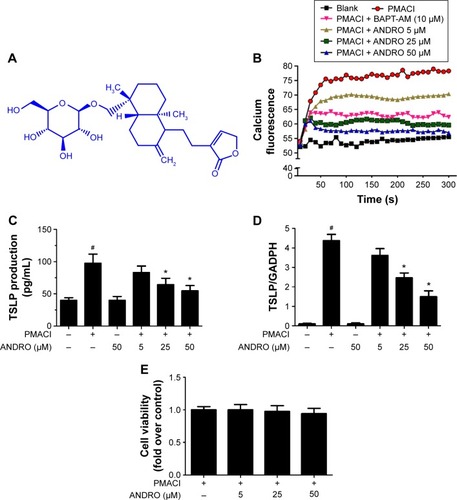
ANDRO inhibited the activation of the Rip2/caspase-1/NF-κB pathway in PMACI-activated HMC-1 cells
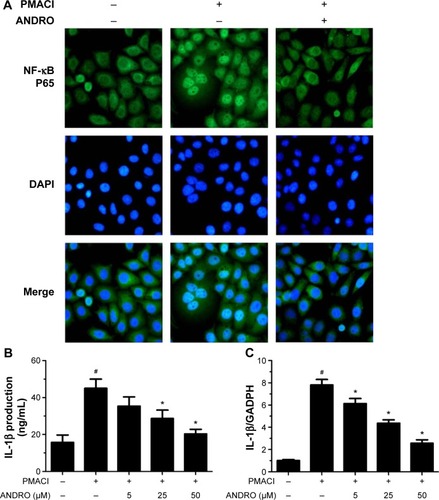
We would like to explore the underlying molecular mechanism through which ANDRO exerted its suppressive effects on TSLP. Previous studies have shown that the activation of the Rip2/caspase-1/NF-κB pathway is essential for the upregulation of TSLP.Citation20,Citation22,Citation23 We examined the effects that ANDRO had on the Rip2/caspase-1/NF-κB pathway. As illustrated in , ANDRO markedly attenuated the increase in the protein levels of Rip2 and caspase-1 induced by PMACI in a dose-dependent manner. In addition, ANDRO markedly inhibited the caspase-1 enzymatic activity (). In an inactivated state, NF-κB p65 is localized in the cytosol where it is combined with its inhibitor IκB. Upon phosphorylation, IκB is ubiquitinated and rapidly degraded, while activated NF-κB p65 is translocated into the nucleus and acts as a transcriptional factor.Citation23 As shown in , while PMACI induced a significant increase in the content of nuclear p65 protein, ANDRO inhibited the increase in a dose-dependent manner. Such an effect of ANDRO was confirmed with the immunofluorescence analysis (). As illustrated in , PMACI-induced IκB degradation was markedly blocked by pretreatment with ANDRO. As caspase-1 mainly serves to cleave IL-1β from their inactive precursors to active forms, and NF-κB is a potent transcriptional factor of IL-1β,Citation24 we studied whether ANDRO would have an inhibitory effect on the expression of IL-1β. As shown in , ANDRO markedly ameliorated the increase in the production and mRNA levels of IL-1β in PMACI-activated HMC-1 cells. These data suggest that ANDRO could inhibit PMACI-induced activation of the Rip2/caspase-1/NF-κB signaling pathway in HMC-1 cells.
Figure 2 ANDRO inhibited the activation of Rip2/caspase-1/NF-κB in PMACI-stimulated HMC-1 cells.
Abbreviations: ANDRO, andrographolide; Rip2, Receptor-interacting protein 2; PMACI, phorbol myristate acetate/calcium ionophore A23187; HMC-1, human mast cell line 1; NE, nuclear extract; CE, cytoplasmic extract.
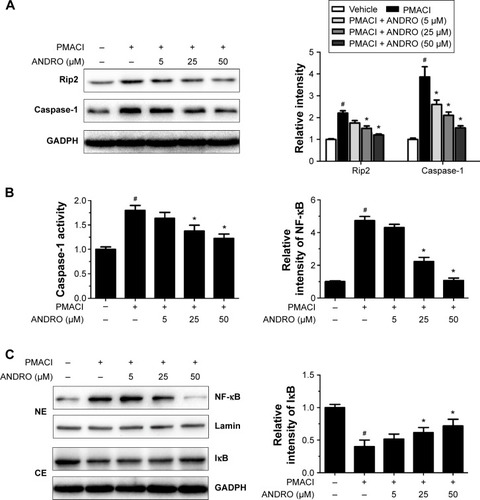
Figure 3 ANDRO suppressed the level of nuclear NF-κB and IL-1β in PMACI-stimulated HMC-1 cells.
