Abstract
Purpose
Epidermal growth factor receptor (EGFR) is usually overexpressed in nasopharyngeal carcinoma (NPC). We tested the antitumor effects of irreversible ErbB family inhibitor afatinib on human NPC using in vitro and in vivo models.
Materials and methods
The effect of afatinib on NPC cells was evaluated using the Cell Counting Kit 8 (CCK8) assay, flow cytometry, and Western blot analyses. The effect of afatinib, as either a single agent or in combination with gemcitabine (GEM), on tumor growth was determined using NPC tumor xenografts in mice.
Results
Afatinib inhibited cell growth in all three NPC cell lines tested in a dose-dependent manner. Afatinib promoted cell cycle arrest at the S and G2/M phases, and it significantly inhibited epidermal growth factor (EGF)-induced activation of EGFR and its downstream signaling factors. Co-treatment with afatinib and GEM more effectively inhibited tumor growth than either drug alone but was associated with increased toxicity.
Conclusion
Afatinib induced cell cycle arrest and inhibited the proliferation of NPC cell lines. Afatinib in combination with GEM demonstrated significant antitumor effect in an NPC xenograft model. The administration of afatinib with GEM in NPC needs to be modified in order to be effective and tolerable.
Introduction
Nasopharyngeal carcinoma (NPC) has a distinct geographic distribution, with low morbidity in most areas worldwide (generally <1/100,000).Citation1 Eighty percent of all NPC cases occur in the People’s Republic of China, with the highest incidence rates in South China.Citation2 NPC-related morbidity in the northern region of the People’s Republic of China is comparable to that observed in the remaining parts of the world. The incidence of NPC in South China is the highest in the world (~20–30/100,000 for nearly 30 years).Citation3
NPC is very sensitive to radiotherapy, and radiotherapy is the treatment of choice for early nonmetastatic NPC.Citation4 With advances in imaging and radiation therapy, the 5-year local control rate of nonmetastatic NPC has risen to 94.9%, and the 5-year relapse-free survival rate has reached 76.7%. The main cause of treatment failure of NPC is distant metastases, whereby ~20% of patients with locally advanced disease present with remote metastasis.Citation5
More than 90% of NPCs are undifferentiated carcinomas, which show relatively good reactivity to chemotherapy. Many cytotoxic chemotherapeutic agents have been tested for NPC, and currently, platinum-based treatment is the basic regimen for palliative care. For salvage therapy after failure of platinum regimen, yew, gemcitabine (GEM), and others are effective.Citation6,Citation7 Despite the relatively high efficacy of these therapies and the eventual availability of various new drugs, the survival of patients with late NPC has not significantly improved, with progression-free survival (PFS) at about 5.6–10.6 months and overall survival (OS) at about 7.6–19.6 months.Citation6,Citation8–Citation10 Identification of novel drugs, especially targeted therapies, is needed to improve the mortality rate in patients with advanced NPC.