Abbreviations: ANDRO, andrographolide; IL, interleukin; PMACI, phorbol myristate acetate/calcium ionophore A23187; HMC-1, human mast cell line 1; PCR, polymerase chain reaction; DAPI, 4′,6-diamidino-2-phenylindole; ELISA, enzyme-linked immunosorbent assay.
ANDRO, both orally and locally administrated, ameliorates clinical symptoms in DNFB-induced AD mice model
We then explored the regulatory effects of ANDRO on AD in vivo. Repeated skin exposure to haptens, such as DNFB, causes AD-like skin lesions in mice.Citation20,Citation22 The TSLP expression levels in skin lesions of DNFB-induced AD-like mice were reported to be elevated.Citation20,Citation22
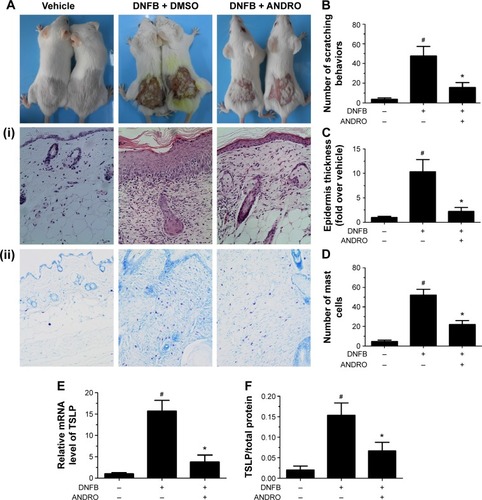
The noticeable AD-associated clinical symptoms such as erythema, excoriation, dryness, and erosion were present in DNFB-induced AD-like skin lesions; however, ANDRO, both orally and locally administrated, greatly ameliorates these symptoms in AD-like skin lesions ( and ). Furthermore, ANDRO significantly relieved scratching behavior induced by DNFB (). We examined the thickness of the epidermis and the infiltration of inflammatory cells in skin samples from the DNFB-challenged mice, with H&E staining analysis. We also examined the infiltration of mast cells with toluidine blue staining analysis. As illustrated in , the epidermis thickness and the number of mast cells were greatly decreased in skin lesions from ANDRO-administrated mice, compared to that of the control group.
Figure 4 ANDRO improved the clinical symptoms in DNFB-induced AD mice via oral administration.
Abbreviations: ANDRO, andrographolide; DNFB, 2,4-dinitrofluorobenzene; H&E, hematoxylin and eosin; mRNA, messenger RNA; TSLP, thymic stromal lymphopoietin; AD, atopic dermatitis; PCR, polymerase chain reaction; ELISA, enzyme-linked immunosorbent assay.
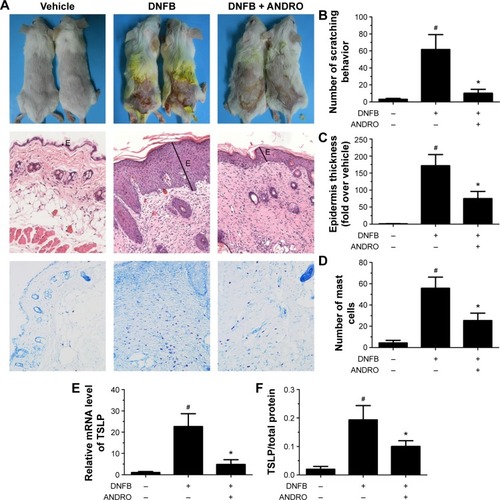
Figure 5 ANDRO improved the clinical symptoms in DNFB-induced AD mice via local administration.
Abbreviations: ANDRO, andrographolide; DMSO, Dimethyl sulfoxide; DNFB, 2,4-dinitrofluorobenzene; H&E, hematoxylin and eosin; mRNA, messenger RNA; TSLP, thymic stromal lymphopoietin; AD, atopic dermatitis; PCR, polymerase chain reaction; ELISA, enzyme-linked immunosorbent assay.
ANDRO decreased the expression level of TSLP in skin lesions from DNFB-induced AD-like mice
Following this process, we examined the effects of ANDRO on TSLP expression levels in skin lesions from DNFB-induced AD-like mice. Our results revealed that ANDRO greatly suppressed the upregulation of TSLP at both mRNA and protein levels in skin lesions induced by DNFB ().
Discussion
AD is one of the most common inflammatory cutaneous diseases. Although topical emollients, steroids, and oral antihistaminic or antiallergic agents were considered to be the first-line therapy for AD patients, their long-term use could induce a variety of adverse effects.Citation25 Thus, in the search for potential new drugs for treating AD without noticeable adverse effects, traditional Chinese medicine has manifested itself with high efficacy and lower risk of adverse effects.Citation26–Citation28 In the present study, we revealed that ANDRO has therapeutic effects on AD both in vitro and in vivo. We found that ANDRO suppressed the expression of caspase-1 and Rip2 as well as caspase-1 enzymatic activity in PMACI-activated HMC-1 cells. ANDRO also inhibited the translocation of NF-κB p65 into the nucleus and degradation of IκB induced by PMACI activation. Our study suggests that ANDRO might have a therapeutic effect on AD by downregulating TSLP via blocking the caspase-1/Rip2/NF-κB pathway. What is more, ANDRO, via oral and local administration, both markedly suppressed the inflammatory response induced by DNFB in vivo, as demonstrated by a decrease in the epidermis thickness and the number of inflammatory cells. ANDRO also suppressed the expression of TSLP in skin lesions induced by DNFB.
TSLP was demonstrated to play an important role in the pathogenesis of AD mainly through triggering dendritic cell-mediated Th2 inflammatory responses.Citation5 Moreover, the TSLP is produced mainly by mast cells, keratinocytes, and epithelial cells.Citation29–Citation31 Human skin mast cells play an essential role in the pathogenesis of AD.Citation32 In this study, we focused on the TSLP produced by mast cells. An increase in the intracellular calcium level has been shown to be important for the activation of mast cells and production of a large number of cytokines, such as TSLP.Citation21 The level of TSLP was increased in stimulated HMC-1 cells.Citation19,Citation33 Furthermore, TSLP was markedly reduced in mast cell-deficient mice.Citation34 Thus, we firstly examined whether ANDRO would attenuate the increase in the intracellular calcium level in activated HMC-1 cells. As expected, ANDRO pretreatment decreased the intracellular calcium level in PMACI-activated HMC-1 cells. In addition, ANDRO suppressed TSLP at both mRNA and protein levels in PMACI-activated HMC-1 cells.
To explore the underlying mechanism of the therapeutic effect of ANDRO on AD, we focused on the Rip2/caspase-1/NF-κB pathway, the activation of which has been proven to be essential for the expression of TSLP.Citation13,Citation22 The inflammasome is a key regulator of pathogen recognition and inflammation.Citation35 Inflammasomes are multiple protein complexes that serve as molecular platforms to activate caspase-1 and regulate maturation of a potent pro-inflammatory cytokine, IL-1β.Citation36 Multiple key proteins of inflammasomes contain caspase recruitment domains.Citation37 Quite unlike the role that most caspases play in apoptosis, caspase-1 is responsible for activating the inactivate precursor of IL-1β.Citation11 The NF-κB signaling pathway activated by caspase-1 contributes to the production of various inflammatory cytokines, including TSLP.Citation13 Caspase-1 transgenic mice, the mice that have an enhanced expression of caspase-1, are highly susceptible to haptens and can spontaneously develop recalcitrant dermatitis and skin ulcers.Citation38,Citation39 All these data suggest that caspase-1 is an important element in the pathogenesis of AD. Rip2 is an adaptor protein of several adaptors that compromise the inflammasome.Citation12,Citation40 Rip2 caspase recruitment domains interact with other caspase recruitment domain-containing proteins such as caspase-1 and lead to the activation of the NF-κB pathway.Citation12,Citation41 Macrophages from Rip2-deficient mice treated with lipopolysaccharide have decreased activation of NF-κB.Citation42 Together, Rip2/caspase-1 pathway is important in the activation of NF-κB.