Epidermal growth factor receptor (EGFR) is overexpressed in 73%–89% of NPC patients, contributing to an increased risk of metastasis and decreased OS.Citation11–Citation14 However, EGFR mutations are rarely detected in NPC,Citation15 suggesting that EGFR mutations might not be a critical factor in NP carcinogenesis. The first-generation EGFR–tyrosine kinase inhibitors (TKIs) were effective in patients with EGFR mutations, which may explain why previous studies found that EGFR-TKIs only stabilized the disease in most patients with refractory metastatic NPC.Citation16,Citation17
Compared to the first-generation reversible EGFR-TKIs gefitinib and erlotinib, afatinib is a potent irreversible ErbB inhibitor that blocks the activity of all kinases related to ErbB family members, including wild-type and mutated EGFR (eg, T790M) and human epidermal growth factor receptor 2 (HER2).Citation18 Owing to its efficacy in the EGFR mutation-associated non-small-cell lung cancer (NSCLC), afatinib was approved as a first-line therapy for advanced NSCLC with mutant EGFR.Citation19
Recent studies have focused on whether afatinib can be applied to EGFR wild-type tumors, and some preclinical and clinical data have shown that afatinib does suppress the growth of cancer cells with wild-type EGFR.Citation19,Citation20 There are ongoing clinical studies investigating the antitumor efficacy of afatinib in multiple solid tumors with ErbB deregulations.Citation21–Citation23 Similar to NPC, overexpression of EGFR is commonly found in squamous cell cancer of the head and neck (SCCHN). Young et alCitation24 demonstrated that the efficacy of afatinib was comparable to that of cetuximab in SCCHN cell lines and xenograft models. A Phase II studyCitation25 comparing the efficacy of cetuximab with afatinib in refractory recurrent SCCHN patients showed similar response and survival rates. Taken together, these data provided the rationale for investigating the use of afatinib for NPC. A Phase III clinical trial compared afatinib with methotrexate (MTX) as a second-line treatment for patients with refractory metastatic head and neck cancer after failure of platinum treatment and found that afatinib significantly prolonged PFS relative to MTX (2.6 months vs 1.7 months; P=0.03). In addition, afatinib improved the objective response rate (ORR: 10.2% vs 5.6%; P=0.10) and the disease control rate (49.1% vs 38.5%; P=0.04), with tolerable side effects,Citation26 relative to MTX. These results showed that afatinib as a second-line therapeutic regimen had a better curative effect than MTX in refractory head and neck cancer and that its application in head and neck cancer required further exploration.
In this study, we explored whether afatinib has curative effects in preclinical studies of NPC, which will shed light on its use for other applications. We selected GEM for combination with afatinib because GEM is a novel and effective agent for the treatment of NPC and is currently being investigated as a first-line therapy in metastatic NPC.Citation6,Citation8–Citation10,Citation27 Moreover, former in vitro studies showed that combination treatment with afatinib and GEM had synergistic effects.Citation23 We hypothesized that combination therapy with afatinib and GEM may have enhanced the antitumor activity relative to the individual administration of these two agents.
Materials and methods
Cell lines
All cell line experiments were approved by the Ethical Committee of Sun Yat-sen University Cancer Center. Three poorly differentiated human NPC cell lines were studied: HNE-1, CNE-2, and SUNE-1. HNE-1 was kindly provided by Professor Kaitai Yao (Southern Medical University, Guangzhou, People’s Republic of China). CNE-2 and SUNE-1 were kindly provided by Professor Musheng Zeng (Sun Yat-Sen University Cancer Center, Guangzhou, People’s Republic of China). Cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 100 units/mL penicillin, and 100 units/mL streptomycin at 37°C in a humidified atmosphere containing 5% CO2. Logarithmically growing cells were used in the experiments.
Reagents and drug preparation
Afatinib was kindly provided by Boehringer Ingelheim Pharmaceuticals (Ingelheim am Rhein, Germany). GEM (Gemzar; Elililly Pharmaceuticals, Indiana, IN, USA) was obtained as a commercial product from our hospital pharmacy.
Afatinib was dissolved in dimethylsulfoxide at a stock concentration of 10 mM and stored at −20°C. For in vivo studies, afatinib was dissolved in plain water at a concentration of 1.25 mg/mL. GEM was dissolved in plain water at a stock concentration of 100 mM and stored at −20°C.
Proliferation assay
Tumor cells were cultured in 96-well plates at an appropriate density per well. Varying concentrations of afatinib, GEM, and the combination were added to the cells 24 hours after plating and incubated for 72 hours, followed by Cell Counting Kit 8 (CCK8; Dojindo, Tokyo, Japan) assay. Afatinib and GEM were added at the same concentration when in the combination assay (eg, both 0.625 μM). The optical density was measured at 450 nm on an enzyme-linked immunosorbent assay reader (SpectraMax M5; Molecular Devices, Sunnyvale, CA, USA). The half-maximal inhibitory concentration (IC50) value was defined as the concentration resulting in 50% of cell growth inhibition relative to untreated control cells. The assay was performed in triplicate in more than three independent experiments. The combination index (CI) was calculated by CalcuSyn (Biosoft, Cambridge, UK). CI <1 indicates synergism; CI =1 indicates additive effects; CI >1 indicates antagonism.Citation28
Cell cycle analysis
Cells were seeded into six-well plates containing RPMI 1640/10% FBS with or without various concentrations of afatinib. Following 48-hour incubation, cells were harvested and fixed with 70% ethanol and stored at −20°C. Tumor cells were incubated with propidium iodide/RNase buffer (Becton Dickinson, Bedford, MA, USA) for 30 minutes at room temperature in the dark. Analysis was performed using the FACScan flow cytometer (Beckman Coulter Inc., Brea, CA, USA) and CellQuest Pro software (Becton, Dickinson and Company, Franklin Lakes, NJ, USA).