This study showed that ANDRO suppressed the level of TSLP by inhibiting the caspase-1/Rip2/NF-κB pathway. These results suggest that ANDRO might be a potential therapeutic agent for AD.
Acknowledgments
This work was supported by National Natural Science Foundation of China for the Youth (81201227), Science and Technology Commission of Shanghai Municipality (12411951600, 13XD1402900), and 2016 CMA-L’OREAL China Skin Grant (S2016131417). The funding sources had no role in the study design; collection, analysis, and interpretation of data; writing of the manuscript; and decision to submit the manuscript for publication.
Disclosure
The authors report no conflicts of interest in this work.
References
- SchmittJLanganSDeckertSHarmonising Outcome Measures for Atopic Dermatitis (HOME) InitiativeAssessment of clinical signs of atopic dermatitis: a systematic review and recommendationJ Allergy Clin Immunol20131321337134724035157
- HarskampCTArmstrongAWImmunology of atopic dermatitis: novel insights into mechanisms and immunomodulatory therapiesSemin Cutan Med Surg20133213213924175400
- KawakamiTAndoTKimuraMWilsonBSKawakamiYMast cells in atopic dermatitisCurr Opin Immunol20092166667819828304
- YamanakaKMizutaniHThe role of cytokines/chemokines in the pathogenesis of atopic dermatitisCurr Probl Dermatol201141809221576949
- JariwalaSPAbramsEBensonAFodemanJZhengTThe role of thymic stromal lymphopoietin in the immunopathogenesis of atopic dermatitisClin Exp Allergy2011411515152021672057
- LeeJNohGLeeSYounYRhimJAtopic dermatitis and cytokines: recent patents in immunoregulatory and therapeutic implications of cytokines in atopic dermatitis – part I: cytokines in atopic dermatitisRecent Pat Inflamm Allergy Drug Discov2012622224722827753
- HarvimaITNilssonGMast cells as regulators of skin inflammation and immunityActa Derm Venereol20119164465021879235
- LiuFTGoodarziHChenHYIgE, mast cells, and eosinophils in atopic dermatitisClin Rev Allergy Immunol20114129831021249468
- IndraAKEpidermal TSLP: a trigger factor for pathogenesis of atopic dermatitisExpert Rev Proteomics20131030931123992412
- LamkanfiMVande WalleLKannegantiTDDeregulated inflammasome signaling in diseaseImmunol Rev201124316317321884175
- BoostKAHoeglSHofstetterCTargeting caspase-1 by inhalation-therapy: effects of Ac-YVAD-CHO on IL-1 beta, IL-18 and downstream proinflammatory parameters as detected in rat endotoxaemiaIntensive Care Med20073386387117384935
- StaalJBekaertTBeyaertRRegulation of NF-κB signaling by caspases and MALT1 paracaspaseCell Res201121405421119681
- MoonPDKimHMThymic stromal lymphopoietin is expressed and produced by caspase-1/NF-κB pathway in mast cellsCytokine20115423924321463955
- BaoGQShenBYPanCPAndrographolide causes apoptosis via inactivation of STAT3 and Akt and potentiates antitumor activity of gemcitabine in pancreatic cancerToxicol Lett2013222233523845849
- ZhangQQDingYLeiYAndrographolide suppress tumor growth by inhibiting TLR4/NF-κB signaling activation in insulinomaInt J Biol Sci20141040441424719558
- LiuWGuoWGuoLAndrographolide sulfonate ameliorates experimental colitis in mice by inhibiting Th1/Th17 responseInt Immunopharmacol20142033734524704625
- NugrohoAELindawatiNYHerlyantiKWidyastutiLPramonoSAnti-diabetic effect of a combination of andrographolide-enriched extract of Andrographis paniculata (Burm f.) Nees and asiaticoside-enriched extract of Centella asiatica L. in high fructose-fat fed ratsIndian J Exp Biol2013511101110824579376
- LiXZhangCShiQImproving the efficacy of conventional therapy by adding andrographolide sulfonate in the treatment of severe hand, foot, and mouth disease: a randomized controlled trialEvid Based Complement Alternat Med2013201331625023401711