Western blotting
Cells were treated with afatinib for 24 hours and then stimulated with 10 nM epidermal growth factor (EGF) (Cell Signaling Technology, Beverley, MA, USA) for 30 minutes before harvest. Cells were harvested and lysed in cell lysis buffer (Cell Signaling Technology). Proteins were resolved on sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membrane (Hoffman-La Roche Ltd., Basel, Switzerland). The membranes were incubated overnight at 4°C with primary antibodies. After being incubated with the appropriate horseradish peroxidase-conjugated secondary antibody for 1 hour at room temperature, bands were detected using an enhanced chemiluminescence system (Cell Signaling Technology). The expression of phosphorylated-EGFR (pEGFR), pAKT, and p-extracellular regulated kinase (pERK) was analyzed to detect the activation of EGFR and its downstream pathways (all antibodies were purchased from Cell Signaling Technology). The quantitative analysis was performed using ImageJ (National Institutes of Health, Bethesda, MD, USA).
Tumor xenograft studies
Six- to 8-week-old male BALB/c nude mice weighing ~16–18 g were maintained under specific pathogen-free conditions. All animal experiments were conducted in accordance with the United Kingdom Co-ordinating Committee on Cancer Research Guidelines for the welfare of animals in experimental neoplasia. All animal experiments were approved by the Experimental Animal Ethical Committee of Sun Yat-sen University. HNE-1 cells (1×107 cells resuspended in 0.2 mL of 0.9% NaCl solution) were inoculated subcutaneously into the right flank of nude mice. When tumors reached ~150 mm3, animals were randomly assigned to one of four groups (n=8 per group): afatinib group, GEM group, combination group, and control group. Afatinib (12.5 mg/kg) was administered by oral gavage for 5 days a week. Intraperitoneal injection of GEM (100 mg/kg) was dosed with 0.9% NaCl every week. NaCl (0.9%, 10 mL/kg) was administered by oral gavage for 5 days a week, and intraperitoneal injection of 0.9% NaCl was administered every week as control treatment. Mice body weight and tumor size were measured and recorded every 3 days. Tumor volume was calculated using the following equation: volume (mm3) = length × widthCitation2 ×0.5. After 4 weeks of treatment, mice were sacrificed, and tumors were harvested, fixed in 10% buffered formalin, and embedded in paraffin.
Statistics
Statistical analyses were performed using SPSS Version 16.0 software (SSPS Inc., Chicago, IL, USA). Data were expressed as mean ± standard deviation. Comparisons in the in vivo studies were analyzed by the unpaired t-test with Welch correction. Two-sided P<0.05 was considered statistically significant.
Results
Afatinib inhibited proliferation in NPC cell lines and showed synergistic effect with GEM
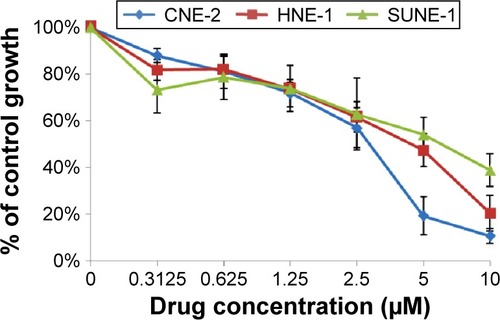
Three human NPC cell lines – CNE-2, HNE-1, and SUNE-1 – were treated with afatinib (0–10 μM) for 72 hours. Afatinib demonstrated a steep dose–response relationship, with dose-dependent growth inhibition in all cell lines (). The IC50 values for afatinib in HNE-1, CNE-2, and SUNE-1 cell lines were 4.41±0.73 μM, 2.81±0.35 μM, and 6.93±0.54 μM, respectively. When combined with GEM, the CI values were separately 0.293, 0.435, and 0.654 when the concentrations were 0.625 μM, 1.25 μM, and 2.5 μM for both drugs, which indicates that synergistic effect exists when afatinib is combined with GEM.
Figure 1 Inhibitory effect of afatinib on growth of NPC cell lines.
Abbreviations: CNE, HNE, SUNE: three human NPC cell lines; NPC, nasopharyngeal carcinoma.
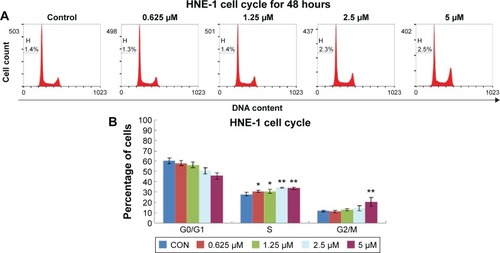
Afatinib arrested the cell cycle at S and G2/M phases
The effects of afatinib on the cell cycle of NPC cells were determined by flow cytometry. HNE-1 cells were treated with various concentrations (0 μM, 0.625 μM, 1.25 μM, 2.5 μM, and 5 μM) of afatinib for 48 hours, resulting in a dose-dependent increase in the percentage of cells in the S and G2/M phases and a concomitant decrease in the proportion of cells in the G0/G1 phase ().
Figure 2 Afatinib induced cell cycle arrest at G1 phase in HNE-1 cell line.
Abbreviations: CON, control; H, percentage of sub-G1 phase; HNE-1, human NPC cell line; NPC, nasopharyngeal carcinoma; SD, standard deviation.
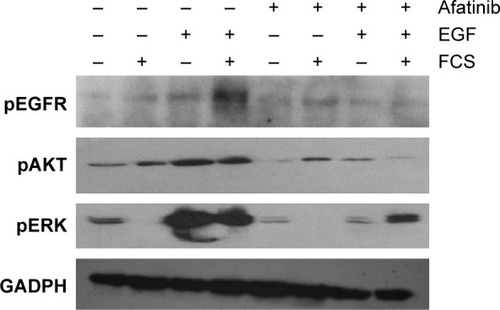
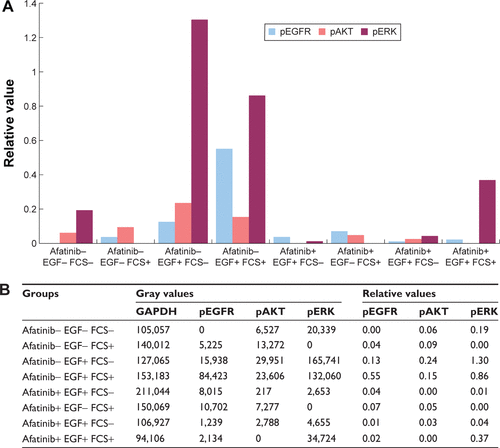
Afatinib inhibited the EGFR signaling pathway
We examined the activity of EGFR and its downstream targets AKT and ERK in HNE-1 cells treated with afatinib. As shown in , after exposure to afatinib for 24 hours, EGF-induced phosphorylation of EGFR, AKT, and ERK was suppressed by afatinib. However, afatinib treatment did not affect the levels of p-EGFR, p-AKT, and p-ERK in the absence of EGF stimulation. The quantitative analysis is provided in .
Figure 3 Effect of afatinib on levels of phosphorylated forms of EGFR, AKT, and ERK in the HNE-1 cell line.
Abbreviations: EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; ERK, extracellular regulated protein kinases; FCS, fetal calf serum; GADPH, glyceraldehyde-3-phosphate dehydrogenase; HNE-1, human NPC cell line; NPC, nasopharyngeal carcinoma; p, phosphorylated.