- HanNRKimHMJeongHJThymic stromal lymphopoietin is regulated by the intracellular calciumCytokine20125921521722578802
- HanNRMoonPDKimHMJeongHJTryptanthrin ameliorates atopic dermatitis through down-regulation of TSLPArch Biochem Biophys2014542142024295961
- MaHTBeavenMARegulators of Ca(2+) signaling in mast cells: potential targets for treatment of mast cell-related diseases?Adv Exp Med Biol2011716629021713652
- HanNRKangSWMoonPDJangJBKimHMJeongHJGenuine traditional Korean medicine, Naju Jjok (Chung-Dae, Polygonum tinctorium) improves 2,4-dinitrofluorobenzene-induced atopic dermatitis-like lesional skinPhytomedicine20142145346024200496
- HeymannMCWinklerSLukschHHuman procaspase-1 variants with decreased enzymatic activity are associated with febrile episodes and may contribute to inflammation via RIP2 and NF-κB signalingJ Immunol20141924379438524706726
- ZhuCXiongZChenXArtemisinin attenuates lipopolysaccharide-stimulated proinflammatory responses by inhibiting NF-κB pathway in microglia cellsPLoS One201274e3512522514713
- BerkeRSinghAGuralnickMAtopic dermatitis: an overviewAm Fam Physician201286354222962911
- WuCTHuangKSYangCHInhibitory effects of cultured Dendrobium tosaense on atopic dermatitis murine modelInt J Pharm201446319320023958754
- LiSKuchtaKTamaruNEfficacy of a novel herbal multi-component traditional Chinese medicine therapy approach in patients with atopic dermatitisForsch Komplementmed20132018919623860020
- GuSYangAWXueCCChinese herbal medicine for atopic eczemaCochrane Database Syst Rev20139CD00864224018636
- Yamamoto-KasaiEYasuiKShichijoMSakataTYoshiokaTImpact of TRPV3 on the development of allergic dermatitis as a dendritic cell modulatorExp Dermatol2013221282082424164484
- JiaXZhangHCaoXYinYZhangBActivation of TRPV1 mediates thymic stromal lymphopoietin release via the Ca2+/NFAT pathway in airway epithelial cellsFEBS Lett2014588173047305424931369
- WilsonSRThéLBatiaLMThe epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itchCell2013155228529524094650
- KritasSKSagginiAVarvaraGImpact of mast cells on the skinInt J Immunopathol Pharmacol20132685585924355220
- HanNRKimHMJeongHJThe β-sitosterol attenuates atopic dermatitis-like skin lesions through down-regulation of TSLPExp Biol Med (Maywood)201423945446424510054
- TakaiTTSLP expression: cellular sources, triggers, and regulatory mechanismsAllergol Int20126131722270071
- DaiXSayamaKTohyamaMMite allergen is a danger signal for the skin via activation of inflammasome in keratinocytesJ Allergy Clin Immunol201112780681421272927
- LeavyOInflammasomes: polymeric assemblyNat Rev Immunol20141428724722480
- MariathasanSNewtonKMonackDMDifferential activation of the inflammasome by caspase-1 adaptors ASC and IpafNature200443021321815190255
- YamanakaKTanakaMTsutsuiHSkin-specific caspase-1-transgenic mice show cutaneous apoptosis and pre-endotoxin shock condition with a high serum level of IL-18J Immunol2000165997100310878376
- MurakamiTYamanakaKTokimeKTopical suplatast tosilate (IPD) ameliorates Th2 cytokine-mediated dermatitis in caspase-1 transgenic mice by downregulating interleukin-4 and interleukin-5Br J Dermatol2006155273216792748
- ThomeMHofmannKBurnsKIdentification of CARDIAK, a RIP-like kinase that associates with caspase-1Curr Biol199888858889705938
- McCarthyJVNiJDixitVMRIP2 is a novel NF-kappaB-activating and cell death-inducing kinaseJ Biol Chem199827316968169759642260
- ChinAIDempseyPWBruhnKMillerJFXuYChengGInvolvement of receptor-interacting protein 2 in innate and adaptive immune responsesNature200241619019411894097