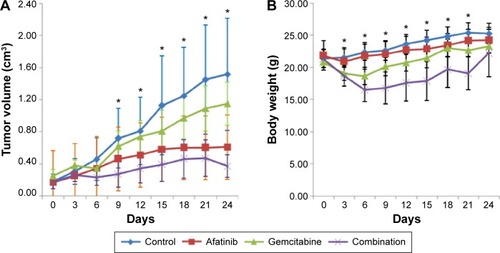
Antitumor activity of afatinib in vivo
We evaluated the efficacy of afatinib in HNE-1 xenograft mouse model, both as a single agent and in combination with GEM. As shown in , afatinib modestly inhibited NPC tumor growth in the HNE-1 xenograft model. In the human NPC cell line xenograft model, combination treatment with afatinib and GEM significantly inhibited tumor growth more than that obtained by the two treatments alone. However, toxicity was more severe, as evidenced by a greater decrease in body weight, in the combination group than in the other treatment groups ().
Figure 4 Effect of afatinib, gemcitabine, or combination on the growth of HNE-1 xenografts.
Abbreviations: HNE-1, human NPC cell line; NPC, nasopharyngeal carcinoma.
Discussion
This preclinical study is the first to investigate the efficacy of afatinib in NPC, and our findings suggest that afatinib is a promising agent for the treatment of NPC. The IC50 values of afatinib were found to be around 3–7 μM in three poorly differentiated human NPC cell lines, suggesting that these cell lines are marginally sensitive to afatinib monotherapy. In the Phase I trials of afatinib monotherapy for advanced solid tumors, different doses of afatinib lower than the maximum tolerated dose achieved an average steady-state maximum concentration (Cmax,ss) of 63.4 ng/mL (~0.13 μM). We observed that the growth inhibition rate of this dose of afatinib was only about 10%–20%. The IC50 of all three cell lines after conversion was 1.37–3.38 μg/mL (the molecular weight of afatinib is ~486 g/mol), and this drug dosage is much higher than the human tolerated blood drug concentration of afatinib. Western blot data also showed that afatinib did not inhibit the EGFR signaling pathway when EGFR was not overactive. In tumors not driven by EGFR mutations, such as gastric cancer, pancreatic cancer, and SCCHN, the combination of afatinib and other agents or radiotherapy might have better response than afatinib alone.Citation21–Citation23 Therefore, we believe that the currently used dose of afatinib may fail to produce satisfactory curative effects as monotherapy in patients with advanced NPC, whereas its use in combination with other drugs (targeted drugs or chemotherapy drug) may be ideal.
Previously, preclinical studiesCitation22,Citation23 reported that afatinib mainly inhibited the growth of tumor cell lines by causing retardation of the cell’s G1 phase. Because chemotherapy drugs mainly act on cells in the proliferating and division phase, there is no appropriate theoretical basis to support the existence of synergic efficiency during concurrent application of small molecule inhibitors and chemotherapy drugs. In addition, both in vitro and in vivo experiments have shown no positive results.Citation29 In our study, cell cycle analysis demonstrated that afatinib induced cell arrest in the G2/M and S phases. This is a very interesting finding, as it provides some support for the use of combination treatments with afatinib and chemotherapeutic agents for NPC patients. Because GEM is an S phase-specific cytotoxic agent, we predicted that this combination might increase efficacy. Our in vivo experiments using combination treatment with afatinib and GEM confirmed this hypothesis.
The human NPC xenograft model demonstrated good sensitivity toward afatinib monotherapy treatment at 12.5 mg/kg. Afatinib in combination with GEM resulted in significant inhibition of tumor growth. However, more weight loss was observed in the combination group, showing that toxicity might limit the clinical applicability of concurrent therapy. To achieve safe and effective results in subsequent clinical studies, the dosages of both afatinib and GEM need to be further explored.
Another option for including afatinib in treatment regimens for NPC is sequential therapy. Previous studies have shown that most EGFR-TKIs induce G0/G1 cell cycle arrest, which might protect cells from the cytotoxic effects of cell cycle phase-dependent chemotherapy agents.Citation29 Sequential administration of EGFR-TKIs following chemotherapy may provide greater efficacy than concurrent administration in NSCLC.Citation30,Citation31 In other preclinical models,Citation32 sequential administrations of two potent agents could delay disease progression. In our preclinical study, afatinib and GEM, both active drugs against NPC, were administered concomitantly. The combination treatment, although effective, induced more toxicity than either agent alone did. On the basis of these observations, we suggest modifying the method of administration. Further studies are needed to evaluate the full potential of this combination.
In conclusion, afatinib effectively arrested the growth of NPC cell lines in vitro. Data from the NPC xenograft model suggested that concurrent administration of afatinib with GEM might lead to significant efficacy but at the cost of enhanced toxicity. Combination therapy of GEM along with afatinib might be an effective treatment option for NPC once alternative dosage or methods of administration (schedules) are optimized in model systems, resulting in a larger therapeutic window for this combination.
Acknowledgments
Cong Xue and Ying Tian are joint first authors. This study was supported by a Chinese National Natural Science Foundation project (grant numbers 81372502 and 81201917), the Specialized Research Fund for the Doctoral Program of Higher Education (20120171120116), the Young Teacher Training Program of Sun Yat-Sen University (14ykpy38), and the Outstanding Young Talent Cultivation Project of Sun Yat-Sen University Cancer Center (04140701).
Supplementary material
Figure S1 The relative quantity of phosphorylated EGFR, AKT, and ERK in HNE-1 cell line.
Abbreviations: EGF, epidermal growth factor; ERK, extracellular regulated protein kinase; FCS, fetal calf serum; GADPH, glyceraldehyde-3-phosphate dehydrogenase; HNE-1, human NPC cell line; pEGFR, phosphorylated epidermal growth factor receptor; p, phosphorylated.
Disclosure
The authors report no conflicts of interest in this work.
References
- Cancer incidence in five continents. Volume VII Iarc SCI Publ1997ixxxiv11240
- WeiKRZhengRSZhangSWLiangZHOuZXChenWQNasopharyngeal carcinoma incidence and mortality in China in 2010Chin J Cancer201433838138725096544
- ZhangLFLiYHXieSHIncidence trend of nasopharyngeal carcinoma from 1987 to 2011 in Sihui County, Guangdong Province, South China: an age-period-cohort analysisChin J Cancer201534835035726058679
- MaBBHuiEPChanATSystemic approach to improving treatment outcome in nasopharyngeal carcinoma: current and future directionsCancer Sci20089971311131818498420
- XiaoWWHuangSMHanFLocal control, survival, and late toxicities of locally advanced nasopharyngeal carcinoma treated by simultaneous modulated accelerated radiotherapy combined with cisplatin concurrent chemotherapy: long-term results of a phase 2 studyCancer201111791874188321509764
- NganRKYiuHHLauWHCombination gemcitabine and cisplatin chemotherapy for metastatic or recurrent nasopharyngeal carcinoma: report of a phase II studyAnn Oncol20021381252125812181249
- TanEHKhooKSWeeJPhase II trial of a paclitaxel and carboplatin combination in Asian patients with metastatic nasopharyngeal carcinomaAnn Oncol199910223523710093695
- ZhangLZhangYHuangPYXuFPengPJGuanZZPhase II clinical study of gemcitabine in the treatment of patients with advanced nasopharyngeal carcinoma after the failure of platinum-based chemotherapyCancer Chemother Pharmacol2008611333817909810
- MaBBHuiEPWongSCMulticenter phase II study of gemcitabine and oxaliplatin in advanced nasopharyngeal carcinoma – correlation with excision repair cross-complementing-1 polymorphismsAnn Oncol200920111854185919549713
- WangCCChangJYLiuTWLinCYYuYCHongRLPhase II study of gemcitabine plus vinorelbine in the treatment of cisplatin-resistant nasopharyngeal carcinomaHead Neck2006281748016331692
- FujiiMYamashitaTIshiguroRTashiroMKameyamaKSignificance of epidermal growth factor receptor and tumor associated tissue eosinophilia in the prognosis of patients with nasopharyngeal carcinomaAuris Nasus Larynx200229217518111893453
- MaBBPoonTCToKFPrognostic significance of tumor angiogenesis, Ki 67, p53 oncoprotein, epidermal growth factor receptor and HER2 receptor protein expression in undifferentiated nasopha-ryngeal carcinoma – a prospective studyHead Neck2003251086487212966511
- SheenTSHuangYTChangYLEpstein-Barr virus-encoded latent membrane protein 1 co-expresses with epidermal growth factor receptor in nasopharyngeal carcinomaJpn J Cancer Res199990121285129210665644
- ChuaDTNichollsJMShamJSAuGKPrognostic value of epidermal growth factor receptor expression in patients with advanced stage nasopharyngeal carcinoma treated with induction chemotherapy and radiotherapyInt J Radiat Oncol Biol Phys2004591112015093894
- LeeSCLimSGSooRLack of somatic mutations in EGFR tyrosine kinase domain in hepatocellular and nasopharyngeal carcinomaPharmacogenet Genomics2006161737416344724
- ChuaDTWeiWIWongMPShamJSNichollsJAuGKPhase II study of gefitinib for the treatment of recurrent and metastatic nasopharyngeal carcinomaHead Neck200830786386718213730
- MaBHuiEPKingAA phase II study of patients with metastatic or locoregionally recurrent nasopharyngeal carcinoma and evaluation of plasma Epstein-Barr virus DNA as a biomarker of efficacyCancer Chemother Pharmacol2008621596417762933
- LiDAmbrogioLShimamuraTBIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer modelsOncogene200827344702471118408761
- SequistLVYangJCYamamotoNPhase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutationsJ Clin Oncol201331273327333423816960
- MillerVAHirshVCadranelJAfatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trialLancet Oncol201213552853822452896
- SchutzeCDorflerAEichelerWCombination of EGFR/HER2 tyrosine kinase inhibition by BIBW 2992 and BIBW 2669 with irradiation in FaDu human squamous cell carcinomaStrahlenther Onkol2007183525626417497097
- KhelwattySAEssapenSSeddonAMModjtahediHGrowth response of human colorectal tumour cell lines to treatment with afatinib (BIBW2992), an irreversible erbB family blocker, and its association with expression of HER family membersInt J Oncol201139248349121617858
- IoannouNDalgleishAGSeddonAMAnti-tumour activity of afatinib, an irreversible ErbB family blocker, in human pancreatic tumour cellsBr J Cancer2011105101554156221970876
- YoungNRSoneruCLiuJAfatinib efficacy against squamous cell carcinoma of the head and neck cell lines in vitro and in vivoTarget Oncol201510450150825559287
- SeiwertTYFayetteJCupissolDA randomized, phase II study of afatinib versus cetuximab in metastatic or recurrent squamous cell carcinoma of the head and neckAnn Oncol20142591813182024928832
- MachielsJPHaddadRIFayetteJAfatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): an open-label, randomised phase 3 trialLancet Oncol201516558359425892145
- XueCHuangYHuangPYPhase II study of sorafenib in combination with cisplatin and 5-fluorouracil to treat recurrent or metastatic nasopharyngeal carcinomaAnn Oncol20132441055106123172635
- ChouTCDrug combination studies and their synergy quantification using the Chou-Talalay methodCancer Res201070244044620068163
- HuiEPLuiVWWongCSPreclinical evaluation of sunitinib as single agent or in combination with chemotherapy in nasopharyngeal carcinomaInvest New Drugs20112961123113120467883
- LiTLingYHGoldmanIDPerez-SolerRSchedule-dependent cytotoxic synergism of pemetrexed and erlotinib in human non-small cell lung cancer cellsClin Cancer Res200713113413342217545550
- WuYLLeeJSThongprasertSIntercalated combination of chemotherapy and erlotinib for patients with advanced stage non-small-cell lung cancer (FASTACT-2): a randomised, double-blind trialLancet Oncol201314877778623782814
- SantosCDTijeras-RaballandASerovaMEffects of preset sequential administrations of sunitinib and everolimus on tumour differentiation in Caki-1 renal cell carcinomaBr J Cancer20151121869425422908
